408:
Decomposition of mixtures of mono- and heterometallic alkoxide derivatives has also been examined. This method represents a prospective approach possessing an advantage of capability of obtaining functional materials with increased phase and chemical homogeneity and controllable grain size (including the preparation of nanosized materials) at relatively low temperature (less than 500−900 °C) as compared with the conventional techniques.
43:
31:
178:
283:
can be used in place of sodium, and most alcohols can be used in place of methanol. Generally, the alcohol is used in excess and left to be used as a solvent in the reaction. Thus, an alcoholic solution of the alkali alkoxide is used. Another similar reaction occurs when an alcohol is reacted with a
407:
powders of oxide or metallic phases. This approach is a basis of processes of fabrication of functional materials intended for aircraft, space, electronic fields, and chemical industry: individual oxides, their solid solutions, complex oxides, powders of metals and alloys active towards sintering.
351:
to bring about an exchange of alkyl groups between metal alkoxide and ester. With the metal alkoxide complex in focus, the result is the same as for alcoholysis, namely the replacement of alkoxide ligands, but at the same time the alkyl groups of the ester are changed, which can also be the primary
145:
is more acidic than a typical alcohol; thus, phenoxides are correspondingly less basic and less nucleophilic than alkoxides. They are, however, often easier to handle, and yield derivatives that are more crystalline than those of the alkoxides.
745:
Unkelbach, Christian; O'Shea, Donal F.; Strohmann, Carsten (2014). "Insights into the
Metalation of Benzene and Toluene by Schlosser's Base: A Superbasic Cluster Comprising PhK, PhLi, and
1356:
1393:
897:
1243:
131:. The nucleophilic center for simple alkoxides is located on the oxygen, whereas the nucleophilic site on enolates is delocalized onto both carbon and oxygen sites.
1423:
1460:
476:
1479:
174:
linkages. In solution, the alkali metal derivatives exhibit strong ion-pairing, as expected for the alkali metal derivative of a strongly basic anion.
1550:
1274:
34:
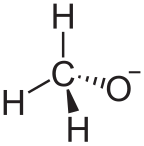
Structure of the methoxide anion. Although alkali metal alkoxides are not salts and adopt complex structures, they behave chemically as sources of
352:
goal of the reaction. Sodium methoxide in solution, for example, is commonly used for this purpose, a reaction that is used in the production of
154:
Alkali metal alkoxides are often oligomeric or polymeric compounds, especially when the R group is small (Me, Et). The alkoxide anion is a good
1415:
1223:
1094:
819:
700:
284:
metal hydride such as NaH. The metal hydride removes the hydrogen atom from the hydroxyl group and forms a negatively charged alkoxide ion.
1529:
1380:
1301:
1248:
890:
1266:
1218:
1541:
1496:
1452:
1436:
1238:
1205:
1195:
1082:
1582:
1491:
1348:
1147:
729:
661:
417:
1444:
1407:
1402:
1368:
1210:
1159:
1124:
1107:
1099:
1035:
975:
1623:
1253:
1026:
1014:
985:
980:
883:
534:
1329:
1233:
1187:
1070:
990:
944:
238:. Highly reducing metals react directly with alcohols to give the corresponding metal alkoxide. The alcohol serves as an
1002:
789:
1521:
1343:
1057:
605:
1613:
721:
306:
102:
1505:
1065:
920:
839:
436:
1293:
680:
558:
483:
1388:
1200:
460:
1228:
751:
400:
86:
564:
Sodium methoxide is produced on an industrial scale and available from a number of chemical companies.
1179:
906:
848:
583:
553:. In this process, vegetable oils or animal fats, which chemically are fatty acid triglycerides, are
504:
1569:
1587:
684:
579:
573:
554:
344:
235:
63:
1592:
1618:
1574:
1320:
925:
864:
368:. Oxo-ligands typically arise via the hydrolysis, often accidentally, and via ether elimination:
141:
are close relatives of the alkoxides, in which the alkyl group is replaced by a phenyl group.
815:
767:
725:
716:
Turova, Nataliya Y.; Turevskaya, Evgeniya P.; Kessler, Vadim G.; Yanovskaya, Maria I. (2002).
696:
657:
530:
856:
759:
622:
520:
403:
in the range ≈100–300 °C. Depending on process conditions, this thermolysis can afford
247:
106:
82:
155:
852:
837:
Turova, Nataliya Y. (2004). "Metal oxoalkoxides. Synthesis, properties and structures".
