873:
506:
360:
661:
534:
191:
622:
774:
287:
954:
685:
1010:
1018:
468:. Since the electrophile is much less acidic than the nucleophile, rapid proton transfer usually transfers the enolate back to the nucleophile if the product is enolizable; however, one may take advantage of the new locus of nucleophilicity if a suitable electrophile is pendant. Depending on the relative acidities of the nucleophile and product, the reaction may be
938:. The resultant species undergoes a Michael addition with another monomer, with the latter acting as an acceptor. This extends the chain by forming another nucleophilic species to act as a donor for the next addition. This process repeats until the reaction is quenched by chain termination. The original Michael donor can be a neutral donor such as
329:
Some authors have broadened the definition of the
Michael addition to essentially refer to any 1,4-addition reaction of α,β-unsaturated carbonyl compounds. Others, however, insist that such a usage is an abuse of terminology, and limit the Michael addition to the formation of carbon–carbon bonds
970:
are some of the earliest applications of the
Michael reaction in polymerizations. A wide variety of Michael donors and acceptors have been used to synthesize a diverse range of polymers. Examples of such polymers include poly(amido amine), poly(amino ester), poly(imido
1664:
Halland, N.; Hansen, T.; Jørgensen, K. (2003). "Organocatalytic asymmetric
Michael reaction of cyclic 1,3-dicarbonyl compounds and α,β-unsaturated ketones--a highly atom-economic catalytic one-step formation of optically active warfarin anticoagulant".
421:
has a large coefficient on the central carbon atom while the LUMO of many alpha, beta unsaturated carbonyl compounds has a large coefficient on the beta carbon. Thus, both reactants can be considered
1801:
Dong, Z.; Wang, L.; Chen, X.; Liu, X.; Lin, L.; Feng, X. (2009). "Organocatalytic
Enantioselective Michael Addition of 4-Hydroxycoumarin to α,β-Unsaturated Ketones: A Simple Synthesis of Warfarin".
5547:
822:
often favours the 1,4-addition. In many syntheses where 1,6-addition was favoured, the substrate contained certain structural features. Research has shown that catalysts can also influence the
544:
claimed priority for the invention. He and T. Komnenos had observed addition products to double bonds as side-products earlier in 1883 while investigating condensation reactions of
1735:
Xie, J.; Yue, L.; Chen, W.; Du, W.; Zhu, J.; Deng, J.; Chen, Y. (2007). "Highly
Enantioselective Michael Addition of Cyclic 1,3-Dicarbonyl Compounds to α,β-Unsaturated Ketones".
306:
of a ketone or aldehyde to an α,β-unsaturated carbonyl compound at the β carbon. The current definition of the
Michael reaction has broadened to include nucleophiles other than
4663:
1003:
861:
in less than 2% yield. This particular catalyst and set of reaction conditions led to the mostly regioselective and enantioselective 1,6-Michael addition of ethyl sorbate
338:
have been used to refer to the 1,4-addition of oxygen and nitrogen nucleophiles, respectively. The
Michael reaction has also been associated with 1,6-addition reactions.
4608:
5376:
1602:
Pansare, S. V.; Pandya, K. (2006). "Simple
Diamine- and Triamine-Protonic Acid Catalysts for the Enantioselective Michael Addition of Cyclic Ketones to Nitroalkenes".
4718:
808:
1364:"1,6-Aza-Michael addition of para-quinone methides with N-heterocycles catalyzed by Zn(OTf)2: A regioselective approach to N-diarylmethyl-substituted heterocycles"
934:
All polymerization reactions have three basic steps: initiation, propagation, and termination. The initiation step is the
Michael addition of the nucleophile to a
4868:
3502:
1348:
5696:
5597:
5371:
4473:
814:-diunsaturated Michael acceptor. The 1,6-addition mechanism is similar to the 1,4-addition, with one exception being the nucleophilic attack occurring at the
3197:
4243:
2394:
5043:
2987:
5138:
3242:
5118:
4613:
3780:
1637:
Ikawa, M.; Stahmann, M. A.; Link, K. P. (1944). "Studies on 4-Hydroxycoumarins. V. The
Condensation of α,β-Unsaturated Ketones with 4-Hydroxycoumarin".
3661:
3217:
5208:
2061:
4963:
2430:
2059:
Lippert, A. R.; Kaeobamrung, J.; Bode, J. W. (2006). "Synthesis of Oligosubstituted Bullvalones: Shapeshifting Molecules Under Basic Conditions".
3795:
1002:, which are used for drug delivery, high performance composites, and coatings. These network polymers are synthesized using a dual chain growth,
272:
172:
5441:
4898:
1828:
Wong, T. C.; Sultana, C. M.; Vosburg, D. A. (2010). "A Green, Enantioselective Synthesis of Warfarin for the Undergraduate Organic Laboratory".
660:
5391:
5003:
4983:
4943:
3750:
872:
5537:
5462:
5346:
3958:
3362:
2693:
1324:
1155:
1058:
5532:
5361:
5018:
4873:
4503:
130:
4348:
3585:
1700:
Kim, H.; Yen, C.; Preston, P.; Chin, J. (2006). "Substrate-directed stereoselectivity in vicinal diamine-catalyzed synthesis of warfarin".
1079:
Mather, B.; Viswanathan, K.; Miller, K.; Long, T. (2006). "Michael addition reactions in macromolecular design for emerging technologies".
4708:
4198:
3873:
5612:
5396:
4418:
314:, and beta-cyanoesters. The resulting product contains a highly useful 1,5-dioxygenated pattern. Non-carbon nucleophiles such as water,
4973:
5607:
5321:
5183:
4938:
533:
5497:
4968:
4883:
4853:
4833:
4698:
4693:
4068:
3993:
3636:
3590:
3457:
2718:
5436:
1586:
5602:
5562:
5512:
4988:
4738:
4668:
3157:
5198:
4803:
3696:
3417:
621:
5188:
2728:
5356:
5113:
5063:
3853:
3785:
3676:
3252:
3007:
2932:
2713:
2245:"Mechanistic Modeling of the Thiol–Michael Addition Polymerization Kinetics: Structural Effects of the Thiol and Vinyl Monomers"
5068:
4878:
4353:
4263:
2387:
1362:
Guin, Soumitra; Saha, Hemonta K.; Patel, Ashvani K.; Gudimella, Santosh K.; Biswas, Subhankar; Samanta, Sampak (17 July 2020).
773:
5642:
5426:
5366:
4768:
4743:
4653:
4233:
4113:
3147:
2643:
5527:
5013:
4808:
3077:
5632:
5218:
4728:
4238:
4183:
4028:
3988:
3820:
3575:
3292:
3142:
2243:
Huang, Sijia; Sinha, Jasmine; Podgórski, Maciej; Zhang, Xinpeng; Claudino, Mauro; Bowman, Christopher N. (14 August 2018).
5592:
5153:
5108:
4598:
4453:
5627:
5542:
5401:
5316:
5213:
4288:
3943:
3611:
3022:
2583:
5517:
5492:
5477:
5173:
5038:
4993:
4758:
4303:
4153:
3367:
3047:
2992:
5522:
5467:
4998:
4413:
4128:
4123:
3616:
3432:
3422:
3137:
2997:
2947:
2942:
2917:
2823:
5691:
5577:
5178:
5098:
4713:
4678:
4523:
3948:
3908:
3805:
3580:
3332:
3277:
2877:
2588:
2578:
2553:
399:
5552:
5253:
5058:
4493:
4058:
4033:
3973:
3565:
3272:
2922:
1247:[On the addition of sodium acetoacetate- and sodium malonic acid esters to the esters of unsaturated acids].
1213:[On the addition of sodium acetoacetate- and sodium malonic acid esters to the esters of unsaturated acids].
220:
In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent
3107:
1039:
Little, R. D.; Masjedizadeh, M. R.; Wallquist, O.; McLoughlin, J. I. (1995). "The Intramolecular Michael Reaction".
4843:
4378:
3830:
3052:
3017:
2613:
2548:
2380:
967:
827:
505:
359:
221:
210:
123:
5411:
5033:
4093:
4018:
3542:
3377:
3062:
2838:
2798:
2543:
5652:
5557:
5291:
5263:
5233:
5148:
5078:
5008:
4928:
4828:
4788:
4483:
4103:
3402:
3397:
2859:
2723:
1171:
Tiano, Martin (2020). "Enantioselective Michael Addition: An Experimental Introduction to Asymmetric Synthesis".
994:
For example, linear step growth polymerization produces the redox active poly(amino quinone), which serves as an
5617:
5487:
5351:
5193:
5053:
4573:
3547:
3097:
3057:
2808:
5507:
5103:
5073:
4948:
4903:
4733:
4643:
4458:
4448:
4278:
3835:
3775:
3740:
3527:
3487:
3262:
3132:
2648:
2638:
2568:
2147:
den Hartog, Tim; Harutyunyan, Syuzanna R.; Font, Daniel; Minnaard, Adriaan J.; Feringa, Ben L. (January 2008).
572:
568:
5083:
4063:
3087:
2633:
2513:
1565:
Reyes, E.; Uria, U.; Vicario, J. L.; Carrillo, L. (2016). "The Catalytic, Enantioselective Michael Reaction".
1443:
5296:
1504:
684:
5701:
5587:
5446:
5238:
5163:
5143:
4863:
4813:
4673:
4638:
4578:
4508:
3810:
3790:
3522:
3442:
3337:
3297:
3267:
3202:
3072:
2982:
2972:
2848:
2558:
902:
functional group as a Michael acceptor. The Michael donor on the drug reacts with a Michael acceptor in the
910:. This is a viable cancer treatment because the target enzyme is inhibited following the Michael reaction.
516:
and realized that this reaction could only work by assuming an addition reaction to the double bond of the
5326:
5048:
4798:
4778:
4753:
4703:
4618:
4593:
4548:
4518:
4498:
4468:
4433:
4388:
4363:
4338:
4223:
4148:
3928:
3621:
3557:
3357:
3082:
3002:
2688:
2663:
2440:
2435:
2347:"Investigations of the redox process of conducting poly(2-methyl-5-amino-1,4-naphthoquinone) (PMANQ) film"
1772:"New Phenylglycine-Derived Primary Amine Organocatalysts for the Preparation of Optically Active Warfarin"
1211:"Ueber die Addition von Natriumacetessig- und Natriummalonsäureäthern zu den Aethern ungesättigter Säuren"
834:
767:
610:
5662:
4408:
3032:
1535:
1410:
1245:"Ueber die Addition von Natriumacetessig- und Natriummalonsäureäther zu den Aethern ungesättigter Säuren"
115:
5248:
5203:
4918:
4888:
4858:
4793:
4773:
4688:
4683:
4648:
4603:
4588:
4583:
4563:
4553:
4488:
4478:
4358:
3878:
3681:
3257:
3212:
3042:
2778:
2498:
2460:
541:
473:
180:
2708:
2703:
2101:"Organocatalytic 1,4-Addition Reaction of α,β-γ,δ-Diunsaturated Aldehydes versus 1,6-Addition Reaction"
310:. Some examples of nucleophiles include doubly stabilized carbon nucleophiles such as beta-ketoesters,
5301:
1363:
1275:
1121:
716:
704:
5431:
5381:
5331:
5311:
5158:
5133:
4848:
4838:
4723:
4538:
4533:
4463:
4248:
4048:
4008:
3938:
3903:
3858:
3825:
3691:
3666:
3646:
3467:
3427:
3387:
3352:
3282:
3037:
2907:
2882:
2420:
2256:
1837:
1180:
609:
sketched below, the base proline is derivatized and works in conjunction with a protic acid such as
5647:
5637:
5622:
5268:
5243:
5228:
5223:
4953:
4908:
4893:
4783:
4763:
4658:
4543:
4528:
4373:
4318:
4308:
4298:
4273:
4038:
3913:
3888:
3800:
3656:
3641:
3626:
3482:
3447:
3392:
3162:
3012:
2957:
2828:
2743:
2603:
2528:
2300:"Indium-Catalyzed Block Copolymerization of Lactide and Methyl Methacrylate by Sequential Addition"
747:
736:
632:
561:
383:
315:
2673:
5386:
5336:
5306:
5168:
4958:
4748:
4633:
4568:
4558:
4323:
4253:
4218:
4213:
4193:
4188:
4133:
4043:
3893:
3755:
3745:
3651:
3437:
3382:
3312:
3232:
3127:
3027:
2962:
2887:
2733:
2598:
2533:
2346:
2327:
2280:
2120:
1507:[Observation on the addition of ethyl malonate to substances with a double carbon bond].
