449:
1351:
682:
29:
588:
As an ACE inhibitor, moexipril causes a decrease in ACE. This blocks the conversion of angiotensin I to angiotensin II. Blockage of angiotensin II limits hypertension within the vasculature. Additionally, moexipril has been found to possess cardioprotective properties. Rats given moexipril one week
1376:
Klutchko S, Blankley CJ, Fleming RW, Hinkley JM, Werner AE, Nordin I, et al. (October 1986). "Synthesis of novel angiotensin converting enzyme inhibitor quinapril and related compounds. A divergence of structure-activity relationships for non-sulfhydryl and sulfhydryl types".
1346:, Hoefle ML, Klutchko S, "Substituted acyl derivatives of 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acids, salts thereof, pharmaceutical compositions containing the derivatives or salts, and the production of the same", published 1982-04-14, assigned to
1136:
Youn TJ, Kim HS, Oh BH (August 1999). "Ventricular remodeling and transforming growth factor-beta 1 mRNA expression after nontransmural myocardial infarction in rats: effects of angiotensin converting enzyme inhibition and angiotensin II type 1 receptor blockade".
666:. Lipophilic ACE inhibitors are able to penetrate membranes more readily, thus tissue ACE may be a target in addition to plasma ACE. A significant reduction in tissue ACE (lung, myocardium, aorta, and kidney) activity has been shown after moexipril use.
604:
and nitric oxide, which cause vasodilation and continue to exert antiproliferative effects. Inhibition of angiotensin II by moexipril decreases remodeling effects on the cardiovascular system. Indirectly, angiotensin II stimulates of the production of
579:
Moexipril is generally well tolerated in elderly patients with hypertension. Hypotension, dizziness, increased cough, diarrhea, flu syndrome, fatigue, and flushing have been found to affect less than 6% of patients who were prescribed moexipril.
1457:
1450:
1443:
2087:
1259:
Cawello W, Boekens H, Waitzinger J, Miller U (January 2002). "Moexipril shows a long duration of action related to an extended pharmacokinetic half-life and prolonged ACE inhibition".
796:
Belal F, Metwaly FH, Younes KM, Amer SM (2009). "Development of
Membrane Electrodes for the Specific Determination of Moexipril Hydrochloride in Dosage Forms and Biological Fluids".
1180:
Kalász H, Petroianu G, Tekes K, Klebovich I, Ludányi K, Gulyás Z (January 2007). "Metabolism of moexipril to moexiprilat: determination of in vitro metabolism using HPLC-ES-MS".
95:
1412:
Kaltenbronn JS, Dejohn D, Krolls U (2009). "Synthesis of – Ethyl Α––Benezenebutanoate, an
Important Intermediate in the Synthesis of Angiotensin Converting Enzyme Inhibitors".
513:
904:
White WB, Stimpel M (November 1995). "Long-term safety and efficacy of moexipril alone and in combination with hydrochlorothiazide in elderly patients with hypertension".
635:
moexipril hydrochloride, and is metabolized in the liver to form the pharmacologically active compound moexiprilat. Formation of moexiprilat is caused by hydrolysis of an
613:), all of which have tissue proliferative effects that are blocked by the actions of moexipril. The antiproliferative effects of moexipril have also been demonstrated by
2080:
51:
828:
Rodgers K, Vinson MC, Davis MW (1996). Breakthroughs: New drug approvals of 1995 -- part 1 (Report). Vol. 140. Advanstar
Communications, Inc. p. 84.
593:, displayed decreased infarct size. The cardioprotective effects of ACE inhibitors are mediated through a combination of angiotensin II inhibition and
2494:
1826:
2504:
2073:
1929:
1877:
534:
2031:
879:
771:
1735:
850:
1631:
2565:
1852:
1761:
1856:
1788:
238:
125:
567:
It was patented in 1980 and approved for medical use in 1995. Moexipril is available from
Schwarz Pharma under the trade name
1830:
1792:
1716:
1661:
1593:
2545:
2499:
1946:
1925:
1881:
1644:
670:
1470:
1017:
Hartman JC (September 1995). "The role of bradykinin and nitric oxide in the cardioprotective action of ACE inhibitors".
1809:
368:
1627:
1623:
2530:
2048:
1435:
428:
2555:
2535:
1054:"Stimulation of endothelial cell prostaglandin production by angiotensin peptides. Characterization of receptors"
1296:"Chemical informatics uncovers a new role for moexipril as a novel inhibitor of cAMP phosphodiesterase-4 (PDE4)"
542:
317:
1885:
1532:
2525:
2036:
1757:
1615:
1536:
83:
1784:
1553:
1848:
1692:
1619:
1611:
1516:
977:
Chrysant SG (February 1998). "Vascular remodeling: the role of angiotensin-converting enzyme inhibitors".
