1143:
1286:
377:
236:
792:
605:
600:
49:
65:
788:
1124:
39:
1151:
1382:, weakness in the arms and legs, and in rare cases may be fatal. The oil is readily absorbed through the skin and may increase heart rate, cause convulsions or rarely death. Ingestion may similarly cause headaches, dizziness, nausea, vomiting and gastrointestinal irritation, loss of sensation/use in limbs and also causes internal bleeding.
996:
1118:
793:
791:
613:
580:
809:
1453:
Ahluwalia, R.; Wanchoo, R. K.; Sharma, S. K.; Vashisht, J. L. (1996). "Density, viscosity, and surface tension of binary liquid systems: Ethanoic acid, propanoic acid, and butanoic acid with nitrobenzene".
1109:. This mixture is sometimes called "mixed acid." The production of nitrobenzene is one of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (Δ
1142:
1609:
Bhattacharya A, Purohit VC, Suarez V, Tichkule R, Parmer G, Rinaldi F (March 2006). "One-step reductive amidation of nitro arenes: application in the synthesis of
Acetaminophen".
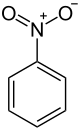
1508:
1439:
911:
1401:
1009:
1848:
794:
426:
1386:
1906:
1179:
reaction of it with benzene. The nitronium ion is generated by the reaction of nitric acid and an acidic dehydration agent, typically sulfuric acid:
1312:. It has been replaced by less toxic chemicals for this purpose. A significant merchant market for nitrobenzene is its use in the production of the
719:
1378:, impair vision, cause liver or kidney damage, anemia and lung irritation. Inhalation of vapors may induce headache, nausea, fatigue, dizziness,
1899:
1397:
1544:
1404:(42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
1988:
1176:
1983:
1393:
which is "possibly carcinogenic to humans". It has been shown to cause liver, kidney, and thyroid adenomas and carcinomas in rats.
1390:
1385:
Nitrobenzene is considered a likely human carcinogen by the United States
Environmental Protection Agency, and is classified by the
816:
391:
1736:
Karwa, Shrikant L.; Rajadhyaksha, Rajeev A. (January 1988). "Selective catalytic hydrogenation of nitrobenzene to hydrazobenzene".
1823:
141:
703:
675:
711:
665:
334:
604:
1900:"40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities"
355:
759:
802:
1993:
1016:
935:
922:
1285:
1998:
1308:, and other materials. Redistilled, as oil of mirbane, nitrobenzene had been used as an inexpensive perfume for
974:
243:
839:
653:
1862:
1978:
731:
553:
723:
231:
1973:
1886:
1368:
627:
599:
592:
193:
48:
751:
1910:
77:
1352:
1304:
Nitrobenzene is used to mask unpleasant odors in shoe and floor polishes, leather dressings, paint
691:
372:
107:
1479:
1290:
683:
657:
153:
1066:-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from
735:
679:
1948:
695:
1842:
1777:
1753:
1714:
1683:
1591:
1540:
1471:
1032:
707:
38:
1745:
1659:
1618:
1581:
1573:
1532:
1463:
1043:
531:
449:
739:
343:
1356:
852:
743:
715:
262:
213:
1938:
727:
755:
649:
376:
235:
173:
117:
1863:"Agents Classified by the IARC Monographs, International Agency for Research on Cancer"
1586:
1561:
1375:
1348:
1344:
1277:
1056:
1035:
987:
978:
671:
542:
64:
1967:
1098:
1079:
1039:
520:
510:
224:
1483:
767:
1336:
1327:. Evidence suggests its use in agriculture as a plant growth/flowering stimulant.