1391:
1342:
676:
347:
184:
2518:
1210:
448:, or more usually, enolate nucleophile. In the latter case, the stabilized carbonyl compound is
190:
953:
5123:
4443:
4328:
4293:
4258:
4203:
4158:
4118:
4073:
4053:
4003:
3998:
3968:
3953:
3863:
3770:
3706:
3671:
3497:
3372:
3247:
3172:
3152:
3067:
2902:
2897:
2843:
2753:
2658:
2618:
2573:
2455:
2450:
2415:
2319:
2272:
2225:
2207:
2168:
2078:
2033:
2009:
1981:
1953:
1925:
1897:
1869:
1752:
1717:
1682:
1619:
1582:
1383:
1330:
1320:
1295:
1151:
1054:
1040:
972:
672:
644:
606:
560:
Researchers have expanded the scope of Michael additions to include elements of chirality via
144:
43:
2148:
2100:
793:
5657:
5502:
5472:
5416:
5341:
5273:
5028:
4978:
4823:
4628:
4403:
4398:
4343:
4333:
4108:
3918:
3898:
3868:
3765:
3701:
3686:
3517:
3472:
3462:
3452:
3347:
3327:
3322:
3307:
3302:
3182:
3177:
3117:
3102:
3092:
2937:
2927:
2793:
2783:
2773:
2683:
2678:
2653:
2593:
2445:
2404:
2358:
2311:
2264:
2215:
2199:
2160:
2149:"Catalytic Enantioselective 1,6-Conjugate Addition of Grignard Reagents to Linear Dienoates"
2112:
2070:
2041:
1845:
1810:
1783:
1744:
1709:
1674:
1646:
1611:
1574:
1547:
1516:
1485:
1455:
1418:
1375:
1287:
1256:
1222:
1188:
1135:
1108:
1088:
1046:
891:
842:
823:
763:
700:
636:
521:
485:
461:
441:
426:
256:
244:
206:
1417:, Conjugate Addition Reactions in Organic Synthesis, vol. 9, Elsevier, pp. 1–61,
5567:
5258:
5093:
5088:
4383:
4368:
4313:
4268:
4228:
4178:
4143:
4138:
4083:
4078:
4013:
3963:
3883:
3711:
3595:
3570:
3532:
3507:
3492:
3477:
3412:
3287:
3237:
3227:
3207:
3167:
2977:
2967:
2952:
2748:
2668:
2493:
2488:
1505:"Bemerkung über die Addition von Aethylmalonat an Körper mit doppelter Kohlenstoffbindung"
1319:. Brent L. Iverson, Eric V. Anslyn, Christopher S. Foote (Eighth ed.). Boston, Mass.
999:
819:
743:
724:
583:
286:
2538:
2508:
1274:
Mather, Brian D.; Viswanathan, Kalpana; Miller, Kevin M.; Long, Timothy E. (1 May 2006).
1244:
2260:
2244:
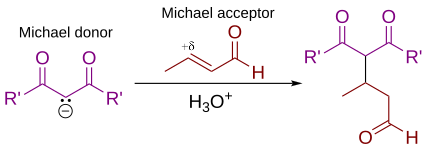
1841:
1184:
5572:
5482:
5421:
4513:
4423:
4393:
4168:
4023:
3760:
3537:
3407:
3222:
3192:
2892:
2788:
2563:
2425:
2298:
Jung, Hyuk-Joon; Yu, Insun; Nyamayaro, Kudzanai; Mehrkhodavandi, Parisa (5 June 2020).
2220:
2187:
1422:
1291:
1240:
1206:
1092:
995:
732:
720:
648:
433:
299:
236:
2362:
2099:
Hayashi, Yujiro; Okamura, Daichi; Umemiya, Shigenobu; Uchimaru, Tadafumi (July 2012).
1538:[On the reaction of aliphatic aldehydes with malonic acid and ethylmalonate].
5685:
5582:
5283:
5128:
5023:
4818:
4208:
4173:
4163:
4098:
4088:
3978:
3815:
3631:
3342:
3317:
3187:
2833:
2818:
2803:
2698:
2628:
2608:
2523:
2331:
2284:
1771:
1395:
1143:
1139:
728:
708:
652:
602:
525:
449:
413:
The reaction is dominated by orbital, rather than electrostatic, considerations. The
368:
2299:
2124:
552:. However, according to biographer Takashi Tokoroyama, this claim is without merit.
4623:
3983:
3735:
3512:
3112:
2912:
2763:
2758:
2623:
2478:
2345:
Pham, M.C; Hubert, S; Piro, B; Maurel, F; Le Dao, H; Takenouti, H (February 2004).
895:
628:
545:
517:
497:
465:
202:
198:
1009:
2268:
3122:
2768:
2738:
2503:
2004:
1976:
1948:
1920:
1892:
1864:
1578:
1446:[On the reaction of 2,3-dibrompropionic acid with malonic acid ester].
1192:
1050:
1017:
903:
500:
derivative (now recognized as involving two successive substitution reactions).
488:
was prompted by an 1884 publication by Conrad & Kuthzeit on the reaction of
456:
and a weak base (soft enolization). The resulting enolate attacks the activated
422:
248:
224:
164:
17:
2203:
2005:"Conversion of Nitro to Carbonyl by Ozonolysis of Nitronates: 2,5-Heptanedione"
1276:"Michael addition reactions in macromolecular design for emerging technologies"
429:
are of similar energy, and react efficiently to form a new carbon–carbon bond.
382:, stabilized by its electron-withdrawing groups. Structures 2a to 2c are three
5406:
4933:
4283:
2372:
1459:
1379:
1334:
899:
453:
2323:
2315:
2276:
2211:
1551:
1520:
1387:
1299:
1260:
1226:
469:
376:
252:
2229:
2172:
2164:
2116:
2082:
2045:
1814:
1787:
1756:
1721:
1686:
1678:
1623:
1489:
1314:
691:
Several asymmetric versions of this reaction exist using chiral catalysts.
512:
Michael was able to obtain the same product by replacing the propionate by
2813:
2483:
2186:
Boike, Lydia; Henning, Nathaniel J.; Nomura, Daniel K. (25 August 2022).
1536:"Ueber die Einwirkung von Fettaldehyden auf Malonsäure und Aethylmalonat"
947:
712:
668:
579:
576:
549:
311:
268:
2074:
1650:
2473:
984:
957:
Polymerization mechanism of a Michael addition with a thiol nucleophile
935:
640:
595:
591:
587:
464:, forming a carbon–carbon bond. This also transfers the enolate to the
418:
387:
323:
307:
303:
240:
160:
2031:
Mukaiyama, T. (1977). "Titanium Tetrachloride in Organic Synthesis ".
1849:
1748:
1713:
1615:
358:
189:
1444:"Ueber die Einwirkung von α-β-Dibrompropionsäure auf Malonsäureester"
988:
907:
565:
457:
264:
699:
Classical examples of the Michael reaction are the reaction between
326:
can also react with an α,β-unsaturated carbonyl in a 1,4-addition.
1008:
980:
976:
952:
943:
939:
890:
A Michael reaction is used as a mechanistic step by many covalent
871:
319:
276:
232:
187:
and is widely used for the mild formation of carbon-carbon bonds.
1770:
Kristensen, T. E.; Vestli, K.; Hansen, F. K.; Hansen, T. (2009).
786:
The 1,6-Michael reaction proceeds via nucleophilic attack on the
1122:"Chapter 18: Enols and Enolates – The Michael Addition reaction"
876:
The Michael addition of ethylmagnesium bromide to ethyl sorbate.
849:)-(–)-3 ligand. This reaction produced the 1,6-addition product
437:
414:
406:
abstracts a proton from protonated base (or solvent) to produce
280:
228:
2857:
2376:
643:(formed between the proline nitrogen and the cycloketone) and
998:
coatings on various metal surfaces. Another example includes
818:
carbon of the Michael acceptor. However, research shows that
1476:
Tokoroyama, T. (2010). "Discovery of the Michael Reaction".
772:
390:
ions. This nucleophile reacts with the electrophilic alkene
285:
564:
versions of the reaction. The most common methods involve
857:
in approximately 99% yield, and the 1,4-addition product
898:
such as ibrutinib, osimertinib, and rociletinib have an
330:
through the addition of carbon nucleophiles. The terms
267:(the Michael acceptor), the R" substituent is usually a
1865:"1,1,2,3-Propanetetracarboxylic acid, tetraethyl ester"
386:
that can be drawn for this species, two of which have
796:
639:
believed to be responsible for this selectivity, the
5548:
Erlenmeyer–Plöchl azlactone and amino-acid synthesis
5455:
5282:
4917:
4432:
3927:
3844:
3724:
3604:
3556:
2866:
833:For example, the image below shows the addition of
655:to the protonated amine in the proline side group.
594:with chiral secondary amines, usually derived from
179:by creating a carbon-carbon bond at the acceptor's
802:
667:A well-known Michael reaction is the synthesis of
4609:Divinylcyclopropane-cycloheptadiene rearrangement
1006:radical and step growth Michael addition system.
707:(Michael acceptor), that of diethyl malonate and
520:. He then confirmed this assumption by reacting
452:with a strong base (hard enolization) or with a
283:), or R" may be any electron withdrawing group.
746:sequence of Michael and aldol additions is the
484:The research done by Arthur Michael in 1887 at
445:
4869:Thermal rearrangement of aromatic hydrocarbons
3503:Thermal rearrangement of aromatic hydrocarbons
1448:Berichte der Deutschen Chemischen Gesellschaft
5598:Lectka enantioselective beta-lactam synthesis
2388:
1471:
1469:
8:
5377:Inverse electron-demand Diels–Alder reaction
3198:Heterogeneous metal catalyzed cross-coupling
4719:Lobry de Bruyn–Van Ekenstein transformation
5279:
3553:
2854:
2395:
2381:
2373:
1347:: CS1 maint: location missing publisher (
436:, the Michael reaction may proceed via an
31:
5209:Petrenko-Kritschenko piperidone synthesis
4664:Fritsch–Buttenberg–Wiechell rearrangement
2219:
1150:(1st ed.). Oxford University Press.