718:
184:
1869:
1805:
1669:
1665:
1657:
1571:
2560:
2540:
2489:
1822:
1589:
1549:
1466:
590:
308:
417:
2484:
2107:
2096:
2000:
1921:
1893:
1873:
706:
207:
1343:
1889:
444:
263:
2243:
2233:
2218:
2208:
2099:
1974:
1162:
1118:
709:
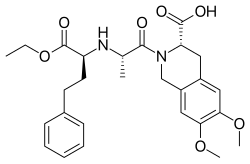
from the adjacent chiral center. Reaction of the product with hydrogen chloride then cleaves the
2446:
2431:
2416:
2268:
2238:
2228:
2173:
2426:
2421:
2168:
1964:
61:
2550:
2451:
2223:
2183:
1394:
1325:
1276:
1238:
1197:
1154:
1110:
1075:
1034:
994:
954:
913:
875:
867:
846:
840:
767:
763:
730:
546:
153:
140:
41:
1421:
1386:
1315:
1307:
1268:
1228:
1189:
1146:
1102:
1065:
1026:
986:
944:
805:
598:
465:
272:
166:
377:
357:
2461:
2128:
1904:
640:
194:
174:
448:
1294:
Cameron RT, Coleman RG, Day JP, Yalla KC, Houslay MD, Adams DR, et al. (May 2013).
689:
The synthesis of the all-important dipeptide-like side chain involves alkylation of the
2133:
2123:
2042:
1363:
1347:
1320:
1295:
1233:
1216:
561:
949:
932:
623:
in rats. Other ACE inhibitors have also been found to produce these actions, as well.
2519:
2466:
1699:
1523:
1494:
1483:
1106:
1030:
756:
557:
75:
1166:
1122:
619:
studies where moexipril inhibits the estrogen-stimulated growth of neonatal cardiac
2401:
2391:
2371:
2321:
1959:
1685:
1474:
1070:
1053:
681:
538:
108:
103:
2065:
297:
2381:
2356:
2346:
2341:
2311:
2258:
2163:
2153:
1969:
1841:
1750:
1604:
636:
620:
69:
1193:
990:
2396:
2386:
2366:
2331:
2316:
2296:
2286:
2253:
2248:
2213:
2198:
2193:
2188:
2178:
2158:
2017:
1993:
1984:
1950:
1836:
1815:
1777:
1772:
1767:
1745:
1709:
1704:
1680:
1582:
1559:
1542:
1509:
1504:
1425:
1358:
1311:
1093:
Phillips PA (July 1999). "Interaction between endothelin and angiotensin II".
659:
651:
606:
594:
498:
348:
647:
and persistent ACE inhibition of moexipril allows once-daily administration.
639:
group. Moexipril is incompletely absorbed after oral administration, and its
597:
proliferation. Increased levels of bradykinin stimulate in the production of
2441:
2436:
2411:
2376:
2351:
2326:
2306:
2291:
2281:
2263:
2143:
2138:
1935:
1914:
1862:
1725:
1675:
1637:
1577:
1528:
1499:
655:
644:
550:
220:
55:
1329:
1280:
1242:
1201:
1158:
1114:
610:
1398:
1150:
1079:
1038:
998:
958:
917:
809:
20:
2361:
2301:
2203:
1798:
1650:
1564:
663:
615:
328:
1390:
1052:
Jaiswal N, Diz DI, Chappell MC, Khosla MC, Ferrario CM (February 1992).
337:
632:
283:
199:
28:
1272:
408:
397:
680:
388:
134:
2069:
1439:
1261:
International
Journal of Clinical Pharmacology and Therapeutics
147:
609:
1 and 3 (ET1, ET3) and the transforming growth factor beta-1 (
705:); the presominane of the desired isomer is attributable to
433:
1095:
933:"The renin-angiotensin system and vascular hypertrophy"
520:
2276:
545:. Moexipril can be administered alone or with other
2475:
2106:
2010:
1983:
1944:
1903:
1734:
1482:
729:-butyl ester in this product is again cleaved with
717:). Coupling of that acid to the secondary amine on
497:
464:
459:
427:
407:
387:
367:
347:
327:
316:
307:
282:
262:
229:
219:
206:
193:
183:
173:
165:
124:
119:
94:
82:
68:
50:
40:
35:
874:. Lippincott Williams & Wilkins. p. 647.