1253:
1205:
687:
1123:
1622:
1335:
Aside from its conversion to aniline, nitrobenzene can be selectively reduced to
699:
307:
1800:
1772:
1709:
1678:
1324:
1316:
1106:
899:
876:
828:
17:
1958:
1943:
1885:
National
Institutes of Health · U.S. Department of Health and Human Services,
1636:
1435:
1340:
1273:
472:
204:
1757:
1663:
1536:
1475:
1500:
1320:
1313:
1261:
1168:
1164:
1094:
565:
1953:
1595:
1577:
645:
637:
1379:
815:
808:
801:
774:
1749:
1150:
1467:
1305:
1269:
1209:
1082:
1075:
1071:
1067:
970:
500:
294:
244:
1257:
1157:
1063:
1046:
184:
986:
Except where otherwise noted, data are given for materials in their
282:
1284:
1117:
1102:
633:
318:
164:
140:
130:
1309:
948:
763:
641:
490:
273:
1265:
1505:
Immediately
Dangerous to Life or Health Concentrations (IDLH)
1355:. It has been used as a mild oxidant in reactions like the
1319:(also known as acetaminophen). Nitrobenzene is also used in
1156:
World capacity for nitrobenzene in 1985 was about 1,700,000
747:
360:
47:
37:
1822:
Division, US EPA, ORD, Integrated Risk
Information System.
1400:
in the United States as defined in
Section 302 of the U.S.
1374:
Prolonged exposure may cause serious damage to the central
1204:
Approximately 95% of nitrobenzene industrially produced is
786:
1650:
Bigelow, H. E.; Palmer, Albert (1931). "Azoxybenzene".
1004:
1562:"a case of poisoning by oil of mirbane (nitro-benzol)"
1509:
National
Institute for Occupational Safety and Health
1440:
National
Institute for Occupational Safety and Health
1637:"Flowering stimulant composition using nitrobenzene"
1371:
5 mg/m) and readily absorbed through the skin.
1074:. In the laboratory, it is occasionally used as a
1062:. It is a water-insoluble pale yellow oil with an
1402:Emergency Planning and Community Right-to-Know Act
1824:"Nitrobenzene CASRN 98-95-3 - IRIS - US EPA, ORD"
306:
1738:Industrial & Engineering Chemistry Research
790:
495:pungent, like paste shoe polish to almond-like
116:
1529:Ullmann's Encyclopedia of Industrial Chemistry
1289:Most aniline is consumed in the production of
1703:
1701:
1527:Booth G (2007). "Nitro Compounds, Aromatic".
8:
1847:: CS1 maint: multiple names: authors list (
525:210.9 °C (411.6 °F; 484.0 K)
1959:https://patents.google.com/patent/US9113628
400:InChI=1S/C6H5NO2/c8-7(9)6-4-2-1-3-5-6/h1-5H
1097:of benzene with a mixture of concentrated
410:InChI=1/C6H5NO2/c8-7(9)6-4-2-1-3-5-6/h1-5H
375:
234:
212:
26:
1887:Nomination: Nitrobenzene Review committee
1585:
515:5.7 °C (42.3 °F; 278.8 K)
342:
1434:NIOSH Pocket Guide to Chemical Hazards.
1939:International Chemical Safety Card 0065
1522:
1520:
1518:
1413:
431:
396:
371:
1944:NIOSH Pocket Guide to Chemical Hazards
1840:
1495:
1493:
1429:
1427:
1425:
1423:
1421:
1419:
1417:
847:480 °C (896 °F; 753 K)
225:
1708:Coleman GH, McCloskey CM, Stuart FA.
1531:(6th ed.). Weinheim: Wiley-VCH.
833:88 °C (190 °F; 361 K)
403:Key: LQNUZADURLCDLV-UHFFFAOYSA-N
192:
172:
7:
1177:electrophilic aromatic substitution
413:Key: LQNUZADURLCDLV-UHFFFAOYAA
297:
281:
1167:process involves formation of the
25:
1677:Bigelow HE, Robinson DB (1955).
1149:
1141:
1122:
1116:
994:
603:
598:
63:
1323:, as it has an unusually large
1293:, a precursor to polyurethanes.