1104:
1102:
795:
5372:Intramolecular Diels–Alder cycloaddition
2062:Journal of the American Chemical Society
1639:Journal of the American Chemical Society
1604:Journal of the American Chemical Society
1016:
987:), poly(enone sulfide) and poly(enamine
979:sulfide), poly(aspartamide), poly(imido
841:using a copper catalyst with a reversed
472:in base. In most cases, the reaction is
54:
2153:Angewandte Chemie International Edition
1975:Horning, E. C.; Finelli, A. F. (1963).
1031:
727:, that of ethyl phenylcyanoacetate and
5392:Metal-centered cycloaddition reactions
5044:Debus–Radziszewski imidazole synthesis
2988:Bodroux–Chichibabin aldehyde synthesis
1409:Perlmutter, P., ed. (1 January 1992),
1340:
853:in 0% yield, the 1,6-addition product
5538:Diazoalkane 1,3-dipolar cycloaddition
5442:Vinylcyclopropane (5+2) cycloaddition
5347:Diazoalkane 1,3-dipolar cycloaddition
5119:Hurd–Mori 1,2,3-thiadiazole synthesis
4614:Dowd–Beckwith ring-expansion reaction
3781:Hurd–Mori 1,2,3-thiadiazole synthesis
2694:LFER solvent coefficients (data page)
2188:"Advances in covalent drug discovery"
2142:
2140:
2138:
2136:
2134:
2094:
2092:
1977:"α-Phenyl-α-carbethoxyglutaronitrile"
1893:"1,3-Cyclohexanedione, 5,5-dimethyl-"
1863:Clarke, H. T.; Murray, T. F. (1941).
1803:European Journal of Organic Chemistry
1776:European Journal of Organic Chemistry
1478:European Journal of Organic Chemistry
1109:Michael Addition | PharmaXChange.info
950:, or alkyl ligands bound to a metal.
302:, the reaction is the addition of an
197:The Michael addition is an important
7:
5697:Carbon-carbon bond forming reactions
4349:Sharpless asymmetric dihydroxylation
3586:Methoxymethylenetriphenylphosphorane
1891:Shriner, R. L.; Todd, H. R. (1943).
1415:Tetrahedron Organic Chemistry Series
1074:
1072:
1070:
183:. It belongs to the larger class of
4474:Allen–Millar–Trippett rearrangement
2003:McMurry, J. E.; Melton, J. (1988).
5613:Nitrone-olefin (3+2) cycloaddition
5608:Niementowski quinazoline synthesis
5397:Nitrone-olefin (3+2) cycloaddition
5322:Azide-alkyne Huisgen cycloaddition
5184:Niementowski quinazoline synthesis
4939:Azide-alkyne Huisgen cycloaddition
4244:Meerwein–Ponndorf–Verley reduction
3796:Leimgruber–Batcho indole synthesis
1540:Justus Liebig's Annalen der Chemie
1423:10.1016/b978-0-08-037067-5.50007-2
1292:10.1016/j.progpolymsci.2006.03.001
1093:10.1016/j.progpolymsci.2006.03.001
1045:. Vol. 47. pp. 315–552.
528:forming the first Michael adduct:
25:
5437:Trimethylenemethane cycloaddition
5139:Johnson–Corey–Chaykovsky reaction
5004:Cadogan–Sundberg indole synthesis
4984:Bohlmann–Rahtz pyridine synthesis
4944:Baeyer–Emmerling indole synthesis
3751:Cadogan–Sundberg indole synthesis
3243:Johnson–Corey–Chaykovsky reaction
1949:"Methyl γ-Methyl-γ-nitrovalerate"
1442:Conrad, M.; Guthzeit, M. (1884).
273:α,β-unsaturated carbonyl compound
5533:Cook–Heilbron thiazole synthesis
5362:Hexadehydro Diels–Alder reaction
5189:Niementowski quinoline synthesis
5019:Cook–Heilbron thiazole synthesis
4964:Bischler–Möhlau indole synthesis
4874:Tiffeneau–Demjanov rearrangement
4504:Baker–Venkataraman rearrangement
3662:Horner–Wadsworth–Emmons reaction
3333:Mizoroki-Heck vs. Reductive Heck
3218:Horner–Wadsworth–Emmons reaction
2729:Neighbouring group participation
715:), that of diethyl malonate and
683:
679:first reported by Link in 1944:
659:
620:
532:
504:
243:groups, which make the adjacent
5069:Fiesselmann thiophene synthesis
4899:Westphalen–Lettré rearrangement
4879:Vinylcyclopropane rearrangement
4709:Kornblum–DeLaMare rearrangement
4354:Epoxidation of allylic alcohols
4264:Noyori asymmetric hydrogenation
4199:Kornblum–DeLaMare rearrangement
3874:Gallagher–Hollander degradation
5528:Chichibabin pyridine synthesis
5014:Chichibabin pyridine synthesis
4974:Blum–Ittah aziridine synthesis
4809:Ring expansion and contraction
3078:Cross dehydrogenative coupling
271:, which makes the compound an
1:
5498:Bischler–Napieralski reaction
5456:Heterocycle forming reactions
5109:Hemetsberger indole synthesis
4969:Bischler–Napieralski reaction
4884:Wagner–Meerwein rearrangement
4854:Sommelet–Hauser rearrangement
4834:Seyferth–Gilbert homologation
4699:Ireland–Claisen rearrangement
4694:Hofmann–Martius rearrangement
4454:2,3-sigmatropic rearrangement
4069:Corey–Winter olefin synthesis
3994:Barton–McCombie deoxygenation
3637:Corey–Winter olefin synthesis
3591:Seyferth–Gilbert homologation
3458:Seyferth–Gilbert homologation
2363:10.1016/S0379-6779(03)00373-4
2192:Nature Reviews Drug Discovery
1830:Journal of Chemical Education
1509:Journal für Praktische Chemie
1313:Brown, William Henry (2018).
1249:Journal für Praktische Chemie
1215:Journal für Praktische Chemie
1173:Journal of Chemical Education
514:2-bromacrylic acid ethylester
209:C–C bond formation, and many
27:Reaction in organic chemistry
5603:Lehmstedt–Tanasescu reaction
5563:Gabriel–Colman rearrangement
5518:Bucherer carbazole synthesis
5513:Borsche–Drechsel cyclization
5493:Bernthsen acridine synthesis
5478:Bamberger triazine synthesis
5463:Algar–Flynn–Oyamada reaction
5174:Nazarov cyclization reaction
5039:De Kimpe aziridine synthesis
4994:Bucherer carbazole synthesis
4989:Borsche–Drechsel cyclization
4759:Nazarov cyclization reaction
4739:Meyer–Schuster rearrangement
4669:Gabriel–Colman rearrangement
4419:Wolffenstein–Böters reaction
4304:Reduction of nitro compounds
4154:Grundmann aldehyde synthesis
3959:Algar–Flynn–Oyamada reaction
3368:Olefin conversion technology
3363:Nozaki–Hiyama–Kishi reaction
3158:Gabriel–Colman rearrangement
3048:Claisen-Schmidt condensation
2993:Bouveault aldehyde synthesis
2269:10.1021/acs.macromol.8b01264
1921:"β-Methylglutaric anhydride"
1411:"Chapter One – Introduction"
830:of a 1,6-addition reaction.
766:and the catalyst is usually
5578:Hantzsch pyridine synthesis
5357:Enone–alkene cycloadditions
5179:Nenitzescu indole synthesis
5099:Hantzsch pyridine synthesis
5064:Ferrario–Ackermann reaction
4714:Kowalski ester homologation
4679:Halogen dance rearrangement
4524:Benzilic acid rearrangement
3949:Akabori amino-acid reaction
3909:Von Braun amide degradation
3854:Barbier–Wieland degradation
3806:Nenitzescu indole synthesis
3786:Kharasch–Sosnovsky reaction
3677:Julia–Kocienski olefination
3581:Kowalski ester homologation
3278:Kowalski ester homologation
3253:Julia–Kocienski olefination
3008:Cadiot–Chodkiewicz coupling
2933:Aza-Baylis–Hillman reaction
2878:Acetoacetic ester synthesis
2589:Dynamic binding (chemistry)
2579:Conrotatory and disrotatory
2554:Charge remote fragmentation
2034:Angew. Chem. Int. Ed. Engl.
1579:10.1002/0471264180.or090.01
1280:Progress in Polymer Science
1193:10.1021/acs.jchemed.0c00164
1081:Progress in Polymer Science
1051:10.1002/0471264180.or047.02
968:step growth polymerizations
556:Asymmetric Michael reaction
490:ethyl 2,3-dibromopropionate
400:conjugate addition reaction
5718:
5643:Robinson–Gabriel synthesis
5593:Kröhnke pyridine synthesis
5427:Retro-Diels–Alder reaction
5367:Imine Diels–Alder reaction
5154:Kröhnke pyridine synthesis
4769:Newman–Kwart rearrangement
4744:Mislow–Evans rearrangement
4654:Fischer–Hepp rearrangement
4599:Di-π-methane rearrangement
4379:Stephen aldehyde synthesis
4114:Eschweiler–Clarke reaction
3831:Williamson ether synthesis
3148:Fujiwara–Moritani reaction
3053:Combes quinoline synthesis
3018:Carbonyl olefin metathesis
2719:More O'Ferrall–Jencks plot
2644:Grunwald–Winstein equation
2614:Electron-withdrawing group
2549:Catalytic resonance theory
2204:10.1038/s41573-022-00542-z
2019:, vol. 6, p. 648
1991:, vol. 4, p. 776
1963:, vol. 4, p. 652
1935:, vol. 4, p. 630
1907:, vol. 2, p. 200
1879:, vol. 1, p. 272
777:Mukaiyama–Michael addition
760:Mukaiyama–Michael addition
754:Mukaiyama-Michael addition
446:Mukaiyama–Michael addition
5653:Urech hydantoin synthesis
5633:Pomeranz–Fritsch reaction
5558:Fischer oxazole synthesis
5292:1,3-Dipolar cycloaddition
5264:Urech hydantoin synthesis
5234:Reissert indole synthesis
5219:Pomeranz–Fritsch reaction
5149:Knorr quinoline synthesis
5079:Fischer oxazole synthesis
5009:Camps quinoline synthesis
4929:1,3-Dipolar cycloaddition
4829:Semipinacol rearrangement
4804:Ramberg–Bäcklund reaction
4789:Piancatelli rearrangement
4729:McFadyen–Stevens reaction
4484:Alpha-ketol rearrangement
4239:McFadyen–Stevens reaction
4184:Kiliani–Fischer synthesis
4104:Elbs persulfate oxidation
4029:Bouveault–Blanc reduction
3989:Baeyer–Villiger oxidation
3821:Schotten–Baumann reaction
3697:Ramberg–Bäcklund reaction
3576:Kiliani–Fischer synthesis
3418:Ramberg–Bäcklund reaction
3403:Pinacol coupling reaction
3398:Piancatelli rearrangement
3293:Liebeskind–Srogl coupling
3143:Fujimoto–Belleau reaction
2860:List of organic reactions
2724:Negative hyperconjugation
2469:
2411:
1460:10.1002/cber.188401701314
1380:10.1016/j.tet.2020.131338
573:quaternary ammonium salts
298:As originally defined by
137:
111:Organic Chemistry Portal
105:
94:
85:
74:
65:
58:
49:
34:
5628:Pictet–Spengler reaction
5543:Einhorn–Brunner reaction
5508:Boger pyridine synthesis
5402:Oxo-Diels–Alder reaction
5317:Aza-Diels–Alder reaction
5214:Pictet–Spengler reaction
5114:Hofmann–Löffler reaction
5104:Hegedus indole synthesis
5074:Fischer indole synthesis
4949:Bartoli indole synthesis
4904:Willgerodt rearrangement
4734:McLafferty rearrangement
4644:Ferrier carbocyclization
4459:2,3-Wittig rearrangement
4449:1,2-Wittig rearrangement
4289:Parikh–Doering oxidation
4279:Oxygen rebound mechanism
3944:Adkins–Peterson reaction
3836:Yamaguchi esterification
3776:Hegedus indole synthesis
3741:Bartoli indole synthesis
3612:Bamford–Stevens reaction
3528:Weinreb ketone synthesis
3488:Stork enamine alkylation
3263:Knoevenagel condensation
3133:Ferrier carbocyclization
3023:Castro–Stephens coupling
2649:Hammett acidity function
2639:Free-energy relationship
2584:Curtin–Hammett principle
2569:Conformational isomerism
2316:10.1021/acscatal.0c01365
1552:10.1002/jlac.18832180204
1521:10.1002/prac.18870350144
1261:10.1002/prac.18940490103
1227:10.1002/prac.18870350136
1124:. University of Calgary.