755:
1414:Organic Preparations and Procedures International
296:
250:)-2-amino]propanoyl]-6,7-dimethoxy-3,4-dihydro-1
16:Antihypertensive drug of the ACE inhibitor class
271:
2081:
1451:
937:Journal of the American College of Cardiology
791:
789:
8:
2108:
1217:"Moexipril and left ventricular hypertrophy"
19:
1254:
1252:
158:In general: ℞ (Prescription only)
2088:
2074:
2066:
1458:
1444:
1436:
868:"Angiotensin-Converting Enzyme Inhibitors"
654:, and is in the same hydrophobic range as
537:(ACE inhibitor) used for the treatment of
447:
356:
2495:Olmesartan/amlodipine/hydrochlorothiazide
1319:
1232:
1069:
1012:
1010:
1008:
972:
970:
968:
948:
823:
821:
819:
556:It works by inhibiting the conversion of
376:
2505:Valsartan/hydrochlorothiazide/amlodipine
899:
897:
701:) with ethyl 2-bromo-4-phenylbutanoate (
1632:+bisoprolol, amlodipine, and indapamide
762:. Sterling Publishing Company. p.
746:
535:angiotensin converting enzyme inhibitor
443:
336:
243:
74:
845:. John Wiley & Sons. p. 468.
18:
725:) gives the corresponding amine. The
416:
215:1 hour; 2-9 hours (active metabolite)
60:
7:
713:-butyl group to give the half acid (
107:
1221:Vascular Health and Risk Management
396:
287:
14:
643:is low. The long pharmacokinetic
1107:10.1046/j.1440-1681.1999.03069.x
754:Hochadel, Maryanne, ed. (2006).
482:
476:
202:(active metabolite, moexiprilat)
27:
1215:Chrysant GS, Nguyen PK (2007).
839:Fischer J, Ganellin CR (2006).
798:Portugaliae Electrochimica Acta
254:-isoquinoline-3-carboxylic acid
1379:Journal of Medicinal Chemistry
1071:10.1161/01.hyp.19.2_suppl.ii49
1019:The Annals of Thoracic Surgery
488:
470:
1:
2500:Valsartan/hydrochlorothiazide
1356:; M. L. Hoefle, S. Klutchko,
950:10.1016/s0735-1097(96)00251-3
931:Rosendorff C (October 1996).
906:Journal of Human Hypertension
842:Analogue-based Drug Discovery
2113:Tooltip Angiotensin receptor
1139:Basic Research in Cardiology
1031:10.1016/0003-4975(95)00192-N
631:Moexipril is available as a
2582:
1628:+bisoprolol and amlodipine
1624:+amlodipine and indapamide
1194:10.2174/157340607779317490
991:10.1053/hj.1998.v135.86971
460:Chemical and physical data
2026:
1426:10.1080/00304948309355428
1312:10.1016/j.bcp.2013.02.026
510:
234:
225:50% (faeces), 13% (urine)
26:
1524:Dicarboxylate-containing
1471:renin–angiotensin system
1300:Biochemical Pharmacology
543:congestive heart failure
2566:Tetrahydroisoquinolines
758:The AARP Guide to Pills
1700:Phosphonate-containing
1467:Antihypertensive drugs
1064:(2 Suppl): II49–II55.
979:American Heart Journal
719:tetrahydroisoquinoline
686:
589:prior to induction of
2490:Olmesartan/amlodipine
1495:Sulfhydryl-containing
1359:U.S. patent 4,344,949
1151:10.1007/s003950050149
810:10.4152/pea.200904463
733:to afford moexipril (
684:
591:myocardial infarction
2546:Norsalsolinol ethers
2485:Amlodipine/valsartan
2097:Angiotensin receptor
870:. In Dart RC (ed.).
707:asymmetric induction
685:Moexipril synthesis:
650:Moexipril is highly
1930:+amlodipine and HCT
1878:+amlodipine and HCT
1853:+amlodipine and HCT
1827:+amlodipine and HCT
1789:+amlodipine and HCT
1762:+amlodipine and HCT
1662:+amlodipine and HCT
1391:10.1021/jm00160a026
1182:Medicinal Chemistry
985:(2 Pt 2): S21–S30.
584:Mechanism of action
143:(Prescription only)
23:
2531:Carboxylate esters
2053:Never to phase III
1348:Warner Lambert Co.
872:Medical toxicology
866:Banerji S (2004).
687:
669:It has additional
2556:Methoxy compounds
2536:Enantiopure drugs
2513:
2512:
2409:Renin inhibitors:
2063:
2062:
1385:(10): 1953–1961.