990:(at 25 °C , 100 kPa).
1949:IARC Monograph: "Nitrobenzene"
1367:Nitrobenzene is highly toxic (
1:
1456:Journal of Solution Chemistry
1398:extremely hazardous substance
916:(US health exposure limits):
1623:10.1016/j.tetlet.2005.09.196
1093:Nitrobenzene is prepared by
1989:Nonlinear optical materials
1560:Hogarth CW (January 1912).
865:or concentration (LD, LC):
33:
2015:
1907:Government Printing Office
1787:, vol. 1, p. 445
1724:, vol. 3, p. 668
1693:, vol. 3, p. 103
1357:Skraup quinoline synthesis
1252:Aniline is a precursor to
477:123.11 g/mol
1984:IARC Group 2B carcinogens
1905:(July 1, 2008 ed.).
984:
975:Benzenediazonium chloride
959:
910:
861:
579:
574:
442:
422:
387:
100:
88:
76:
71:
62:
32:
1664:10.15227/orgsyn.011.0016
1537:10.1002/14356007.a17_411
1300:Specialized applications
1038:and the simplest of the
666:Precautionary statements
1799:Clarke, HT; Davis, AW.
1773:"β-Phenylhydroxylamine"
1566:British Medical Journal
1396:It is classified as an
887:590 mg/kg (mouse, oral)
554:Magnetic susceptibility
537:0.19 g/100 ml at 20 °C
485:yellowish, oily liquid
1578:10.1136/bmj.1.2665.183
1294:
906:750 mg/kg (dog, oral)
797:
53:
43:
1369:Threshold Limit Value
1288:
885:600 mg/kg (rat, oral)
883:780 mg/kg (rat, oral)
796:
51:
41:
1916:on February 25, 2012
942:TWA 1 ppm (5 mg/m)
929:TWA 1 ppm (5 mg/m)
779:(fire diamond)
78:Preferred IUPAC name
1750:10.1021/ie00073a005
1611:Tetrahedron Letters
1391:Group 2B carcinogen
1353:phenylhydroxylamine
532:Solubility in water
154:Beilstein Reference
29:
1468:10.1007/BF00972581
1295:
1291:methylenedianiline
1175:), followed by an
1070:as a precursor to
1017:Infobox references
960:Related compounds
951:(Immediate danger)
798:
54:
44:
27:
1994:Aromatic solvents
1805:Organic Syntheses
1785:Collected Volumes
1778:Organic Syntheses
1722:Collected Volumes
1715:Organic Syntheses
1691:Collected Volumes
1684:Organic Syntheses
1652:Organic Syntheses
1617:(11): 1861–1864.
1546:978-3-527-30385-4
1331:Organic reactions
1078:, especially for
1025:Chemical compound
1023:
1022:
966:Related compounds
628:Hazard statements
560:-61.80·10 cm/mol
356:CompTox Dashboard
142:Interactive image
58:
57:
16:(Redirected from
2006:
1999:Phenyl compounds
1954:US EPA factsheet
1926:
1925:
1923:
1921:
1915:
1909:. Archived from
1904:
1896:
1890:
1883:
1877:
1876:
1874:
1872:
1867:
1859:
1853:
1852:
1846:
1838:
1836:
1834:
1819:
1813:
1812:
1796:
1790:
1788:
1781:
1768:
1762:
1761:
1733:
1727:
1725:
1718:
1710:"Nitrosobenzene"
1705:
1696:
1694:
1687:
1674:
1668:
1667:
1647:
1641:
1640:
1633:
1627:
1626:
1606:
1600:
1599:
1589:
1557:
1551:
1550:
1524:
1513:
1512:
1497:
1488:
1487:
1450:
1444:
1443:
1431:
1154:
1153:
1145:
1135:
1126:
1120:
1113:= −117 kJ/mol).