914:Polymerization reactions
601:In the reaction between
586:, which is activated by
569:phase transfer catalysis
173:α,β-unsaturated carbonyl
155:is a reaction between a
5588:Knorr pyrrole synthesis
5523:Bucherer–Bergs reaction
5468:Allan–Robinson reaction
5447:Wagner-Jauregg reaction
5239:Ring-closing metathesis
5164:Larock indole synthesis
5144:Knorr pyrrole synthesis
4999:Bucherer–Bergs reaction
4864:Stieglitz rearrangement
4844:Skattebøl rearrangement
4814:Ring-closing metathesis
4674:Group transfer reaction
4639:Favorskii rearrangement
4579:Cornforth rearrangement
4509:Bamberger rearrangement
4414:Wolff–Kishner reduction
4234:Markó–Lam deoxygenation
4129:Fleming–Tamao oxidation
4124:Fischer–Tropsch process
3811:Oxymercuration reaction
3791:Knorr pyrrole synthesis
3617:Barton–Kellogg reaction
3523:Wagner-Jauregg reaction
3443:Ring-closing metathesis
3433:Reimer–Tiemann reaction
3423:Rauhut–Currier reaction
3338:Nef isocyanide reaction
3298:Malonic ester synthesis
3268:Knorr pyrrole synthesis
3203:High dilution principle
3138:Friedel–Crafts reaction
3073:Cross-coupling reaction
2998:Bucherer–Bergs reaction
2983:Blanc chloromethylation
2973:Blaise ketone synthesis
2948:Baylis–Hillman reaction
2943:Barton–Kellogg reaction
2918:Allan–Robinson reaction
2824:Woodward–Hoffmann rules
2559:Charge-transfer complex
1947:Moffett, R. B. (1963).
803:{\displaystyle \gamma }
762:, the nucleophile is a
647:are co-facial with the
524:and the ethyl ester of
5553:Feist–Benary synthesis
5327:Bradsher cycloaddition
5297:4+4 Photocycloaddition
5254:Simmons–Smith reaction
5199:Paternò–Büchi reaction
5059:Feist–Benary synthesis
5049:Dieckmann condensation
4799:Pummerer rearrangement
4779:Oxy-Cope rearrangement
4754:Myers allene synthesis
4704:Jacobsen rearrangement
4619:Electrocyclic reaction
4594:Demjanov rearrangement
4549:Buchner ring expansion
4519:Beckmann rearrangement
4499:Aza-Cope rearrangement
4494:Arndt–Eistert reaction
4469:Alkyne zipper reaction
4389:Transfer hydrogenation
4364:Sharpless oxyamination
4339:Selenoxide elimination
4224:Lombardo methylenation
4149:Griesbaum coozonolysis
4059:Corey–Itsuno reduction
4034:Boyland–Sims oxidation
3974:Angeli–Rimini reaction
3622:Boord olefin synthesis
3566:Arndt–Eistert reaction
3558:Homologation reactions
3358:Nitro-Mannich reaction
3273:Kolbe–Schmitt reaction
3083:Cross-coupling partner
3003:Buchner ring expansion
2923:Arndt–Eistert reaction
2689:Kinetic isotope effect
2436:Rearrangement reaction
2165:10.1002/anie.200703702
2117:10.1002/cctc.201200161
2046:10.1002/anie.197708171
1815:10.1002/ejoc.200900831
1788:10.1002/ejoc.200900664
1679:10.1002/anie.200352136
1490:10.1002/ejoc.200901130
1022:
1014:
958:
877:
835:ethylmagnesium bromide
804:
778:
768:titanium tetrachloride
363:
290:
255:when reacted with the
194:
5412:Pauson–Khand reaction
5249:Sharpless epoxidation
5204:Pechmann condensation
5084:Friedländer synthesis
5034:Davis–Beirut reaction
4889:Wallach rearrangement
4859:Stevens rearrangement
4794:Pinacol rearrangement
4774:Overman rearrangement
4689:Hofmann rearrangement
4684:Hayashi rearrangement
4649:Ferrier rearrangement
4604:Dimroth rearrangement
4589:Curtius rearrangement
4584:Criegee rearrangement
4564:Claisen rearrangement
4554:Carroll rearrangement
4489:Amadori rearrangement
4479:Allylic rearrangement
4359:Sharpless epoxidation
4094:Dess–Martin oxidation
4019:Bohn–Schmidt reaction
3879:Hofmann rearrangement
3682:Kauffmann olefination
3605:Olefination reactions
3543:Wurtz–Fittig reaction
3378:Palladium–NHC complex
3258:Kauffmann olefination
3213:Homologation reaction
3063:Corey–House synthesis
3043:Claisen rearrangement
2839:Yukawa–Tsuno equation
2799:Swain–Lupton equation
2779:Spherical aromaticity
2714:Möbius–Hückel concept
2499:Aromatic ring current
2461:Substitution reaction
1534:Komnenos, T. (1883).
1020:
1012:
956:
875:
805:
776:
614:-toluenesulfonic acid
542:Rainer Ludwig Claisen
494:diethyl sodiomalonate
362:
289:
193:
5618:Paal–Knorr synthesis
5488:Barton–Zard reaction
5432:Staudinger synthesis
5382:Ketene cycloaddition
5352:Diels–Alder reaction
5332:Cheletropic reaction
5312:Alkyne trimerisation
5194:Paal–Knorr synthesis
5159:Kulinkovich reaction
5134:Jacobsen epoxidation
5054:Diels–Alder reaction
4849:Smiles rearrangement
4839:Sigmatropic reaction
4724:Lossen rearrangement
4574:Corey–Fuchs reaction
4539:Boekelheide reaction
4534:Bergmann degradation
4464:Achmatowicz reaction
4249:Methionine sulfoxide
4049:Clemmensen reduction
4009:Bergmann degradation
3939:Acyloin condensation
3904:Strecker degradation
3859:Bergmann degradation
3826:Ullmann condensation
3692:Peterson olefination
3667:Hydrazone iodination
3647:Elimination reaction
3548:Zincke–Suhl reaction
3468:Sonogashira coupling
3428:Reformatsky reaction
3388:Peterson olefination
3353:Nierenstein reaction
3283:Kulinkovich reaction
3098:Diels–Alder reaction
3058:Corey–Fuchs reaction
3038:Claisen condensation
2908:Alkyne trimerisation
2883:Acyloin condensation
2849:Σ-bishomoaromaticity
2809:Thorpe–Ingold effect
2421:Elimination reaction
1919:James Cason (1963).
1503:Claisen, L. (1887).
794:
782:1,6-Michael reaction
703:(Michael donor) and
631:is favored with 99%
476:at low temperature.
384:resonance structures
354:as the nucleophile:
336:aza-Michael reaction
332:oxa-Michael reaction
222:electron-withdrawing
153:Michael 1,4 addition
5638:Prilezhaev reaction
5623:Pellizzari reaction
5302:(4+3) cycloaddition
5269:Van Leusen reaction
5244:Robinson annulation
5229:Pschorr cyclization
5224:Prilezhaev reaction
4954:Bergman cyclization
4909:Wolff rearrangement
4894:Weerman degradation
4784:Pericyclic reaction
4764:Neber rearrangement
4659:Fries rearrangement
4544:Brook rearrangement
4529:Bergman cyclization
4374:Staudinger reaction
4319:Rosenmund reduction
4309:Reductive amination
4274:Oppenauer oxidation
4064:Corey–Kim oxidation
4039:Cannizzaro reaction
3914:Weerman degradation
3889:Isosaccharinic acid
3801:Mukaiyama hydration
3657:Hofmann elimination
3642:Dehydrohalogenation
3627:Chugaev elimination
3448:Robinson annulation
3393:Pfitzinger reaction
3163:Gattermann reaction
3108:Wulff–Dötz reaction
3088:Dakin–West reaction
3013:Carbonyl allylation
2958:Bergman cyclization
2744:Kennedy J. P. Orton
2664:Hammond's postulate
2634:Flippin–Lodge angle
2604:Electromeric effect
2529:Beta-silicon effect
2514:Baker–Nathan effect
2261:2018MaMol..51.5979H
2069:(46): 14738–14739.
1842:2010JChEd..87..194W
1651:10.1021/ja01234a019
1185:2020JChEd..97.2291T
1013:Poly(amino quinone)
748:Robinson annulation
737:methyl vinyl ketone
402:. Finally, enolate
375:by a base leads to
185:conjugate additions
5692:Addition reactions
5387:McCormack reaction
5337:Conia-ene reaction
5169:Madelung synthesis
4959:Biginelli reaction
4749:Mumm rearrangement
4634:Favorskii reaction
4569:Cope rearrangement
4559:Chan rearrangement
4324:Rubottom oxidation
4254:Miyaura borylation
4219:Lipid peroxidation
4214:Lindgren oxidation
4194:Kornblum oxidation
4189:Kolbe electrolysis
4134:Fukuyama reduction
4044:Carbonyl reduction
3894:Marker degradation
3756:Diazonium compound
3746:Boudouard reaction
3725:Carbon-heteroatom
3652:Grieco elimination
3438:Rieche formylation
3383:Passerini reaction
3313:Meerwein arylation
3233:Hydroxymethylation
3128:Favorskii reaction
3028:Chan rearrangement
2963:Biginelli reaction
2888:Aldol condensation
2734:2-Norbornyl cation
2709:Möbius aromaticity
2704:Markovnikov's rule
2599:Effective molarity
2544:Bürgi–Dunitz angle
2534:Bicycloaromaticity
1023:
1015:
959:
878:
828:enantioselectivity
800:
779:
677:benzylideneacetone
425:. These polarized
364:
348:reaction mechanism
291:
203:diastereoselective
195:
5679:
5678:
5675:
5674:
5671:
5670:
5663:Wohl–Aue reaction
5307:6+4 Cycloaddition
5124:Iodolactonization
4444:1,2-rearrangement
4409:Wohl–Aue reaction
4329:Sabatier reaction
4294:Pinnick oxidation
4259:Mozingo reduction
4204:Leuckart reaction
4159:Haloform reaction
4074:Criegee oxidation
4054:Collins oxidation
4004:Benkeser reaction
3999:Bechamp reduction
3969:Andrussow process
3954:Alcohol oxidation
3864:Edman degradation
3771:Haloform reaction
3720:
3719:
3707:Takai olefination
3672:Julia olefination
3498:Takai olefination
3373:Olefin metathesis
3248:Julia olefination
3173:Grignard reaction
3153:Fukuyama coupling
3068:Coupling reaction
3033:Chan–Lam coupling
2903:Alkyne metathesis
2898:Alkane metathesis
2754:Phosphaethynolate
2659:George S. Hammond
2619:Electronic effect
2574:Conjugated system
2456:Stereospecificity
2451:Stereoselectivity
2416:Addition reaction
2405:organic reactions
2310:(11): 6488–6496.