881:978-0-7817-2845-4
773:978-1-4027-1740-6
731:hydrogen chloride
696:
547:antihypertensives
528:
527:
429:CompTox Dashboard
151:
138:
2573:
2278:
2114:
2110:
2090:
2083:
2076:
2067:
1998:
1919:
1905:Renin inhibitors
1867:
1846:
1820:
1803:
1782:
1755:
1723:Other/ungrouped:
1714:
1690:
1655:
1642:
1609:
1587:
1569:
1547:
1514:
1460:
1453:
1446:
1437:
1430:
1429:
1409:
1403:
1402:
1373:
1367:
1361:
1355:
1354:
1350:
1340:
1334:
1333:
1323:
1306:(9): 1297–1305.
1291:
1285:
1284:
1273:10.5414/cpp40009
1256:
1247:
1246:
1236:
1212:
1206:
1205:
1177:
1171:
1170:
1133:
1127:
1126:
1090:
1084:
1083:
1073:
1049:
1043:
1042:
1014:
1003:
1002:
974:
963:
962:
952:
928:
922:
921:
901:
892:
891:
889:
888:
863:
857:
856:
836:
830:
829:
825:
814:
813:
793:
784:
783:
781:
780:
761:
751:
694:
693:-butyl ester of
524:
523:
516:
505:
490:
484:
478:
472:
452:
451:
437:
435:
420:
400:
380:
360:
340:
320:
300:
290:
289:
275:
211:
149:
146:
136:
133:
111:
78:
64:
31:
24:
22:
2581:
2580:
2576:
2575:
2574:
2572:
2571:
2570:
2516:
2515:
2514:
2509:
2471:
2462:Angiotensinogen
2129:Angiotensin III
2112:
2102:
2094:
2064:
2059:
2058:
2043:Clinical trials
2022:
2006:
1996:
1979:
1940:
1917:
1907:
1899:
1865:
1844:
1818:
1801:
1780:
1753:
1738:
1730:
1712:
1688:
1653:
1640:
1607:
1585:
1567:
1545:
1512:
1486:
1478:
1464:
1434:
1433:
1411:
1410:
1406:
1375:
1374:
1370:
1357:
1352:
1342:
1341:
1337:
1293:
1292:
1288:
1258:
1257:
1250:
1214:
1213:
1209:
1179:
1178:
1174:
1135:
1134:
1130:
1092:
1091:
1087:
1051:
1050:
1046:
1016:
1015:
1006:
976:
975:
966:
930:
929:
925:
912:(11): 879–884.
903:
902:
895:
886:
884:
882:
865:
864:
860:
853:
838:
837:
833:
827:
826:
817:
795:
794:
787:
778:
776:
774:
753:
752:
748:
743:
679:
671:PDE4-inhibiting
641:bioavailability
629:
602:
599:prostaglandin E
586:
577:
519:
517:
514:(what is this?)
511:
503:
493:
487:
481:
475:
455:
431:
423:
403:
383:
363:
343:
323:
303:
286:
278:
258:
255:
242:
241:
209:
185:Protein binding
175:Bioavailability
167:Pharmacokinetic
161:
115:
85:
17:
12:
11:
5:
2579:
2577:
2569:
2568:
2563:
2558:
2553:
2548:
2543:
2538:
2533:
2528:
2526:ACE inhibitors
2518:
2517:
2511:
2510:
2508:
2507:
2502:
2497:
2492:
2487:
2481:
2479:
2473:
2472:
2470:
2469:
2464:
2455:
2454:
2449:
2444:
2439:
2434:
2429:
2424:
2419:
2414:
2405:
2404:
2399:
2394:
2389:
2384:
2379:
2374:
2369:
2364:
2359:
2354:
2349:
2344:
2339:
2334:
2329:
2324:
2319:
2314:
2309:
2304:
2299:
2294:
2289:
2284:
2272:
2271:
2266:
2261:
2256:
2251:
2246:
2241:
2236:
2231:
2226:
2221:
2216:
2211:
2206:
2201:
2196:
2191:
2186:
2181:
2176:
2171:
2166:
2161:
2156:
2147:
2146:
2141:
2136:
2134:Angiotensin IV
2131:
2126:
2124:Angiotensin II
2117:
2115:
2104:
2103:
2095:
2093:
2092:
2085:
2078:
2070:
2061:
2060:
2057:
2056:
2055:
2054:
2051:
2040:
2034:
2028:
2027:
2024:
2023:
2021:
2020:
2014:
2012:
2008:
2007:
2005:
2004:
1990:
1988:
1981:
1980:
1978:
1977:
1972:
1967:
1962:
1956:
1954:
1942:
1941:
1939:
1938:
1933:
1911:
1909:
1901:
1900:
1898:
1897:
1886:+lercanidipine
1860:
1839:
1834:
1813:
1796:
1775:
1770:
1765:
1748:
1742:
1740:
1732:
1731:
1729:
1728:
1720:
1707:
1696:
1683:
1678:
1673:
1648:
1635:
1602:
1597:
1580:
1575:
1562:
1557:
1540:
1533:+lercanidipine
1520:
1507:
1502:
1490:
1488:
1484:ACE inhibitors
1480:
1479:
1469:acting on the
1465:
1463:
1462:
1455:
1448:
1440:
1432:
1431:
1420:(1–2): 35–40.
