301:
191:
108:
24:
633:
565:
657:. Recent studies have shown that monensin may transport sodium ion through the membrane in both electrogenic and electroneutral manner. This approach explains ionophoric ability and in consequence antibacterial properties of not only parental monensin, but also its derivatives that do not possess carboxylic groups. It blocks
324:
InChI=1S/C36H62O11/c1-10-34(31-20(3)16-26(43-31)28-19(2)15-21(4)36(41,18-37)46-28)12-11-27(44-34)33(8)13-14-35(47-33)17-25(38)22(5)30(45-35)23(6)29(42-9)24(7)32(39)40/h19-31,37-38,41H,10-18H2,1-9H3,(H,39,40)/t19-,20-,21+,22+,23-,24-,25-,26+,27+,28-,29+,30-,31+,33-,34-,35+,36-/m0/s1
334:
InChI=1/C36H62O11/c1-10-34(31-20(3)16-26(43-31)28-19(2)15-21(4)36(41,18-37)46-28)12-11-27(44-34)33(8)13-14-35(47-33)17-25(38)22(5)30(45-35)23(6)29(42-9)24(7)32(39)40/h19-31,37-38,41H,10-18H2,1-9H3,(H,39,40)/t19-,20-,21+,22+,23-,24-,25-,26+,27+,28-,29+,30-,31+,33-,34-,35+,36-/m0/s1
1393:
1292:
Kallen, K. J.; Quinn, P.; Allan, D. (1993-02-24). "Monensin inhibits synthesis of plasma membrane sphingomyelin by blocking transport of ceramide through the Golgi: evidence for two sites of sphingomyelin synthesis in BHK cells".
652:
such as: Li, Na, K, Rb, Ag, and Tl. Monensin A is able to transport these cations across lipid membranes of cells in an electroneutral (i.e. non-depolarizing) exchange, playing an important role as an Na/H
1237:"Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus"
1431:
466:
578:
1336:
Zhang, G. F.; Driouich, A.; Staehelin, L. A. (December 1996). "Monensin-induced redistribution of enzymes and products from Golgi stacks to swollen vesicles in plant cells".
616:
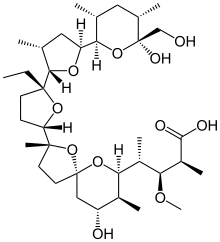
The structure of monensin was first described by
Agtarap et al. in 1967, and was the first polyether antibiotic to have its structure elucidated in this way. The first
730:
1130:"A sodium ion selective electrode based on a highly lipophilic monensin derivative and its application to the measurement of sodium ion concentrations in serum"
350:
1417:
1065:
709:
Monensin has some degree of activity on mammalian cells and thus toxicity is common. This is especially pronounced in horses, where monensin has a
1063:
Huczyński, A.; Stefańska, J.; Przybylski, P.; Brzezinski, B.; Bartl, F. (2008). "Synthesis and antimicrobial properties of
Monensin A esters".
1161:
Kim, N.; Park, K.; Park, I.; Cho, Y.; Bae, Y. (2005). "Application of a taste evaluation system to the monitoring of Kimchi fermentation".
1441:
713:
1/100th that of ruminants. Accidental poisoning of equines with monensin is a well-documented occurrence which has resulted in deaths.
1424:
673:
properties of monensin and its derivatives are a result of their ability to transport metal cations through cellular and subcellular
881:
315:
1489:
689:, increase the production of propionic acid and prevent bloat. Furthermore, monensin, but also its derivatives monensin methyl
496:
1128:
Tohda, Koji; Suzuki, Koji; Kosuge, Nobutaka; Nagashima, Hitoshi; Watanabe, Kazuhiko; Inoue, Hidenari; Shirai, Tsuneo (1990).
903:
1163:
1101:
585:
939:
258:
1200:
814:"Antimicrobial Growth Promoters Used in Animal Feed: Effects of Less Well Known Antibiotics on Gram-Positive Bacteria"
279:
609:
1509:
1499:
937:
Pinkerton, M.; Steinrauf, L. K. (1970). "Molecular structure of monovalent metal cation complexes of monensin".
1504:
546:
694:
1099:
Matsuoka, T.; Novilla, M.N.; Thomson, T.D.; Donoho, A.L. (1996). "Review of monensin toxicosis in horses".
186:
901:(2007). "Molecular structure of the 1:1 inclusion complex of Monensin A lithium salt with acetonitrile".