2255:(15): 5979–5988.
2075:10.1021/ja063900+
2017:Collected Volumes
2010:Organic Syntheses
1989:Collected Volumes
1982:Organic Syntheses
1961:Collected Volumes
1954:Organic Syntheses
1933:Collected Volumes
1926:Organic Syntheses
1905:Collected Volumes
1898:Organic Syntheses
1877:Collected Volumes
1870:Organic Syntheses
1850:10.1021/ed800040m
1749:10.1021/ol062718a
1714:10.1021/ol062000v
1708:(23): 5239–5242.
1673:(40): 4955–4957.
1667:Angewandte Chemie
1616:10.1021/ja062701n
1610:(30): 9624–9625.
1567:Organic Reactions
1484:(10): 2009–2016.
1326:978-1-337-51640-2
1316:Organic chemistry
1157:978-0-19-850346-0
1148:Organic Chemistry
1138:; Greeves, Nick;
1136:Clayden, Jonathan
1060:978-0-471-26418-7
1021:Poly(amido amine)
837:to ethyl sorbate
790:carbon of an α,β-
673:4-hydroxycoumarin
575:derived from the
540:In the same year
427:frontier orbitals
251:enough to form a
145:organic chemistry
141:
140:
101:
100:
44:Addition reaction
35:Michael Addition
16:(Redirected from
5709:
5658:Wenker synthesis
5648:Stollé synthesis
5503:Bobbitt reaction
5473:Auwers synthesis
5417:Povarov reaction
5342:Cyclopropanation
5280:
5274:Wenker synthesis
5029:Darzens reaction
4979:Bobbitt reaction
4824:Schmidt reaction
4629:Enyne metathesis
4404:Whiting reaction
4399:Wharton reaction
4344:Shapiro reaction
4334:Sarett oxidation
4299:Prévost reaction
4109:Emde degradation
3919:Wohl degradation
3899:Ruff degradation
3869:Emde degradation
3766:Grignard reagent
3702:Shapiro reaction
3687:McMurry reaction
3554:
3518:Ullmann reaction
3483:Stollé synthesis
3473:Stetter reaction
3463:Shapiro reaction
3453:Sakurai reaction
3348:Negishi coupling
3328:Minisci reaction
3323:Michael reaction
3308:McMurry reaction
3303:Mannich reaction
3183:Hammick reaction
3178:Grignard reagent
3118:Enyne metathesis
3103:Doebner reaction
3093:Darzens reaction
2938:Barbier reaction
2928:Auwers synthesis
2855:
2829:Woodward's rules
2794:Superaromaticity
2784:Spiroaromaticity
2684:Inductive effect
2679:Hyperconjugation
2654:Hammett equation
2594:Edwards equation
2446:Regioselectivity
2397:
2390:
2383:
2374:
2367:
2366:
2357:(2–3): 183–197.
2351:Synthetic Metals
2342:
2336:
2335:
2295:
2289:
2288:
2240:
2234:
2233:
2223:
2183:
2177:
2176:
2144:
2129:
2128:
2096:
2087:
2086:
2056:
2050:
2049:
2028:
2022:
2020:
2013:
2000:
1994:
1992:
1985:
1972:
1966:
1964:
1957:
1944:
1938:
1936:
1929:
1916:
1910:
1908:
1901:
1888:
1882:
1880:
1873:
1860:
1854:
1853:
1825:
1819:
1818:
1798:
1792:
1791:
1767:
1761:
1760:
1732:
1726:
1725:
1697:
1691:
1690:
1661:
1655:
1654:
1634:
1628:
1627:
1599:
1593:
1592:
1562:
1556:
1555:
1531:
1525:
1524:
1500:
1494:
1493:
1473:
1464:
1463:
1454:(1): 1185–1188.
1439:
1433:
1432:
1431:
1429:
1406:
1400:
1399:
1359:
1353:
1352:
1346:
1338:
1310:
1304:
1303:
1271:
1265:
1264:
1237:
1231:
1230:
1203:
1197:
1196:
1179:(8): 2291–2295.
1168:
1162:
1161:
1132:
1126:
1125:
1117:
1111:
1106:
1097:
1096:
1076:
1065:
1064:
1036:
1000:network polymers
931:
930:
926:
824:regioselectivity
809:
807:
806:
801:
764:silyl enol ether
717:methyl crotonate
705:diethyl fumarate
701:diethyl malonate
687:
663:
637:transition state
624:
536:
522:diethyl malonate
508:
486:Tufts University
462:regioselectivity
442:silyl enol ether
207:enantioselective
169:Michael acceptor
149:Michael reaction
133:
118:
116:michael-addition
89:
69:Michael Acceptor
56:
55:
32:
21:
18:Michael acceptor
5717:
5716:
5712:
5711:
5710:
5708:
5707:
5706:
5682:
5681:
5680:
5667:
5568:Gewald reaction
5451:
5278:
5259:Skraup reaction
5094:Graham reaction
5089:Gewald reaction
4920:
4913:
4435:
4428:
4384:Swern oxidation
4369:Stahl oxidation
4314:Riley oxidation
4269:Omega oxidation
4229:Luche reduction
4179:Jones oxidation
4144:Glycol cleavage
4139:Ganem oxidation
4084:Davis oxidation
4079:Dakin oxidation
4014:Birch reduction
3964:Amide reduction
3930:
3923:
3884:Hooker reaction
3846:
3840:
3728:
3726:
3716:
3712:Wittig reaction
3600:
3596:Wittig reaction
3571:Hooker reaction
3552:
3533:Wittig reaction
3508:Thorpe reaction
3493:Suzuki reaction
3478:Stille reaction
3413:Quelet reaction
3288:Kumada coupling
3238:Ivanov reaction
3228:Hydrovinylation
3208:Hiyama coupling
3168:Glaser coupling
2978:Blaise reaction
2968:Bingel reaction
2953:Benary reaction
2870:
2868:
2862:
2853:
2749:Passive binding
2669:Homoaromaticity
2519:Baldwin's rules
2494:Antiaromaticity
2489:Anomeric effect
2465:
2407:
2401:
2371:
2370:
2344:
2343:
2339:
2297:
2296:
2292:
2242:
2241:
2237:
2198:(12): 881–898.
2185:
2184:
2180:
2146:
2145:
2132:
2098:
2097:
2090:
2058:
2057:
2053:
2040:(12): 817–826.
2030:
2029:
2025:
2015:
2002:
2001:
1997:
1987:
1974:
1973:
1969:
1959:
1946:
1945:
1941:
1931:
1918:
1917:
1913:
1903:
1890:
1889:
1885:
1875:
1862:
1861:
1857:
1827:
1826:
1822:
1800:
1799:
1795:
1769:
1768:
1764:
1737:Organic Letters
1734:
1733:
1729:
1702:Organic Letters
1699:
1698:
1694:
1663:
1662:
1658:
1636:
1635:
1631:
1601:
1600:
1596:
1589:
1564:
1563:
1559:
1533:
1532:
1528:
1502:
1501:
1497:
1475:
1474:
1467:
1441:
1440:
1436:
1427:
1425:
1408:
1407:
1403:
1361:
1360:
1356:
1339:
1327:
1312:
1311:
1307:
1273:
1272:
1268:
1239:
1238:
1234:
1205:
1204:
1200:
1170:
1169:
1165:
1158:
1134:
1133:
1129:
1119:
1118:
1114:
1107:
1100:
1078:
1077:
1068:
1061:
1038:
1037:
1033:
1028:
964:
932:
928:
924:
922:
921:
916:
888:
886:Pharmaceuticals
883:
820:organocatalysis
792:
791:
784:
756:
725:methyl acrylate
697:
653:hydrogen bonded
584:organocatalysis
558:
482:
344:
296:
213:variants exist
199:atom-economical
175:) to produce a
129:
114:
87:
80:
76:
67:
28:
23:
22:
15:
12:
11:
5:
5715:
5713:
5705:
5704:
5702:Name reactions
5699:
5694:
5684:
5683:
5677:
5676:
5673:
5672:
5669:
5668:
5666:
5665:
5660:
5655:
5650:
5645:
5640:
5635:
5630:
5625:
5620:
5615:
5610:
5605:
5600:
5595:
5590:
5585:
5580:
5575:
5573:Hantzsch ester
5570:
5565:
5560:
5555:
5550:
5545:
5540:
5535:
5530:
5525:
5520:
5515:
5510:
5505:
5500:
5495:
5490:
5485:
5483:Banert cascade
5480:
5475:
5470:
5465:
5459:
5457:
5453:
5452:
5450:
5449:
5444:
5439:
5434:
5429:
5424:
5422:Prato reaction
5419:
5414:
5409:
5404:
5399:
5394:
5389:
5384:
5379:
5374:
5369:
5364:
5359:
5354:
5349:
5344:
5339:
5334:
5329:
5324:
5319:
5314:
5309:
5304:
5299:
5294:
5288:
5286:
5277:
5276:
5271:
5266:
5261:
5256:
5251:
5246:
5241:
5236:
5231:
5226:
5221:
5216:
5211:
5206:
5201:
5196:
5191:
5186:
5181:
5176:
5171:
5166:
5161:
5156:
5151:
5146:
5141:
5136:
5131:
5126:
5121:
5116:
5111:
5106:
5101:
5096:
5091:
5086:
5081:
5076:
5071:
5066:
5061:
5056:
5051:
5046:
5041:
5036:
5031:
5026:
5021:
5016:
5011:
5006:
5001:
4996:
4991:
4986:
4981:
4976:
4971:
4966:
4961:
4956:
4951:
4946:
4941:
4936:
4931:
4925:
4923:
4915:
4914:
4912:
4911:
4906:
4901:
4896:
4891:
4886:
4881:
4876:
4871:
4866:
4861:
4856:
4851:
4846:
4841:
4836:
4831:
4826:
4821:
4816:
4811:
4806:
4801:
4796:
4791:
4786:
4781:
4776:
4771:
4766:
4761:
4756:
4751:
4746:
4741:
4736:
4731:
4726:
4721:
4716:
4711:
4706:
4701:
4696:
4691:
4686:
4681:
4676:
4671:
4666:
4661:
4656:
4651:
4646:
4641:
4636:
4631:
4626:
4621:
4616:
4611:
4606:
4601:
4596:
4591:
4586:
4581:
4576:
4571:
4566:
4561:
4556:
4551:
4546:
4541:
4536:
4531:
4526:
4521:
4516:
4514:Banert cascade
4511:
4506:
4501:
4496:
4491:
4486:
4481:
4476:
4471:
4466:
4461:
4456:
4451:
4446:
4440:
4438:
4434:Rearrangement
4430:
4429:
4427:
4426:
4424:Zinin reaction
4421:
4416:
4411:
4406:
4401:
4396:
4394:Wacker process
4391:
4386:
4381:
4376:
4371:
4366:
4361:
4356:
4351:
4346:
4341:
4336:
4331:
4326:
4321:
4316:
4311:
4306:
4301:
4296:
4291:
4286:
4281:
4276:
4271:
4266:
4261:
4256:
4251:
4246:
4241:
4236:
4231:
4226:
4221:
4216:
4211:
4206:
4201:
4196:
4191:
4186:
4181:
4176:
4171:
4169:Hydrogenolysis
4166:
4161:
4156:
4151:
4146:
4141:
4136:
4131:
4126:
4121:
4119:Étard reaction
4116:
4111:
4106:
4101:
4096:
4091:
4086:
4081:
4076:
4071:
4066:
4061:
4056:
4051:
4046:
4041:
4036:
4031:
4026:
4024:Bosch reaction
4021:
4016:
4011:
4006:
4001:
3996:
3991:
3986:
3981:
3976:
3971:
3966:
3961:
3956:
3951:
3946:
3941:
3935:
3933:
3929:Organic redox
3925:
3924:
3922:
3921:
3916:
3911:
3906:
3901:
3896:
3891:
3886:
3881:
3876:
3871:
3866:
3861:
3856:
3850:
3848:
3842:
3841:
3839:
3838:
3833:
3828:
3823:
3818:
3813:
3808:
3803:
3798:
3793:
3788:
3783:
3778:
3773:
3768:
3763:
3761:Esterification
3758:
3753:
3748:
3743:
3738:
3732:
3730:
3722:
3721:
3718:
3717:
3715:
3714:
3709:
3704:
3699:
3694:
3689:
3684:
3679:
3674:
3669:
3664:
3659:
3654:
3649:
3644:
3639:
3634:
3629:
3624:
3619:
3614:
3608:
3606:
3602:
3601:
3599:
3598:
3593:
3588:
3583:
3578:
3573:
3568:
3562:
3560:
3551:
3550:
3545:
3540:
3538:Wurtz reaction
3535:
3530:
3525:
3520:
3515:
3510:
3505:
3500:
3495:
3490:
3485:
3480:
3475:
3470:
3465:
3460:
3455:
3450:
3445:
3440:
3435:
3430:
3425:
3420:
3415:
3410:
3408:Prins reaction
3405:
3400:
3395:
3390:
3385:
3380:
3375:
3370:
3365:
3360:
3355:
3350:
3345:
3340:
3335:
3330:
3325:
3320:
3315:
3310:
3305:
3300:
3295:
3290:
3285:
3280:
3275:
3270:
3265:
3260:
3255:
3250:
3245:
3240:
3235:
3230:
3225:
3223:Hydrocyanation
3220:
3215:
3210:
3205:
3200:
3195:
3193:Henry reaction
3190:
3185:
3180:
3175:
3170:
3165:
3160:
3155:
3150:
3145:
3140:
3135:
3130:
3125:
3120:
3115:
3110:
3105:
3100:
3095:
3090:
3085:
3080:
3075:
3070:
3065:
3060:
3055:
3050:
3045:
3040:
3035:
3030:
3025:
3020:
3015:
3010:
3005:
3000:
2995:
2990:
2985:
2980:
2975:
2970:
2965:
2960:
2955:
2950:
2945:
2940:
2935:
2930:
2925:
2920:
2915:
2910:
2905:
2900:
2895:
2893:Aldol reaction
2890:
2885:
2880:
2874:
2872:
2867:Carbon-carbon
2864:
2863:
2858:
2852:
2851:
2846:
2844:Zaitsev's rule
2841:
2836:
2831:
2826:
2821:
2816:
2811:
2806:
2801:
2796:
2791:
2789:Steric effects
2786:
2781:
2776:
2771:
2766:
2761:
2756:
2751:
2746:
2741:
2736:
2731:
2726:
2721:
2716:
2711:
2706:
2701:
2696:
2691:
2686:
2681:
2676:
2671:
2666:
2661:
2656:
2651:
2646:
2641:
2636:
2631:
2626:
2621:
2616:
2611:
2606:
2601:
2596:
2591:
2586:
2581:
2576:
2571:
2566:
2561:
2556:
2551:
2546:
2541:
2536:
2531:
2526:
2521:
2516:
2511:
2506:
2501:
2496:
2491:
2486:
2481:
2476:
2470:
2467:
2466:
2464:
2463:
2458:
2453:
2448:
2443:
2441:Redox reaction
2438:
2433:
2428:
2426:Polymerization
2423:
2418:
2412:
2409:
2408:
2402:
2400:
2399:
2392:
2385:
2377:
2369:
2368:
2337:
2290:
2249:Macromolecules
2235:
2178:
2159:(2): 398–401.
2130:
2111:(7): 959–962.
2088:
2051:
2023:
1995:
1967:
1939:
1911:
1883:
1855:
1820:
1793:
1762:
1743:(3): 413–415.
1727:
1692:
1656:
1629:
1594:
1587:
1557:
1546:(2): 145–167.
1526:
1515:(1): 413–415.
1511:. 2nd series.
1495:
1465:
1434:
1401:
1374:(28): 131338.
1354:
1325:
1305:
1286:(5): 487–531.
1266:
1251:. 2nd series.
1232:
1217:. 2nd series.
1198:
1163:
1156:
1144:Wothers, Peter
1140:Warren, Stuart
1127:
1112:
1098:
1087:(5): 487–531.
1066:
1059:
1030:
1029:
1027:
1024:
996:anti-corrosion
983:), poly(amino
963:
960:
920:
917:
915:
912:
887:
884:
882:
879:
799:
783:
780:
755:
752:
721:2-nitropropane
696:
693:
689:
688:
665:
664:
645:β-nitrostyrene
626:
625:
607:β-nitrostyrene
557:
554:
538:
537:
510:
509:
481:
478:
434:aldol addition
417:of stabilized
366:
365:
343:
340:
300:Arthur Michael
295:
292:
218:
217:
177:Michael adduct
139:
138:
135:
134:
127:
120:
119:
112:
108:
107:
103:
102:
99:
98:
96:Michael adduct
92:
91:
83:
82:
78:
72:
71:
63:
62:
52:
51:
47:
46:
41:
40:Reaction type
37:
36:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
5714:
5703:
5700:
5698:
5695:
5693:
5690:
5689:
5687:
5664:
5661:
5659:
5656:
5654:
5651:
5649:
5646:
5644:
5641:
5639:
5636:
5634:
5631:
5629:
5626:
5624:
5621:
5619:
5616:
5614:
5611:
5609:
5606:
5604:
5601:
5599:
5596:
5594:
5591:
5589:
5586:
5584:
5583:Herz reaction
5581:
5579:
5576:
5574:
5571:
5569:
5566:
5564:
5561:
5559:
5556:
5554:
5551:
5549:
5546:
5544:
5541:
5539:
5536:
5534:
5531:
5529:
5526:
5524:
5521:
5519:
5516:
5514:
5511:
5509:
5506:
5504:
5501:
5499:
5496:
5494:
5491:
5489:
5486:
5484:
5481:
5479:
5476:
5474:
5471:
5469:
5466:
5464:
5461:
5460:
5458:
5454:
5448:
5445:
5443:
5440:
5438:
5435:
5433:
5430:
5428:
5425:
5423:
5420:
5418:
5415:
5413:
5410:
5408:
5405:
5403:
5400:
5398:
5395:
5393:
5390:
5388:
5385:
5383:
5380:
5378:
5375:
5373:
5370:
5368:
5365:
5363:
5360:
5358:
5355:
5353:
5350:
5348:
5345:
5343:
5340:
5338:
5335:
5333:
5330:
5328:
5325:
5323:
5320:
5318:
5315:
5313:
5310:
5308:
5305:
5303:
5300:
5298:
5295:
5293:
5290:
5289:
5287:
5285:
5284:Cycloaddition
5281:
5275:
5272:
5270:
5267:
5265:
5262:
5260:
5257:
5255:
5252:
5250:
5247:
5245:
5242:
5240:
5237:
5235:
5232:
5230:
5227:
5225:
5222:
5220:
5217:
5215:
5212:
5210:
5207:
5205:
5202:
5200:
5197:
5195:
5192:
5190:
5187:
5185:
5182:
5180:
5177:
5175:
5172:
5170:
5167:
5165:
5162:
5160:
5157:
5155:
5152:
5150:
5147:
5145:
5142:
5140:
5137:
5135:
5132:
5130:
5129:Isay reaction
5127:
5125:
5122:
5120:
5117:
5115:
5112:
5110:
5107:
5105:
5102:
5100:
5097:
5095:
5092:
5090:
5087:
5085:
5082:
5080:
5077:
5075:
5072:
5070:
5067:
5065:
5062:
5060:
5057:
5055:
5052:
5050:
5047:
5045:
5042:
5040:
5037:
5035:
5032:
5030:
5027:
5025:
5024:Cycloaddition
5022:
5020:
5017:
5015:
5012:
5010:
5007:
5005:
5002:
5000:
4997:
4995:
4992:
4990:
4987:
4985:
4982:
4980:
4977:
4975:
4972:
4970:
4967:
4965:
4962:
4960:
4957:
4955:
4952:
4950:
4947:
4945:
4942:
4940:
4937:
4935:
4932:
4930:
4927:
4926:
4924:
4922:
4919:Ring forming
4916:
4910:
4907:
4905:
4902:
4900:
4897:
4895:
4892:
4890:
4887:
4885:
4882:
4880:
4877:
4875:
4872:
4870:
4867:
4865:
4862:
4860:
4857:
4855:
4852:
4850:
4847:
4845:
4842:
4840:
4837:
4835:
4832:
4830:
4827:
4825:
4822:
4820:
4819:Rupe reaction
4817:
4815:
4812:
4810:
4807:
4805:
4802:
4800:
4797:
4795:
4792:
4790:
4787:
4785:
4782:
4780:
4777:
4775:
4772:
4770:
4767:
4765:
4762:
4760:
4757:
4755:
4752:
4750:
4747:
4745:
4742:
4740:
4737:
4735:
4732:
4730:
4727:
4725:
4722:
4720:
4717:
4715:
4712:
4710:
4707:
4705:
4702:
4700:
4697:
4695:
4692:
4690:
4687:
4685:
4682:
4680:
4677:
4675:
4672:
4670:
4667:
4665:
4662:
4660:
4657:
4655:
4652:
4650:
4647:
4645:
4642:
4640:
4637:
4635:
4632:
4630:
4627:
4625:
4622:
4620:
4617:
4615:
4612:
4610:
4607:
4605:
4602:
4600:
4597:
4595:
4592:
4590:
4587:
4585:
4582:
4580:
4577:
4575:
4572:
4570:
4567:
4565:
4562:
4560:
4557:
4555:
4552:
4550:
4547:
4545:
4542:
4540:
4537:
4535:
4532:
4530:
4527:
4525:
4522:
4520:
4517:
4515:
4512:
4510:
4507:
4505:
4502:
4500:
4497:
4495:
4492:
4490:
4487:
4485:
4482:
4480:
4477:
4475:
4472:
4470:
4467:
4465:
4462:
4460:
4457:
4455:
4452:
4450:
4447:
4445:
4442:
4441:
4439:
4437:
4431:
4425:
4422:
4420:
4417:
4415:
4412:
4410:
4407:
4405:
4402:
4400:
4397:
4395:
4392:
4390:
4387:
4385:
4382:
4380:
4377:
4375:
4372:
4370:
4367:
4365:
4362:
4360:
4357:
4355:
4352:
4350:
4347:
4345:
4342:
4340:
4337:
4335:
4332:
4330:
4327:
4325:
4322:
4320:
4317:
4315:
4312:
4310:
4307:
4305:
4302:
4300:
4297:
4295:
4292:
4290:
4287:
4285:
4282:
4280:
4277:
4275:
4272:
4270:
4267:
4265:
4262:
4260:
4257:
4255:
4252:
4250:
4247:
4245:
4242:
4240:
4237:
4235:
4232:
4230:
4227:
4225:
4222:
4220:
4217:
4215:
4212:
4210:
4209:Ley oxidation
4207:
4205:
4202:
4200:
4197:
4195:
4192:
4190:
4187:
4185:
4182:
4180:
4177:
4175:
4174:Hydroxylation
4172:
4170:
4167:
4165:
4164:Hydrogenation
4162:
4160:
4157:
4155:
4152:
4150:
4147:
4145:
4142:
4140:
4137:
4135:
4132:
4130:
4127:
4125:
4122:
4120:
4117:
4115:
4112:
4110:
4107:
4105:
4102:
4100:
4099:DNA oxidation
4097:
4095:
4092:
4090:
4089:Deoxygenation
4087:
4085:
4082:
4080:
4077:
4075:
4072:
4070:
4067:
4065:
4062:
4060:
4057:
4055:
4052:
4050:
4047:
4045:
4042:
4040:
4037:
4035:
4032:
4030:
4027:
4025:
4022:
4020:
4017:
4015:
4012:
4010:
4007:
4005:
4002:
4000:
3997:
3995:
3992:
3990:
3987:
3985:
3982:
3980:
3979:Aromatization
3977:
3975:
3972:
3970:
3967:
3965:
3962:
3960:
3957:
3955:
3952:
3950:
3947:
3945:
3942:
3940:
3937:
3936:
3934:
3932:
3926:
3920:
3917:
3915:
3912:
3910:
3907:
3905:
3902:
3900:
3897:
3895:
3892:
3890:
3887:
3885:
3882:
3880:
3877:
3875:
3872:
3870:
3867:
3865:
3862:
3860:
3857:
3855:
3852:
3851:
3849:
3843:
3837:
3834:
3832:
3829:
3827:
3824:
3822:
3819:
3817:
3816:Reed reaction
3814:
3812:
3809:
3807:
3804:
3802:
3799:
3797:
3794:
3792:
3789:
3787:
3784:
3782:
3779:
3777:
3774:
3772:
3769:
3767:
3764:
3762:
3759:
3757:
3754:
3752:
3749:
3747:
3744:
3742:
3739:
3737:
3734:
3733:
3731:
3727:bond forming
3723:
3713:
3710:
3708:
3705:
3703:
3700:
3698:
3695:
3693:
3690:
3688:
3685:
3683:
3680:
3678:
3675:
3673:
3670:
3668:
3665:
3663:
3660:
3658:
3655:
3653:
3650:
3648:
3645:
3643:
3640:
3638:
3635:
3633:
3632:Cope reaction
3630:
3628:
3625:
3623:
3620:
3618:
3615:
3613:
3610:
3609:
3607:
3603:
3597:
3594:
3592:
3589:
3587:
3584:
3582:
3579:
3577:
3574:
3572:
3569:
3567:
3564:
3563:
3561:
3559:
3555:
3549:
3546:
3544:
3541:
3539:
3536:
3534:
3531:
3529:
3526:
3524:
3521:
3519:
3516:
3514:
3511:
3509:
3506:
3504:
3501:
3499:
3496:
3494:
3491:
3489:
3486:
3484:
3481:
3479:
3476:
3474:
3471:
3469:
3466:
3464:
3461:
3459:
3456:
3454:
3451:
3449:
3446:
3444:
3441:
3439:
3436:
3434:
3431:
3429:
3426:
3424:
3421:
3419:
3416:
3414:
3411:
3409:
3406:
3404:
3401:
3399:
3396:
3394:
3391:
3389:
3386:
3384:
3381:
3379:
3376:
3374:
3371:
3369:
3366:
3364:
3361:
3359:
3356:
3354:
3351:
3349:
3346:
3344:
3343:Nef synthesis
3341:
3339:
3336:
3334:
3331:
3329:
3326:
3324:
3321:
3319:
3318:Methylenation
3316:
3314:
3311:
3309:
3306:
3304:
3301:
3299:
3296:
3294:
3291:
3289:
3286:
3284:
3281:
3279:
3276:
3274:
3271:
3269:
3266:
3264:
3261:
3259:
3256:
3254:
3251:
3249:
3246:
3244:
3241:
3239:
3236:
3234:
3231:
3229:
3226:
3224:
3221:
3219:
3216:
3214:
3211:
3209:
3206:
3204:
3201:
3199:
3196:
3194:
3191:
3189:
3188:Heck reaction
3186:
3184:
3181:
3179:
3176:
3174:
3171:
3169:
3166:
3164:
3161:
3159:
3156:
3154:
3151:
3149:
3146:
3144:
3141:
3139:
3136:
3134:
3131:
3129:
3126:
3124:
3121:
3119:
3116:
3114:
3111:
3109:
3106:
3104:
3101:
3099:
3096:
3094:
3091:
3089:
3086:
3084:
3081:
3079:
3076:
3074:
3071:
3069:
3066:
3064:
3061:
3059:
3056:
3054:
3051:
3049:
3046:
3044:
3041:
3039:
3036:
3034:
3031:
3029:
3026:
3024:
3021:
3019:
3016:
3014:
3011:
3009:
3006:
3004:
3001:
2999:
2996:
2994:
2991:
2989:
2986:
2984:
2981:
2979:
2976:
2974:
2971:
2969:
2966:
2964:
2961:
2959:
2956:
2954:
2951:
2949:
2946:
2944:
2941:
2939:
2936:
2934:
2931:
2929:
2926:
2924:
2921:
2919:
2916:
2914:
2911:
2909:
2906:
2904:
2901:
2899:
2896:
2894:
2891:
2889:
2886:
2884:
2881:
2879:
2876:
2875:
2873:
2869:bond forming
2865:
2861:
2856:
2850:
2847:
2845:
2842:
2840:
2837:
2835:
2834:Y-aromaticity
2832:
2830:
2827:
2825:
2822:
2820:
2819:Walsh diagram
2817:
2815:
2812:
2810:
2807:
2805:
2804:Taft equation
2802:
2800:
2797:
2795:
2792:
2790:
2787:
2785:
2782:
2780:
2777:
2775:
2774:Σ-aromaticity
2772:
2770:
2767:
2765:
2762:
2760:
2757:
2755:
2752:
2750:
2747:
2745:
2742:
2740:
2737:
2735:
2732:
2730:
2727:
2725:
2722:
2720:
2717:
2715:
2712:
2710:
2707:
2705:
2702:
2700:
2699:Marcus theory
2697:
2695:
2692:
2690:
2687:
2685:
2682:
2680:
2677:
2675:
2674:Hückel's rule
2672:
2670:
2667:
2665:
2662:
2660:
2657:
2655:
2652:
2650:
2647:
2645:
2642:
2640:
2637:
2635:
2632:
2630:
2629:Evelyn effect
2627:
2625:
2622:
2620:
2617:
2615:
2612:
2610:
2609:Electron-rich
2607:
2605:
2602:
2600:
2597:
2595:
2592:
2590:
2587:
2585:
2582:
2580:
2577:
2575:
2572:
2570:
2567:
2565:
2562:
2560:
2557:
2555:
2552:
2550:
2547:
2545:
2542:
2540:
2537:
2535:
2532:
2530:
2527:
2525:
2524:Bema Hapothle
2522:
2520:
2517:
2515:
2512:
2510:
2507:
2505:
2502:
2500:
2497:
2495:
2492:
2490:
2487:
2485:
2482:
2480:
2477:
2475:
2472:
2471:
2468:
2462:
2459:
2457:
2454:
2452:
2449:
2447:
2444:
2442:
2439:
2437:
2434:
2432:
2429:
2427:
2424:
2422:
2419:
2417:
2414:
2413:
2410:
2406:
2398:
2393:
2391:
2386:
2384:
2379:
2378:
2375:
2364:
2360:
2356:
2352:
2348:
2341:
2338:
2333:
2329:
2325:
2321:
2317:
2313:
2309:
2305:
2304:ACS Catalysis
2301:
2294:
2291:
2286:
2282:
2278:
2274:
2270:
2266:
2262:
2258:
2254:
2250:
2246:
2239:
2236:
2231:
2227:
2222:
2217:
2213:
2209:
2205:
2201:
2197:
2193:
2189:
2182:
2179:
2174:
2170:
2166:
2162:
2158:
2154:
2150:
2143:
2141:
2139:
2137:
2135:
2131:
2126:
2122:
2118:
2114:
2110:
2106:
2102:
2095:
2093:
2089:
2084:
2080:
2076:
2072:
2068:
2064:
2063:
2055:
2052:
2047:
2043:
2039:
2036:
2035:
2027:
2024:
2018:
2012:
2011:
2006:
1999:
1996:
1990:
1984:
1983:
1978:
1971:
1968:
1962:
1956:
1955:
1950:
1943:
1940:
1934:
1928:
1927:
1922:
1915:
1912:
1906:
1900:
1899:
1894:
1887:
1884:
1878:
1872:
1871:
1866:
1859:
1856:
1851:
1847:
1843:
1839:
1835:
1831:
1824:
1821:
1816:
1812:
1808:
1804:
1797:
1794:
1789:
1785:
1781:
1777:
1773:
1766:
1763:
1758:
1754:
1750:
1746:
1742:
1738:
1731:
1728:
1723:
1719:
1715:
1711:
1707:
1703:
1696:
1693:
1688:
1684:
1680:
1676:
1672:
1668:
1660:
1657:
1652:
1648:
1644:
1640:
1633:
1630:
1625:
1621:
1617:
1613:
1609:
1605:
1598:
1595:
1590:
1588:9780471264187
1584:
1580:
1576:
1572:
1568:
1561:
1558:
1553:
1549:
1545:
1541:
1537:
1530:
1527:
1522:
1518:
1514:
1510:
1506:
1499:
1496:
1491:
1487:
1483:
1479:
1472:
1470:
1466:
1461:
1457:
1453:
1449:
1445:
1438:
1435:
1424:
1420:
1416:
1412:
1405:
1402:
1397:
1393:
1389:
1385:
1381:
1377:
1373:
1369:
1365:
1358:
1355:
1350:
1344:
1336:
1332:
1328:
1322:
1318:
1317:
1309:
1306:
1301:
1297:
1293:
1289:
1285:
1281:
1277:
1270:
1267:
1262:
1258:
1254:
1250:
1246:
1242:
1236:
1233:
1228:
1224:
1220:
1216:
1212:
1208:
1202:
1199:
1194:
1190:
1186:
1182:
1178:
1174:
1167:
1164:
1159:
1153:
1149:
1145:
1141:
1137:
1131:
1128:
1123:
1116:
1113:
1110:
1105:
1103:
1099:
1094:
1090:
1086:
1082:
1075:
1073:
1071:
1067:
1062:
1056:
1052:
1048:
1044:
1043:
1035:
1032:
1025:
1019:
1011:
1007:
1005:
1004:photo-induced
1001:
997:
992:
990:
986:
982:
978:
974:
969:
961:
955:
951:
949:
945:
941:
937:
927:
918:
913:
911:
909:
905:
901:
897:
893:
885:
880:
874:
870:
868:
864:
860:
856:
852:
848:
844:
840:
836:
831:
829:
825:
821:
817:
813:
797:
789:
781:
775:
771:
769:
765:
761:
753:
751:
749:
745:
740:
738:
734:
730:
729:acrylonitrile
726:
722:
718:
714:
710:
709:mesityl oxide
706:
702:
694:
692:
686:
682:
681:
680:
678:
674:
670:
662:
658:
657:
656:
654:
650:
646:
642:
638:
634:
630:
623:
619:
618:
617:
615:
613:
608:
604:
603:cyclohexanone
599:
597:
593:
589:
585:
581:
578:
574:
570:
567:
563:
555:
553:
551:
547:
543:
535:
531:
530:
529:
527:
526:cinnamic acid
523:
519:
515:
507:
503:
502:
501:
499:
495:
491:
487:
479:
477:
475:
471:
467:
463:
459:
455:
451:
447:
443:
439:
435:
430:
428:
424:
420:
416:
411:
409:
405:
401:
397:
393:
389:
385:
381:
378:
374:
370:
369:Deprotonation
361:
357:
356:
355:
353:
349:
341:
339:
337:
333:
327:
325:
321:
317:
313:
309:
305:
301:
293:
288:
284:
282:
278:
274:
270:
266:
262:
258:
254:
250:
246:
242:
238:
234:
230:
226:
223:
216:
215:
214:
212:
208:
204:
200:
192:
188:
186:
182:
178:
174:
170:
166:
162:
158:
157:Michael donor
154:
150:
146:
136:
132:
128:
125:
122:
121:
117:
113:
110:
109:
104:
97:
93:
90:
84:
73:
70:
64:
61:
60:Michael Donor
57:
53:
48:
45:
42:
39:
38:
33:
30:
19:
4624:Ene reaction
3984:Autoxidation
3845:Degradation
3736:Azo coupling
3513:Ugi reaction
3113:Ene reaction
2913:Alkynylation
2764:Polyfluorene
2759:Polar effect
2624:Electrophile
2539:Bredt's rule
2509:Baird's rule
2479:Alpha effect
2354:
2350:
2340:
2307:
2303:
2293:
2252:
2248:
2238:
2195:
2191:
2181:
2156:
2152:
2108:
2104:
2066:
2060:
2054:
2037:
2032:
2026:
2016:
2008:
1998:
1988:
1980:
1970:
1960:
1952:
1942:
1932:
1924:
1914:
1904:
1896:
1886:
1876:
1868:
1858:
1833:
1829:
1823:
1809:(30): 5192.