1404:
1368:
1364:Warner-Lambert
1335:
1286:
1248:
1207:
1188:(1): 101–106.
1172:
1145:(4): 246–253.
1128:
1085:
1044:
1004:
964:
943:(4): 803–812.
923:
893:
880:
858:
851:
831:
815:
804:(4): 463–475.
785:
772:
745:
744:
742:
739:
678:
675:
628:
625:
600:
585:
582:
576:
573:
562:angiotensin II
526:
525:
508:
507:
501:
495:
494:
491:
485:
479:
473:
468:
462:
461:
457:
456:
454:
453:
440:
438:
425:
424:
422:
421:
413:
411:
405:
404:
402:
401:
393:
391:
385:
384:
382:
381:
373:
371:
365:
364:
362:
361:
353:
351:
345:
344:
342:
341:
333:
331:
325:
324:
322:
321:
313:
311:
305:
304:
302:
301:
293:
291:
280:
279:
277:
276:
268:
266:
260:
259:
257:
256:
245:
237:
236:
235:
232:
231:
227:
226:
223:
217:
216:
213:
204:
203:
197:
191:
190:
187:
181:
180:
177:
171:
170:
163:
162:
160:
159:
156:
144:
130:
128:
122:
121:
117:
116:
114:
113:
100:
98:
92:
91:
88:
86:administration
80:
79:
72:
66:
65:
58:
48:
47:
44:
38:
37:
33:
32:
15:
13:
10:
9:
6:
4:
3:
2:
2578:
2567:
2564:
2562:
2559:
2557:
2554:
2552:
2549:
2547:
2544:
2542:
2539:
2537:
2534:
2532:
2529:
2527:
2524:
2523:
2521:
2506:
2503:
2501:
2498:
2496:
2493:
2491:
2488:
2486:
2483:
2482:
2480:
2478:
2477:Combinations:
2474:
2468:
2467:Angiotensin I
2465:
2463:
2460:
2457:
2456:
2453:
2450:
2448:
2445:
2443:
2440:
2438:
2435:
2433:
2430:
2428:
2425:
2423:
2420:
2418:
2415:
2413:
2410:
2407:
2406:
2403:
2400:
2398:
2395:
2393:
2390:
2388:
2385:
2383:
2380:
2378:
2375:
2373:
2370:
2368:
2365:
2363:
2360:
2358:
2355:
2353:
2350:
2348:
2345:
2343:
2340:
2338:
2335:
2333:
2330:
2328:
2325:
2323:
2320:
2318:
2315:
2313:
2310:
2308:
2305:
2303:
2300:
2298:
2295:
2293:
2290:
2288:
2285:
2283:
2280:
2274:
2273:
2270:
2267:
2265:
2262:
2260:
2257:
2255:
2252:
2250:
2247:
2245:
2242:
2240:
2237:
2235:
2232:
2230:
2227:
2225:
2222:
2220:
2217:
2215:
2212:
2210:
2207:
2205:
2202:
2200:
2197:
2195:
2192:
2190:
2187:
2185:
2182:
2180:
2177:
2175:
2172:
2170:
2167:
2165:
2162:
2160:
2157:
2155:
2152:
2149:
2148:
2145:
2142:
2140:
2137:
2135:
2132:
2130:
2127:
2125:
2122:
2119:
2118:
2116:
2111:
2105:
2101:
2098:
2091:
2086:
2084:
2079:
2077:
2072:
2071:
2068:
2052:
2050:
2047:
2046:
2044:
2041:
2038:
2035:
2033:
2030:
2029:
2025:
2019:
2016:
2015:
2013:
2009:
2002:
1995:
1992:
1991:
1989:
1986:
1982:
1976:
1973:
1971:
1968:
1966:
1963:
1961:
1958:
1957:
1955:
1952:
1948:
1943:
1937:
1934:
1931:
1927:
1923:
1916:
1913:
1912:
1910:
1906:
1902:
1895:
1891:
1887:
1883:
1879:
1875:
1871:
1864:
1861:
1858:
1854:
1850:
1843:
1840:
1838:
1835:
1832:
1828:
1824:
1817:
1814:
1811:
1807:
1800:
1797:
1794:
1790:
1786:
1779:
1776:
1774:
1771:
1769:
1766:
1763:
1759:
1752:
1749:
1747:
1744:
1743:
1741:
1737:
1733:
1727:
1724:
1721:
1718:
1711:
1708:
1706:
1702:
1701:
1697:
1694:
1687:
1684:
1682:
1679:
1677:
1674:
1671:
1667:
1663:
1659:
1652:
1649:
1646:
1639:
1636:
1633:
1629:
1625:
1621:
1617:
1613:
1606:
1603:
1601:
1598:
1595:
1591:
1584:
1581:
1579:
1576:
1573:
1566:
1563:
1561:
1558:
1555:
1551:
1544:
1541:
1538:
1537:+nitrendipine
1534:
1530:
1527:
1525:
1521:
1518:
1511:
1508:
1506:
1503:
1501:
1498:
1496:
1492:
1491:
1489:
1485:
1481:
1476:
1472:
1468:
1461:
1456:
1454:
1449:
1447:
1442:
1441:
1438:
1427:
1423:
1419:
1415:
1408:
1405:
1400:
1396:
1392:
1388:
1384:
1380:
1372:
1369:
1365:
1360:
1349:
1345:
1339:
1336:
1331:
1327:
1322:
1317:
1313:
1309:
1305:
1301:
1297:
1290:
1287:
1282:
1278:
1274:
1270:
1266:
1262:
1255:
1253:
1249:
1244:
1240:
1235:
1230:
1226:
1222:
1218:
1211:
1208:
1203:
1199:
1195:
1191:
1187:
1183:
1176:
1173:
1168:
1164:
1160:
1156:
1152:
1148:
1144:
1140:
1132:
1129:
1124:
1120:
1116:
1112:
1108:
1104:
1100:
1096:
1089:
1086:
1081:
1077:
1072:
1067:
1063:
1059:
1055:
1048:
1045:
1040:
1036:
1032:
1028:
1025:(3): 789–92.
1024:
1020:
1013:
1011:
1009:
1005:
1000:
996:
992:
988:
984:
980:
973:
971:
969:
965:
960:
956:
951:
946:
942:
938:
934:
927:
924:
919:
915:
911:
907:
900:
898:
894:
883:
877:
873:
869:
862:
859:
854:
852:9783527607495
848:
844:
843:
835:
832:
824:
822:
820:
816:
811:
807:
803:
799:
792:
790:
786:
775:
769:
765:
760:
759:
750:
747:
740:
738:
736:
732:
728:
724:
720:
716:
712:
708:
704:
700:
692:
683:
676:
674:
672:
667:
665:
661:
657:
653:
648:
646:
642:
638:
634:
626:
624:
622:
618:
617:
612:
608:
603:
596:
592:
583:
581:
574:
572:
570:
565:
563:
559:
558:angiotensin I
554:
552:
548:
544:
540:
536:
532:
522:
515:
509:
502:
500:
496:
469:
467:
463:
458:
450:
446:
445:DTXSID9023330
442:
441:
439:
430:
426:
419:
415:
414:
412:
410:
406:
399:
395:
394:
392:
390:
386:
379:
375:
374:
372:
370:
366:
359:
355:
354:
352:
350:
346:
339:
335:
334:
332:
330:
326:
319:
315:
314:
312:
310:
306:
299:
295:
294:
292:
285:
281:
274:
270:
269:
267:
265:
261:
253:
249:
244:
240:
233:
228:
224:
222:
218:
214:
212:
205:
201:
198:
196:
192:
188:
186:
182:
178:
176:
172:
168:
164:
157:
155:
145:
142:
132:
131:
129:
127:
123:
118:
110:
105:
102:
101:
99:
97:
93:
89:
87:
81:
77:
73:
71:
67:
63:
59:
57:
53:
49:
45:
43:
39:
36:Clinical data
34:
30:
25:
2561:Carboxamides
2541:Ethyl esters
2476:
2459:Propeptides:
2458:
2408:
2402:Zofenoprilat
2392:Trandolapril
2372:Rescinnamine
2336:
2322:Gemopatrilat
2275:
2151:Antagonists:
2150:
2120:
1960:Gemopatrilat
1722:
1698:
1686:Trandolapril
1599:
1522:
1493:
1417:
1413:
1407:
1382:
1378:
1371:
1338:
1303:
1299:
1289:
1264:
1260:
1227:(1): 23–30.