1044:chemical formula
1007:
1001:
998:
997:
900:lowest published
853:Explosive limits
818:
811:
804:
789:
769:
765:
761:
757:
753:
749:
745:
741:
737:
733:
729:
725:
721:
717:
713:
709:
705:
701:
697:
693:
689:
685:
681:
677:
673:
659:
655:
651:
647:
643:
639:
635:
607:
602:
547:0.3 mmHg (25°C)
450:Chemical formula
380:
379:
364:
362:
346:
310:
299:
285:
263:Gmelin Reference
246:
238:
227:
216:
196:
176:
144:
120:
67:
34:
30:
21:
18:Mononitrobenzene
2014:
2013:
2009:
2008:
2007:
2005:
2004:
2003:
1964:
1963:
1935:
1930:
1929:
1919:
1917:
1913:
1902:
1898:
1897:
1893:
1884:
1880:
1870:
1868:
1865:
1861:
1860:
1856:
1839:
1832:
1830:
1821:
1820:
1816:
1798:
1797:
1793:
1783:
1770:
1769:
1765:
1735:
1734:
1730:
1720:
1707:
1706:
1699:
1689:
1676:
1675:
1671:
1649:
1648:
1644:
1635:
1634:
1630:
1608:
1607:
1603:
1559:
1558:
1554:
1547:
1526:
1525:
1516:
1499:
1498:
1491:
1452:
1451:
1447:
1433:
1432:
1415:
1410:
1365:
1333:
1302:
1278:pharmaceuticals
1247:
1243:
1239:
1235:
1231:
1227:
1223:
1219:
1202:
1194:
1190:
1186:
1174:
1148:
1147:
1146:
1138:
1137:
1133:
1131:
1115:
1091:
1060:
1054:
1050:
1026:
1019:
1014:
1013:
1012: ?)
1003:
999:
995:
991:
977:
973:
967:
952:
939:
926:
903:
897:
888:
886:
884:
880:
874:
844:
841:
823:
822:
821:
820:
813:
806:
799:
795:
787:
668:
630:
616:
595:
557:
534:
466:
462:
458:
452:
438:
435:
430:
429:
418:
415:
414:
411:
405:
404:
401:
395:
394:
383:
365:
358:
349:
329:
313:
300:
288:
265:
256:
219:
199:
179:
156:
147:
134:
123:
110:
96:
94:
92:
84:
83:
23:
22:
15:
12:
11:
5:
2012:
2010:
2002:
2001:
1996:
1991:
1986:
1981:
1979:Nitro solvents
1976:
1966:
1965:
1962:
1961:
1956:
1951:
1946:
1941:
1934:
1933:External links
1931:
1928:
1927:
1891:
1878:
1854:
1814:
1791:
1763:
1728:
1697:
1669:
1642:
1628:
1601:
1552:
1545:
1514:
1501:"Nitrobenzene"
1489:
1462:(9): 905–917.