1806:
1802:
1796:
1782:(30): 5185.
1779:
1775:
1765:
1740:
1736:
1730:
1705:
1701:
1695:
1670:
1666:
1659:
1642:
1638:
1632:
1607:
1603:
1597:
1570:
1566:
1560:
1543:
1539:
1529:
1512:
1508:
1498:
1481:
1477:
1451:
1447:
1437:
1426:, retrieved
1414:
1404:
1371:
1367:
1357:
1315:
1308:
1283:
1279:
1269:
1252:
1248:
1235:
1218:
1214:
1201:
1176:
1172:
1166:
1147:
1130:
1115:
1084:
1080:
1041:
1034:
993:
965:
933:
896:Cancer drugs
889:
881:Applications
866:
862:
858:
854:
850:
846:
838:
832:
815:
811:
787:
785:
759:
757:
741:
733:nitropropane
731:and that of
698:
690:
666:
629:Syn addition
627:
611:
600:
559:
546:malonic acid
539:
518:acrylic acid
513:
511:
498:cyclopropane
493:
489:
483:
474:irreversible
466:electrophile
450:deprotonated
431:
412:
407:
403:
395:
391:
379:
372:
367:
351:
345:
335:
331:
328:
297:
260:
225:substituents
219:
196:
176:
171:(usually an
168:
156:
152:
148:
142:
131:RXNO:0000009
126:ontology ID
106:Identifiers
95:
86:
68:
59:
29:
3123:Ethenolysis
2769:Ring strain
2739:Nucleophile
2564:Clar's rule
2504:Aromaticity
2105:ChemCatChem
1368:Tetrahedron
1241:Michael, A.
1221:: 349–356.
1207:Michael, A.
904:active site
865:to product
350:, there is
275:(either an
201:method for
165:nucleophile
5686:Categories
5407:Ozonolysis
4934:Annulation
4284:Ozonolysis
2403:Topics in
1836:(2): 194.
1645:(6): 902.
1335:1200494733
1042:Org. React
1026:References
900:acrylamide
742:A classic
719:, that of
571:, such as
562:asymmetric
496:forming a
454:Lewis acid
294:Definition
263:. For the
211:asymmetric
4921:reactions
4436:reactions
3931:reactions
3847:reactions
3729:reactions
2871:reactions
2332:219762406
2324:2155-5435
2285:105834506
2277:0024-9297
2212:1474-1776
1573:: 1–898.
1428:7 October
1396:225589003
1388:0040-4020
1343:cite book
1300:0079-6700
1255:: 20–25.
1120:Hunt, I.
948:alkoxides
919:Mechanism
892:inhibitor
798:γ
711:(forming
635:. In the
580:alkaloids
550:aldehydes
470:catalytic
460:with 1,4-
432:Like the
377:carbanion
342:Mechanism
312:malonates
253:carbanion
247:hydrogen
245:methylene
163:or other
50:Reaction
2814:Vinylogy
2484:Annulene
2431:Reagents
2230:36008483
2173:18041800
2125:98643888
2083:17105247
1757:17249775
1722:17078687
1687:14579449
1624:16866504
1243:(1894).
1209:(1887).
1146:(2001).
975:), poly(
962:Examples
843:josiphos
713:Dimedone
695:Examples
669:warfarin
577:Cinchona
419:enolates
394:to form
324:enamines
316:alcohols
308:enolates
269:carbonyl
227:such as
181:β-carbon
167:) and a
2474:A value
2257:Bibcode
2221:9403961
1838:Bibcode
1181:Bibcode
985:quinone
973:sulfide
966:Linear
936:monomer
894:drugs.
758:In the
641:enamine
596:proline
592:iminium
588:enamine
480:History
444:in the
388:enolate
346:In the
304:enolate
241:sulfone
161:enolate
2330:
2322:
2283:
2275:
2228:
2218:
2210:
2171:
2123:
2081:
1755:
1720:
1685:
1622:
1585:
1394:
1386:
1333:
1323:
1298:
1154:
1057:
989:ketone
946:, and
944:thiols
940:amines
923:": -->
908:enzyme
906:of an
744:tandem
651:group
566:chiral
458:olefin
322:, and
320:amines
279:or an
265:alkene
249:acidic
147:, the
2328:S2CID
2281:S2CID
2121:S2CID
1392:S2CID
981:ether
977:ester
671:from
649:nitro
582:; or
548:with
492:with
398:in a
277:enone
239:, or
237:nitro
233:cyano
2320:ISSN
2273:ISSN
2226:PMID
2208:ISSN
2169:PMID
2079:PMID
1807:2009
1780:2009
1753:PMID
1718:PMID
1683:PMID
1620:PMID
1583:ISBN
1482:2010
1430:2022
1384:ISSN
1349:link
1331:OCLC
1321:ISBN
1296:ISSN
1152:ISBN
1055:ISBN
925:edit
826:and
735:and
723:and
675:and
605:and
438:enol
423:soft
415:HOMO
334:and
281:enal
257:base
229:acyl
205:and
159:(an
2359:doi
2355:140
2312:doi
2265:doi
2216:PMC
2200:doi
2161:doi
2113:doi
2071:doi
2067:128
2042:doi
1846:doi
1811:doi
1784:doi
1745:doi
1710:doi
1675:doi
1647:doi
1612:doi
1608:128
1575:doi
1548:doi
1544:218
1517:doi
1486:doi
1456:doi
1419:doi
1376:doi
1288:doi
1257:doi
1223:doi
1189:doi
1089:doi
1047:doi
991:).
847:R,S
590:or
371:of
151:or
143:In
124:RSC
81:O)
5688::
2353:.
2349:.
2326:.
2318:.
2308:10
2306:.
2302:.
2279:.
2271:.
2263:.
2253:51
2251:.
2247:.
2224:.
2214:.
2206:.
2196:21
2194:.
2190:.
2167:.
2157:47
2155:.
2151:.
2133:^
2119:.
2107:.
2103:.
2091:^
2077:.
2065:.
2038:16
2014:;
2007:.
1986:;
1979:.
1958:;
1951:.
1930:;
1923:.
1902:;
1895:.
1874:;
1867:.
1844:.
1834:87
1832:.
1805:.
1778:.
1774:.
1751:.
1739:.
1716:.
1704:.
1681:.
1671:42
1669:.
1643:66
1641:.
1618:.
1606:.
1581:.
1571:90
1569:.
1542:.
1513:35
1480:.
1468:^
1452:17
1450:.
1413:,
1390:.
1382:.
1372:76
1370:.
1366:.
1345:}}
1341:{{
1329:.
1294:.
1284:31
1282:.
1278:.
1253:49
1219:35
1187:.
1177:97
1175:.
1142:;
1101:^
1085:31
1083:.
1069:^
1053:.
942:,
869:.
816:𝛿
812:𝛿
788:𝛿
770::
750:.
739:.
633:ee
616::
598:.
440:,
410:.
318:,
261:B:
259:,
235:,
231:,
77:(H
2396:e
2389:t
2382:v
2365:.
2361::
2334:.
2314::
2287:.
2267::
2259::
2232:.
2202::
2175:.
2163::
2127:.
2115::
2109:4
2085:.
2073::
2048:.
2044::
2021:.
1993:.
1965:.
1937:.
1909:.
1881:.
1852:.
1848::
1840::
1817:.
1813::
1790:.
1786::
1759:.
1747::
1741:9
1724:.
1712::
1706:8
1689:.
1677::
1653:.
1649::
1626:.
1614::
1591:.
1577::
1554:.
1550::
1523:.
1519::
1492:.
1488::
1462:.
1458::
1421::
1398:.
1378::
1351:)
1337:.
1302:.
1290::
1263:.
1259::
1229:.
1225::
1195:.
1191::
1183::
1160:.
1095:.
1091::
1063:.
1049::
929:]
867:3
863:1
859:4
855:3
851:2
845:(
839:1
810:,
612:p
408:5
404:4
396:4
392:3
380:2
373:1
352:1
88:↓
79:3
75:+
66:+
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.