1224:
1220:
1210:
1185:
1181:
1175:
1142:
1138:
1131:
1101:(7): 517–8.
1098:
1094:
1088:
1061:
1058:Hypertension
1057:
1047:
1022:
1018:
982:
978:
940:
936:
926:
909:
905:
885:. Retrieved
871:
861:
841:
834:
801:
797:
777:. Retrieved
757:
749:
734:
726:
722:
714:
710:
702:
698:
690:
688:
668:
649:
630:
627:Pharmacology
614:
587:
578:
575:Side effects
568:
566:
555:
539:hypertension
530:
529:
518:
512:
251:
247:
208:Elimination
126:Legal status
120:Legal status
2382:Spiraprilat
2357:Quinaprilat
2347:Perindopril
2342:Omapatrilat
2312:Enalaprilat
2279:inhibitors:
2259:Telmisartan
2244:Saprisartan
2234:Pratosartan
2219:Olodanrigan
2209:Milfasartan
2164:Candesartan
2154:Abitesartan
2039:from market
1975:Sampatrilat
1970:Omapatrilat
1922:+amlodipine
1894:+sacubitril
1874:+amlodipine
1849:+amlodipine
1842:Telmisartan
1823:+amlodipine
1806:+amlodipine
1785:+amlodipine
1758:+amlodipine
1751:Candesartan
1739:("-sartan")
1670:+felodipine
1666:+bisoprolol
1658:+amlodipine
1620:+indapamide
1616:+bisoprolol
1612:+amlodipine
1605:Perindopril
1590:+amlodipine
1572:+manidipine
1554:+pimobendan
1550:+amlodipine
1267:(1): 9–17.
637:ethyl ester
621:fibroblasts
506: g·mol
273:103775-10-6
230:Identifiers
70:MedlinePlus
42:Trade names
2520:Categories
2447:Terlakiren
2432:Imarikiren
2417:Ciprokiren
2397:Zofenopril
2387:Temocapril
2367:Rentiapril
2332:Lisinopril
2317:Fosinopril
2297:Cilazapril
2287:Benazepril
2269:Zolasartan
2254:Tasosartan
2249:Sparsentan
2239:Ripisartan
2229:Pomisartan
2214:Olmesartan
2199:Irbesartan
2194:Forasartan
2189:Fimasartan
2179:Eprosartan
2174:Embusartan
2159:Azilsartan
2100:modulators
2018:Sparsentan
2001:+valsartan
1994:Sacubitril
1987:inhibitors
1985:Neprilysin
1953:inhibitors
1908:("-kiren")
1890:+nebivolol
1870:+aliskiren
1837:Tasosartan
1816:Olmesartan
1778:Irbesartan
1773:Fimasartan
1768:Eprosartan
1746:Azilsartan
1710:Fosinopril
1705:Ceronapril
1693:+verapamil
1681:Temocapril
1583:Lisinopril
1560:Cilazapril
1543:Benazepril
1517:+nebivolol
1510:Zofenopril
1505:Rentiapril
887:2009-10-09
779:2009-10-09
741:References
697:-alanine (
660:benazepril
652:lipophilic
607:endothelin
595:bradykinin
499:Molar mass
418:ChEMBL1165
378:WT87C52TJZ
349:ChemSpider
309:IUPHAR/BPS
264:CAS Number
239:IUPAC name
195:Metabolism
2442:Remikiren
2437:Pepstatin
2427:Enalkiren
2422:Ditekiren
2412:Aliskiren
2377:Spirapril
2352:Quinapril
2337:Moexipril
2327:Imidapril
2307:Enalapril
2292:Captopril
2282:Alacepril
2264:Valsartan
2169:Elisartan
2144:Saralasin
2139:L-163,491
2121:Agonists:
2049:Phase III
2037:Withdrawn
1965:Ilepatril
1936:Remikiren
1915:Aliskiren
1863:Valsartan
1726:Alacepril
1676:Spirapril
1638:Quinapril
1600:Moexipril
1578:Imidapril
1529:Enalapril
1500:Captopril
1487:("-pril")
1362:(1982 to
677:Synthesis
673:effects.