1445:
1412:
1411:
1409:
1406:
1376:nervous system
1364:
1361:
1349:hydrazobenzene
1345:nitrosobenzene
1332:
1329:
1301:
1298:
1297:
1296:
1268:(particularly
1250:
1249:
1245:
1241:
1237:
1233:
1229:
1225:
1221:
1217:
1201:
1198:
1197:
1196:
1192:
1188:
1184:
1172:
1140:
1139:
1132:
1129:
1128:
1127:
1090:
1087:
1058:
1052:
1048:
1036:nitro compound
1024:
1021:
1020:
1015:
993:
992:
988:standard state
985:
982:
981:
979:Nitrosobenzene
968:
965:
962:
961:
957:
956:
953:
947:
944:
943:
940:
934:
931:
930:
927:
921:
918:
917:
908:
907:
904:
895:
893:
890:
889:
881:
872:
870:
867:
866:
859:
858:
855:
849:
848:
845:
838:
835:
834:
831:
825:
824:
814:
807:
800:
785:
784:
783:
782:
780:
771:
770:
669:
664:
661:
660:
631:
626:
623:
622:
617:
612:
609:
608:
596:
591:
588:
587:
577:
576:
572:
571:
568:
562:
561:
558:
552:
549:
548:
545:
543:Vapor pressure
539:
538:
535:
530:
527:
526:
523:
517:
516:
513:
507:
506:
503:
497:
496:
493:
487:
486:
483:
479:
478:
475:
469:
468:
464:
460:
456:
453:
448:
445:
444:
440:
439:
437:
436:
434:c1ccc(cc1)(=O)
433:
425:
424:
423:
420:
419:
417:
416:
412:
409:
408:
406:
402:
399:
398:
390:
389:
388:
385:
384:
382:
381:
368:
366:
354:
351:
350:
348:
347:
339:
337:
331:
330:
328:
327:
323:
321:
315:
314:
312:
311:
303:
301:
293:
290:
289:
287:
286:
278:
276:
270:
269:
266:
261:
258:
257:
255:
254:
250:
248:
240:
239:
229:
221:
220:
218:
217:
209:
207:
201:
200:
198:
197:
189:
187:
181:
180:
178:
177:
169:
167:
161:
160:
157:
152:
149:
148:
146:
145:
137:
135:
128:
125:
124:
122:
121:
113:
111:
106:
103:
102:
98:
97:
95:Oil of mirbane
93:Nitritebenzene
90:
86:
85:
81:
80:
74:
73:
69:
68:
60:
59:
56:
55:
45:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2011:
2000:
1997:
1995:
1992:
1990:
1987:
1985:
1982:
1980:
1977:
1975:
1974:Nitrobenzenes
1972:
1971:
1969:
1960:
1957:
1955:
1952:
1950:
1947:
1945:
1942:
1940:
1937:
1936:
1932:
1912:
1908:
1901:
1895:
1892:
1888:
1882:
1879:
1864:
1858:
1855:
1850:
1844:
1829:
1828:cfpub.epa.gov
1825:
1818:
1815:
1810:
1806:
1802:
1795:
1792:
1786:
1780:
1779:
1774:
1767:
1764:
1759:
1755:
1751:
1747:
1743:
1739:
1732:
1729:
1723:
1717:
1716:
1711:
1704:
1702:
1698:
1692:
1686:
1685:
1680:
1673:
1670:
1665:
1661:
1657:
1653:
1646:
1643:
1638:
1632:
1629:
1624:
1620:
1616:
1612:
1605:
1602:
1597:
1593:
1588:
1583:
1579:
1575:
1572:(2665): 183.
1571:
1567:
1563:
1556:
1553:
1548:
1542:
1538:
1534:
1530:
1523:
1521:
1519:
1515:
1510:
1506:
1502:
1496:
1494:
1490:
1485:
1481:
1477:
1473:
1469:
1465:
1461:
1457:
1449:
1446:
1441:
1437:
1430:
1428:
1426:
1424:
1422:
1420:
1418:
1414:
1407:
1405:
1403:
1399:
1394:
1392:
1388:
1383:
1381:
1377:
1372:
1370:
1362:
1360:
1358:
1354:
1350:
1346:
1342:
1338:
1330:
1328:
1326:
1325:Kerr constant
1322:
1318:
1315:
1311:
1307:
1299:
1292:
1287:
1283:
1282:
1281:
1279:
1275:
1271:
1267:
1263:
1259:
1255:
1215:
1214:
1213:
1211:
1207:
1199:
1182:
1181:
1180:
1178:
1170:
1166:
1161:
1159:
1152:
1144:
1125:
1119:
1114:
1112:
1108:
1104:
1100:
1099:sulfuric acid
1096:
1088:
1086:
1084:
1081:
1080:electrophilic
1077:
1073:
1069:
1065:
1061:
1055:
1045:
1041:
1040:nitrobenzenes
1037:
1034:
1030:
1018:
1011:
1006:
989:
983:
980:
976:
972:
969:
964:
963:
958:
954:
950:
946:
945:
941:
938:(Recommended)
937:
933:
932:
928:
925:(Permissible)
924:
920:
919:
915:
914:
909:
905:
901:
892:
891:
882:
878:
869:
868:
864:
860:
856:
854:
851:
850:
846:
843:
837:
836:
832:
830:
827:
826:
819:
812:
805:
781:
778:
777:
773:
772:
670:
667:
663:
662:
632:
629:
625:
624:
621:
618:
615:
611:
610:
606:
601:
597:
594:
590:
589:
585:
583:
578:
573:
570:1.8112 mPa·s
569:
567:
564:
563:
559:
555:
551:
550:
546:
544:
541:
540:
536:
533:
529:
528:
524:
522:
521:Boiling point
519:
518:
514:
512:
511:Melting point
509:
508:
504:
502:
499:
498:
494:
492:
489:
488:
484:
481:
480:
476:
474:
471:
470:
454:
451:
447:
446:
441:
432:
428:
421:
407:
397:
393:
386:
378:
374:
373:DTXSID3020964
370:
369:
367:
357:
353:
352:
345:
341:
340:
338:
336:
333:
332:
325:
324:
322:
320:
317:
316:
309:
305:
304:
302:
296:
292:
291:
284:
280:
279:
277:
275:
272:
271:
267:
264:
260:
259:
252:
251:
249:
247:
242:
241:
237:
233:
230:
228:
226:ECHA InfoCard
223:
222:
215:
211:
210:
208:
206:
203:
202:
195:
191:
190:
188:
186:
183:
182:
175:
171:
170:
168:
166:
163:
162:
158:
155:
151:
150:
143:
139:
138:
136:
132:
127:
126:
119:
115:
114:
112:
109:
105:
104:
99:
87:
79:
75:
70:
66:
61:
50:
46:
40:
36:
35:
31:
28:Nitrobenzene
19:
1918:. Retrieved
1911:the original
1894:
1889:, 02/02/2010
1881:
1869:. Retrieved
1857:
1831:. Retrieved
1827:
1817:
1808:
1804:
1794:
1784:
1776:
1766:
1744:(1): 21–24.
1741:
1737:
1731:
1721:
1713:
1690:
1682:
1679:"Azobenzene"
1672:
1655:
1651:
1645:
1631:
1614:
1610:
1604:
1569:
1565:
1555:
1528:
1504:
1459:
1455:
1448:
1395:
1384:
1373:
1366:
1337:azoxybenzene
1334:
1303:
1251:
1206:hydrogenated
1203:
1187:+ H ⇌ NO
1162:
1155:
1110:
1092:
1029:Nitrobenzene
1028:
1027:
912:
862:
840:Autoignition
775:
619:
581:
319:RTECS number
101:Identifiers
89:Other names
82:Nitrobenzene
52:Nitrobenzene
42:Nitrobenzene
1920:October 29,
1801:"Quinoline"
1317:paracetamol
1260:chemicals,
1107:nitric acid
1042:, with the
877:median dose
863:Lethal dose
842:temperature
829:Flash point
614:Signal word
505:1.199 g/cm
482:Appearance
443:Properties
232:100.002.469
194:ChEMBL15750
174:CHEBI:27798
91:Nitrobenzol
1968:Categories
1408:References
1341:azobenzene
1321:Kerr cells
1274:explosives
1262:pesticides
1256:polymers,
1089:Production
593:Pictograms
473:Molar mass
344:E57JCN6SSY
205:ChemSpider
129:3D model (
108:CAS Number
1871:10 August
1833:10 August
1758:0888-5885
1476:0095-9782
1314:analgesic
1169:nitronium
1165:nitration
1095:nitration
760:P403+P233
724:P308+P313
720:P304+P340
716:P302+P352
712:P301+P310
584:labelling
566:Viscosity
326:DA6475000
253:202-716-0
245:EC Number
1843:cite web
1771:Kamm O.