1338:
1052:
692:
538:
196:
cluster, highlighting the tendency of alkoxides to aggregate and bind ether ligands.
98:
59:
1607:
1047:
952:
868:
653:
545:
with high molecular weight. Both sodium methoxide and its counterpart prepared with
860:
298:
280:
42:
632:
529:
methylate and sodium methanolate, is a white powder when pure. It is used as an
101:
such as water, occur widely as intermediates in various reactions, including the
1112:
90:
78:
785:
626:
614:
318:
177:
138:
17:
578:
Potassium methoxide in alcoholic solution is commonly used as a catalyst for
550:
546:
542:
404:
353:
51:
30:
771:
763:
66:
and therefore consists of an organic group bonded to a negatively charged
875:
255:
243:
128:
110:
448:
365:
297:
The alkoxide ion and its salts react with primary alkyl halides in an
132:
116:
75:
526:
348:
251:
142:
124:
94:
67:
609:
549:
are frequently used as catalysts for commercial-scale production of
176:
41:
135:
are also unsaturated alkoxides derived from acetylenic alcohols.
239:
879:
234:
Alkoxides can be produced by several routes starting from an
648:
Boyd, Robert
Neilson; Morrison, Robert Thornton (1992).
119:
are unsaturated alkoxides derived by deprotonation of a
786:"Sodium Methoxide Material Safety Data Sheet (MSDS)"
1562:
1472:
1313:
1286:
1172:
1140:
968:
937:
913:
421:
810:G. Knothe; J. Krahl; J. Van Gerpen, eds. (2010),
246:is produced as a by-product. A classic case is
364:Many metal alkoxide compounds also feature oxo-
503:basic reagent in alcohol solution for organic
109:alkoxides are widely used for coatings and as
97:. Alkoxides, although generally not stable in
891:
8:
600:
598:
898:
884:
876:
321:as summarized in this idealized equation:
689:Alkoxo and Aryloxo Derivatives of Metals
652:(6th ed.). Englewood Cliffs, N.J.:
386:
382:
378:
374:
335:
331:
327:
272:
268:
264:
192:
188:
184:
169:
161:
29:
594:
687:; Rothwell, Ian P.; Singh, A. (2001).
643:
641:
675:
673:
7:
347:process, metal alkoxides react with
46:The structure of the methoxide ion.
477:Meerwein–Ponndorf–Verley reduction
313:Hydrolysis and transesterification
25:
418:Transition metal alkoxide complex
718:The Chemistry of Metal Alkoxides
451:processing of Si oxides; Si(OMe)
861:10.1070/RC2004v073n11ABEH000855
792:from the original on 2009-02-18
535:anionic addition polymerization
525:Sodium methoxide, also called
455:is avoided for safety reasons
158:, thus many alkoxides feature
1:
814:(2nd ed.), AOCS Press,
293:Reactions with alkyl halides
250:produced by the addition of
27:Conjugate base of an alcohol
70:atom. They are written as
1640:
722:Kluwer Academic Publishers
610:"Theory of Ætherification"
571:
518:
415:
360:Formation of oxo-alkoxides
317:Aliphatic metal alkoxides
307:Williamson ether synthesis
103:Williamson ether synthesis
627:10.1080/14786445008646627
305:to form an ether via the
840:Russian Chemical Reviews
559:fatty acid methyl esters
437:Tetraethyl orthosilicate
81:. Alkoxides are strong
584:production of biodiesel
1624:Coordination chemistry
907:Coordination complexes
812:The Biodiesel Handbook
764:10.1002/anie.201306884
557:with methanol to give
461:Aluminium isopropoxide
412:Illustrative alkoxides
221:
47:
39:
752:Angew. Chem. Int. Ed.
606:Williamson, Alexander
505:elimination reactions
399:Many metal alkoxides
180:
45:
33:
656:. pp. 241–242.
230:From reducing metals
853:2004RuCRv..73.1041T
580:transesterification
574:Potassium methoxide
568:Potassium methoxide
401:thermally decompose
345:transesterification
123:bond adjacent to a
85:and, when R is not
428:molecular formula
319:decompose in water
267:OH + 2 Na → 2 CH
222:
219: Hydrogen (H)
207: Lithium (Li)
48:
40:
1614:Functional groups
1601:
1600:
847:(11): 1041–1064.
821:978-1-893997-62-2
702:978-0-08-048832-5
650:Organic Chemistry
510:
509:
395:Thermal stability
181:Structure of the
74:, where R is the
16:(Redirected from
1631:
900:
893:
886:
877:
872:
825:
824:
807:
801:
800:
798:
797:
782:
776:
775:
742:
736:
735:
713:
707:
706:
685:Mehrotra, Ram C.