656:quinapril
645:half-life
551:diuretics
531:Moexipril
221:Excretion
210:half-life
84:Routes of
62:Monograph
56:Drugs.com
21:Moexipril
2551:Prodrugs
2452:Zankiren
2362:Ramipril
2302:Delapril
2224:PD123319
2204:Losartan
2184:EXP-3174
1799:Losartan
1651:Ramipril
1565:Delapril
1344:EP 49605
1330:23473803
1281:11837383
1243:17583172
1202:17266629
1167:24853463
1159:10505424
1123:27296727
1115:10405777
664:ramipril
616:in vitro
521:(verify)
329:DrugBank
96:ATC code
1399:3020249
1321:3625111
1234:1994034
1080:1735595
1039:7545893
999:9488609
959:8837552
918:8583466
633:prodrug
569:Univasc
533:was an
504:498.576
466:Formula
338:DB00691
284:PubChem
200:Hepatic
112:)
106: (
104:C09AA13
76:a695018
46:Univasc
2032:WHO-EM
1997:
1918:
1866:
1845:
1819:
1802:
1781:
1754:
1736:AIIRAs
1713:
1689:
1654:
1641:
1608:
1586:
1568:
1546:
1513:
1397:
1353:
1328:
1318:
1279:
1241:
1231:
1200:
1165:
1157:
1121:
1113:
1078:
1037:
997:
957:
916:
878:
849:
770:
662:, and
611:TGF-β1
409:ChEMBL
398:D08225
179:13-22%
154:℞-only
152:
139:
2011:Other
1945:Dual
1163:S2CID
1119:S2CID
358:82418
298:91270
1926:+HCT
1882:+HCT
1857:+HCT
1831:+HCT
1810:+HCT
1793:+HCT
1717:+HCT
1645:+HCT
1594:+HCT
1395:PMID
1326:PMID
1277:PMID
1239:PMID
1198:PMID
1155:PMID
1111:PMID
1076:PMID
1035:PMID
995:PMID
955:PMID
914:PMID
876:ISBN
847:ISBN
768:ISBN
727:tert
711:tert
691:tert
541:and
389:KEGG
369:UNII
318:6571
169:data
90:Oral
52:AHFS
2277:ACE
2109:ATR
1951:NEP
1947:ACE
1475:C09
1422:doi
1387:doi
1316:PMC
1308:doi
1269:doi
1229:PMC
1190:doi
1147:doi
1103:doi
1066:doi
1027:doi
987:doi
983:135
945:doi
806:doi
764:640
737:).
564:.
560:to
553:.
549:or
434:EPA
288:CID
189:90%
141:POM
109:WHO
2522::
2045::
1928:,
1924:,
1892:,
1888:,
1884:,
1880:,
1876:,
1872:,
1855:,
1851:,
1829:,
1825:,
1808:,
1791:,
1787:,
1760:,
1703::
1668:,
1664:,
1660:,
1630:,
1626:,
1622:,
1618:,
1614:,
1592:,
1552:,
1535:,
1526::
1418:15
1416:.
1393:.
1383:29
1381:.
1366:).
1324:.
1314:.
1304:85
1302:.
1298:.
1275:.
1265:40
1263:.
1251:^
1237:.
1223:.
1219:.
1196:.
1184:.
1161:.
1153:.
1143:94
1141:.
1117:.
1109:.
1099:26
1097:.
1074:.
1062:19
1060:.
1056:.
1033:.
1023:60
1021:.
1007:^
993:.
981:.
967:^
953:.
941:28
939:.
935:.
908:.
896:^
818:^
802:27
800:.
788:^
766:.
658:,
571:.
480:34
474:27
246:(3
148:US
135:UK
2089:e
2082:t
2075:v
2003:)
1999:(
1949:/
1932:)
1920:(
1896:)
1868:(
1859:)
1847:(
1833:)
1821:(
1812:)
1804:(
1795:)
1783:(
1764:)
1756:(
1719:)
1715:(
1695:)
1691:(
1672:)
1656:(
1647:)
1643:(
1634:)
1610:(
1596:)
1588:(
1574:)
1570:(
1556:)
1548:(
1539:)
1531:(
1519:)
1515:(
1497::
1477:)
1473:(
1459:e
1452:t
1445:v
1428:.
1424::
1401:.
1389::
1332:.
1310::
1283:.
1271::
1245:.
1225:3
1204:.
1192::
1186:3
1169:.
1149::
1125:.
1105::
1082:.
1068::
1041:.
1029::
1001:.
989::
961:.
947::
920:.
910:9
890:.
855:.
812:.
808::
782:.
735:5
723:4
721:(
715:3
703:1
699:2
695:L
601:2
492:7
489:O
486:2
483:N
477:H
471:C
436:)
432:(
252:H
248:S
150::
137::
54:/
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.