1596:20765985
1511:(NIOSH).
1484:95126469
1442:(NIOSH).
1380:cyanosis
1306:solvents
1270:azo dyes
1254:urethane
1083:reagents
1033:aromatic
955:200 ppm
776:NFPA 704
575:Hazards
556:(χ)
1587:2344391
1436:"#0450"
1351:, and
1210:aniline
1171:ion (NO
1134:
1076:solvent
1072:aniline
1068:benzene
1010:what is
1008: (
971:Aniline
857:1.8%-?
501:Density
467:
295:PubChem
159:507540
118:98-95-3
1811:: 478.
1756:
1658:: 16.
1594:
1584:
1543:
1482:
1474:
1363:Safety
1276:, and
1258:rubber
1244:+ 2 H
1228:+ 3 H
1158:tonnes
1130:
1105:, and
1064:almond
1031:is an
1005:verify
1002:
620:Danger
427:SMILES
283:C06813
268:50357
185:ChEMBL
72:Names
1914:(PDF)
1903:(PDF)
1866:(PDF)
1480:S2CID
1389:as a
1310:soaps
1232:→ C
1103:water
913:NIOSH
392:InChI
165:ChEBI
131:JSmol
1922:2011
1873:2017
1849:link
1835:2017
1754:ISSN
1592:PMID
1541:ISBN
1472:ISSN
1387:IARC
1266:dyes
1200:Uses
1191:+ H
1163:The
949:IDLH
768:P501
764:P405
756:P363
752:P361
748:P330
744:P322
740:P321
736:P314
732:P312
728:P311
708:P281
704:P280
700:P273
696:P271
692:P270
688:P264
684:P261
680:P260
676:P202
672:P201
658:H412
654:H372
650:H360
646:H351
642:H331
638:H311
634:H301
491:Odor
335:UNII
308:7416
274:KEGG
214:7138
1746:doi
1660:doi
1619:doi
1582:PMC
1574:doi
1533:doi
1464:doi
1272:),
1212::
1208:to
1183:HNO
936:REL
923:PEL
582:GHS
361:EPA
298:CID
1970::
1845:}}
1841:{{
1826:.
1807:.
1803:.
1782:;
1775:.
1752:.
1742:27
1740:.
1719:;
1712:.
1700:^
1688:;
1681:.
1656:11
1654:.
1615:47
1613:.
1590:.
1580:.
1568:.
1564:.
1539:.
1517:^
1507:.
1503:.
1492:^
1478:.
1470:.
1460:25
1458:.
1438:.
1416:^
1359:.
1347:,
1343:,
1339:,
1280:.
1264:,
1240:NH
1224:NO
1160:.
1121:+
1101:,
1085:.
1057:NO
896:Lo
894:LD
873:50
871:LD
766:,
762:,
758:,
754:,
750:,
746:,
742:,
738:,
734:,
730:,
726:,
722:,
718:,
714:,
710:,
706:,
702:,
698:,
694:,
690:,
686:,
682:,
678:,
674:,
656:,
652:,
648:,
644:,
640:,
636:,
586::
463:NO
1924:.
1875:.
1851:)
1837:.
1809:1
1789:.
1760:.
1748::
1726:.
1695:.
1666:.
1662::
1639:.
1625:.
1621::
1598:.
1576::
1570:1
1549:.
1535::
1486:.
1466::
1248:O
1246:2
1242:2
1238:5
1236:H
1234:6
1230:2
1226:2
1222:5
1220:H
1218:6
1216:C
1195:O
1193:2
1189:2
1185:3
1173:2
1136:H
1111:H
1059:2
1053:5
1051:H
1049:6
1047:C
1000:N
902:)
898:(
879:)
875:(
817:1
810:2
803:3
465:2
461:5
459:H
457:6
455:C
363:)
359:(
133:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.