677:
668:
667:
645:
636:
633:Link to excerpt.
630:
621:(251): 350–356.
602:
521:Sodium methoxide
515:Sodium methoxide
422:
390:
339:
275:
248:sodium methoxide
218:
213: Oxygen (O)
212:
206:
201: Carbon (C)
200:
195:
173:
165:
122:
107:Transition metal
73:
37:
21:
1639:
1638:
1634:
1633:
1632:
1630:
1629:
1628:
1604:
1603:
1602:
1597:
1578:
1558:
1545:
1537:
1533:
1525:
1517:
1513:
1509:
1500:
1487:
1483:
1468:
1464:
1456:
1448:
1440:
1431:
1427:
1419:
1411:
1397:
1384:
1376:
1372:
1364:
1360:
1352:
1333:
1324:
1309:
1305:
1297:
1282:
1270:
1261:
1257:
1214:
1191:
1183:
1168:
1163:
1155:
1151:
1136:
1132:
1128:
1120:
1116:
1103:
1090:
1086:
1078:
1074:
1061:
1043:
1039:
1030:
1022:
1018:
1010:
1006:
998:
994:
964:
960:
956:
948:
933:
929:
909:
904:
836:
833:
831:Further reading
828:
822:
809:
808:
804:
795:
793:
784:
783:
779:
744:
743:
739:
732:
715:
714:
710:
703:
681:Bradley, Don C.
679:
678:
671:
664:
647:
646:
639:
604:
603:
596:
592:
576:
570:
555:transesterified
523:
517:
500:
496:
472:
468:
454:
444:
420:
414:
397:
388:
384:
380:
376:
372:
362:
337:
333:
329:
325:
315:
302:
295:
290:
274:
270:
266:
262:
232:
227:
220:
216:
214:
210:
208:
204:
202:
198:
194:
190:
186:
182:
171:
167:
163:
159:
156:bridging ligand
152:
120:
99:protic solvents
71:
35:
28:
23:
22:
15:
12:
11:
5:
1637:
1635:
1627:
1626:
1621:
1616:
1606:
1605:
1599:
1598:
1596:
1595:
1590:
1585:
1580:
1576:
1572:
1566:
1564:
1563:Halide donors:
1560:
1559:
1557:
1556:
1548:
1543:
1539:
1535:
1531:
1527:
1523:
1519:
1515:
1511:
1507:
1503:
1498:
1494:
1489:
1485:
1481:
1476:
1474:
1470:
1469:
1467:
1466:
1462:
1458:
1454:
1450:
1446:
1442:
1438:
1434:
1429:
1425:
1421:
1417:
1413:
1409:
1405:
1400:
1395:
1391:
1386:
1382:
1378:
1374:
1370:
1366:
1362:
1358:
1354:
1350:
1346:
1341:
1336:
1331:
1327:
1322:
1317:
1315:
1311:
1310:
1308:
1307:
1303:
1299:
1295:
1290:
1288:
1284:
1283:
1281:
1280:
1272:
1268:
1264:
1259:
1255:
1251:
1246:
1241:
1236:
1231:
1226:
1221:
1216:
1212:
1208:
1203:
1198:
1193:
1189:
1185:
1181:
1176:
1174:
1170:
1169:
1167:
1166:
1161:
1157:
1153:
1149:
1144:
1142:
1138:
1137:
1135:
1134:
1130:
1126:
1122:
1118:
1114:
1110:
1105:
1101:
1097:
1092:
1088:
1084:
1080:
1076:
1072:
1068:
1063:
1059:
1055:
1050:
1045:
1041:
1037:
1033:
1028:
1024:
1020:
1016:
1012:
1008:
1004:
1000:
996:
992:
988:
983:
978:
972:
970:
966:
965:
963:
962:
958:
954:
950:
946:
941:
939:
935:
934:
932:
931:
927:
923:
917:
915:
911:
910:
905:
903:
902:
895:
888:
880:
874:
873:
832:
829:
827:
826:
820:
802:
777:
758:(2): 553–556.
737:
730:
708:
701:
693:Academic Press
669:
662:
637:
593:
591:
588:
572:Main article:
569:
566:
539:ethylene oxide
519:Main article:
516:
513:
508:
507:
501:
498:
494:
491:
480:
479:
473:
470:
466:
463:
457:
456:
452:
445:
442:
439:
433:
432:
429:
426:
413:
410:
396:
393:
392:
391:
361:
358:
341:
340:
314:
311:
300:
294:
291:
289:
286:
277:
276:
231:
228:
226:
223:
215:
209:
203:
197:
151:
148:
60:conjugate base
26:
24:
18:Metal alkoxide
14:
13:
10:
9:
6:
4:
3:
2:
1636:
1625:
1622:
1620:
1617:
1615:
1612:
1611:
1609:
1594:
1591:
1589:
1586:
1584:
1581:
1579:
1573:
1571:
1568:
1567:
1565:
1561:
1555:
1554:
1549:
1547:
1540:
1538:
1528:
1526:
1520:
1518:
1504:
1502:
1495:
1493:
1490:
1488:
1478:
1477:
1475:
1471:
1465:
1459:
1457:
1451:
1449:
1443:
1441:
1435:
1433:
1422:
1420:
1414:
1412:
1406:
1404:
1401:
1399:
1392:
1390:
1387:
1385:
1379:
1377:
1367:
1365:
1355:
1353:
1347:
1345:
1342:
1340:
1337:
1335:
1328:
1326:
1319:
1318:
1316:
1312:
1306:
1300:
1298:
1292:
1291:
1289:
1285:
1279:
1277:
1273:
1271:
1265:
1263:
1252:
1250:
1247:
1245:
1242:
1240:
1237:
1235:
1232:
1230:
1227:
1225:
1222:
1220:
1217:
1215:
1209:
1207:
1204:
1202:
1199:
1197:
1194:
1192:
1186:
1184:
1178:
1177:
1175:
1171:
1165:
1158:
1156:
1146:
1145:
1143:
1139:
1133:
1123:
1121:
1111:
1109:
1106:
1104:
1098:
1096:
1093:
1091:
1081:
1079:
1069:
1067:
1064:
1062:
1056:
1054:
1051:
1049:
1046:
1044:
1034:
1032:
1025:
1023:
1013:
1011:
1001:
999:
989:
987:
984:
982:
979:
977:
974:
973:
971:
967:
961:
951:
949:
943:
942:
940:
936:
930:
924:
922:
919:
918:
916:
912:
908:
901:
896:
894:
889:
887:
882:
881:
878:
870:
866:
862:
858:
854:
850:
846:
842:
841:
835:
834:
830:
823:
817:
813:
806:
803:
791:
787:
781:
778:
773:
769:
765:
761:
757:
754:
753:
748:
741:
738:
733:
731:9780792375210
727:
723:
720:. Dordrecht:
719:
712:
709:
704:
698:
694:
691:. San Diego:
690:
686:
682:
676:
674:
670:
665:
663:9780136436690
659:
655:
654:Prentice Hall
651:
644:
642:
638:
634:
628:
624:
620:
617:
616:
611:
607:
601:
599:
595:
589:
587:
585:
581:
575:
567:
565:
562:
560:
556:
552:
548:
544:
540:
536:
532:
528:
522:
514:
512:
506:
502:
492:
489:
487:
482:
481:
478:
474:
464:
462:
459:
458:
450:
446:
440:
438:
435:
434:
430:
427:
424:
423:
419:
411:
409:
406:
402:
394:
371:
370:
369:
367:
359:
357:
355:
350:
346:
334:O → Al(OH)
324:
323:
322:
320:
312:
310:
308:
304:
292:
287:
285:
282:
281:alkali metals
261:
260:
259:
257:
253:
249:
245:
241:
237:
229:
224:
179:
175:
157:
149:
147:
144:
140:
136:
134:
130:
126:
118:
114:
112:
108:
104:
100:
96:
92:
88:
84:
80:
77:
69:
65:
61:
57:
53:
44:
32:
19:
1552:
1275:
844:
838:
811:
805:
794:. Retrieved
788:. NOAA.gov.
780:
755:
750:
746:
740:
717:
711:
688:
649:
618:
613:
577:
563:
541:, forming a
524:
511:
485:
475:reagent for
398:
363:
342:
316:
296:
278:
233:
153:
137:
115:
91:nucleophiles
55:
49:
381:O → RCO
225:Preparation
79:substituent
1608:Categories
1224:amino acid
1141:Si donors:
796:2010-04-13
615:Phil. Mag.
590:References
484:Potassium
416:See also:
303:2 reaction
288:Properties
139:Phenoxides
1619:Alkoxides
1473:S donors:
1314:O donors:
1287:P donors:
1249:porphyrin
1196:imidazole
1173:N donors:
969:C donors:
938:B donors:
914:H donors:
869:250920020
561:(FAMEs).
551:biodiesel
547:potassium
543:polyether
531:initiator
488:-butoxide
405:nanosized
377:R' + CH
354:biodiesel
254:metal to
150:Structure
111:catalysts
93:and good
52:chemistry
790:Archived
772:24273149
749:BuOLi".
608:(1850).
431:comment
338:+ 3 ROH
256:methanol
244:hydrogen
133:Ynolates
129:aldehyde
117:Enolates
56:alkoxide
1087:& C
849:Bibcode
582:in the
449:sol-gel
441:Si(OEt)
389:+ R'OH
366:ligands
343:In the
271:ONa + H
236:alcohol
187:(OBu-t)
95:ligands
89:, good
76:organyl
64:alcohol
58:is the
995:=CH-CH
867:
818:
770:
728:
699:
660:
533:of an
527:sodium
349:esters
330:+ 3 H
326:Al(OR)
279:Other
252:sodium
242:, and
217:
211:
205:
199:
143:Phenol
125:ketone
68:oxygen
62:of an
986:HC(O)
981:RC(O)
865:S2CID
537:with
497:(OBu)
469:(OPr)
425:name
191:(thf)
87:bulky
83:bases
54:, an
1389:acac
1361:/HCO
1244:bipy
1003:C(CH
816:ISBN
768:PMID
726:ISBN
697:ISBN
658:ISBN
486:tert
447:for
263:2 CH
240:acid
1484:NCS
1461:OPR
1437:OSR
1416:ClO
1403:ONO
1381:RCO
1258:Si)
1254:(Me
1239:RCN
1206:RNO
1154:4−n
1152:SiR
1108:≡CR
1100:=CR
1095:RNC
1019:=CH
857:doi
760:doi
623:doi
373:RCO
166:or
127:or
121:C−H
105:.
50:In
1610::
1588:Br
1583:Cl
1551:NC
1542:SR
1522:SO
1492:RS
1453:PO
1445:SO
1432:NO
1408:NO
1398:CO
1357:CO
1339:RO
1302:PR
1294:PR
1278:CS
1234:RN
1219:py
1211:NO
1201:NO
1180:NH
1164:Si
1089:70
1085:60
1058:CO
1053:CO
1048:CN
1027:RC
1015:CH
991:CH
945:BR
863:.
855:.
845:73
843:.
766:.
756:53
724:.
695:.
683:;
672:^
640:^
619:37
612:.
597:^
586:.
490:,
471:12
465:Al
385:CH
356:.
309:.
258::
183:Li
113:.
72:RO
36:RO
1593:I
1577:2
1575:F
1570:F
1553:S
1546:O
1544:2
1536:3
1534:O
1532:2
1530:S
1524:2
1516:2
1514:S
1512:2
1510:C
1508:2
1506:R
1501:S
1499:2
1497:R
1486:2
1482:2
1480:R
1463:3
1455:4
1447:4
1439:2
1430:5
1428:H
1426:5
1424:C
1418:4
1410:3
1396:2
1394:R
1383:2
1375:4
1373:O
1371:2
1369:C
1363:3
1359:3
1351:2
1349:O
1344:O
1334:O
1332:2
1330:R
1325:O
1323:2
1321:H
1304:2
1296:3
1276:N
1269:2
1267:N
1262:N
1260:2
1256:3
1229:N
1213:2
1190:3
1188:N
1182:3
1162:3
1160:R
1150:n
1148:H
1131:7
1129:H
1127:9
1125:C
1119:5
1117:H
1115:5
1113:C
1102:2
1083:C
1077:6
1075:R
1073:6
1071:C
1066:C
1060:2
1042:4
1040:H
1038:6
1036:C
1031:R
1029:2
1021:2
1017:2
1009:3
1007:)
1005:2
997:2
993:2
976:R
959:n
957:H
955:m
953:B
947:2
928:2
926:H
921:H
899:e
892:t
885:v
871:.
859::
851::
799:.
774:.
762::
747:t
734:.
705:.
666:.
635:)
631:(
629:.
625::
499:4
495:4
493:K
467:4
453:4
443:4
387:3
383:2
379:3
375:2
336:3
332:2
328:3
301:N
299:S
273:2
269:3
265:3
193:3
189:4
185:4
172:O
170:3
168:M
164:O
162:2
160:M
38:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.