148:
898:
763:"Structure and Antimicrobial Properties of Monensin A and Its Derivatives: Summary of the Achievements"
1016:"Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity"
53:)-4--3′-methyl-5-yl}-9-hydroxy-2,8-dimethyl-1,6-dioxaspirodecan-7-yl]-3-methoxy-2-methylpentanoic acid
1209:
911:
36:
1494:
1398:
873:
674:
296:
74:
710:
1353:
1345:
1318:
1310:
1274:
1256:
1180:
1081:
1045:
996:
955:
877:
843:
794:
510:
1409:
1377:
1302:
1264:
1248:
1217:
1172:
1141:
1110:
1073:
1035:
1027:
986:
947:
919:
865:
833:
825:
784:
774:
425:
373:
168:
267:
84:
698:
617:
487:
477:
866:
300:
190:
1213:
915:
128:
1269:
1236:
1040:
1015:
861:
789:
762:
556:
1372:
1221:
1114:
838:
813:
1483:
1306:
1031:
951:
735:
670:
658:
621:
449:
414:
179:
923:
482:
472:
247:
829:
991:
975:"Monensin A acid complexes as a model of electrogenic transport of sodium cation"
974:
1453:
686:
645:
530:
1176:
1077:
662:
654:
604:
534:
436:
396:
159:
1349:
1314:
1260:
1468:
1463:
641:
601:
1184:
1085:
1000:
847:
798:
1357:
1322:
1278:
1146:
1129:
1049:
959:
779:
1252:
973:
Huczyński, Adam; Jan
Janczak; Daniel Łowicki; Bogumil Brzezinski (2012).
685:
Monensin is used extensively in the beef and dairy industries to prevent
206:
198:
666:
453:
445:
441:
234:
23:
731:"Health product highlights 2021: Annexes of products approved in 2021"
693:(MME), and particularly monensin decyl ester (MDE) are widely used in
632:
649:
358:
O=C(O)(C)(OC)(C)5O1(O(C)(CC1)2O(CC)(CC2)4O(3O(O)(CO)(C3C)C)C4C)C(O)5C
139:
555:
Except where otherwise noted, data are given for materials in their
222:
690:
631:
119:
107:
97:
1295:
Biochimica et
Biophysica Acta (BBA) - Lipids and Lipid Metabolism
697:. In laboratory research, monensin is used extensively to block
213:
1413:
1020:
Biochimica et
Biophysica Acta (BBA) - Reviews on Biomembranes
1394:"Nearly 70 horses die after eating feed containing monensin"
284:
1373:"Tainted feed blamed for 4 horse deaths at Florida stable"
505:
636:
The structure of the sodium (Na) complex of monensin A.
573:
1014:
Mollenhauer, H. H.; Morre, D. J.; Rowe, L. D. (1990).
812:
Butaye, P.; Devriese, L. A.; Haesebrouck, F. (2003).
199:
897:
Huczyński, A.; Ratajczak-Sitarz, M.; Katrusiak, A.;
648:
with a preference to form complexes with monovalent
1235:Griffiths, G.; Quinn, P.; Warren, G. (March 1983).
979:Biochimica et Biophysica Acta (BBA) - Biomembranes
246:
613:. It is widely used in ruminant animal feeds.
83:
1425:
8:
486:
476:
1432:
1418:
1410:
761:Daniel Łowicki and Adam Huczyński (2013).
756:
754:
299:
189:
167:
15:
1268:
1145:
1039:
990:
837:
788:
778:
266:
722:
669:, and other biological activities. The
355:
320:
295:
419:104 °C (219 °F; 377 K)
180:
327:Key: GAOZTHIDHYLHMS-KEOBGNEYSA-N
147:
127:
7:
1440:Nonribosomally synthesized porters (
1102:Journal of Equine Veterinary Science
620:of monensin was reported in 1979 by
872:. Weinheim, Germany: VCH. pp.
337:Key: GAOZTHIDHYLHMS-KEOBGNEYBF
237:
221:
14:
1201:Sensors and Actuators B: Chemical
1198:Toko, K. (2000). "Taste Sensor".
1338:European Journal of Cell Biology
661:protein transport, and exhibits
563:
401:670.871 g/mol
22:
559:(at 25 °C , 100 kPa).
924:10.1016/j.molstruc.2006.07.046
1:
1222:10.1016/S0925-4005(99)00508-0
1164:Biosensors and Bioelectronics
1115:10.1016/S0737-0806(96)80059-1
830:10.1128/CMR.16.2.175-188.2003
818:Clinical Microbiology Reviews
767:BioMed Research International
1392:Lacy Vilhauer (2024-08-27).
1307:10.1016/0005-2760(93)90111-l
1032:10.1016/0304-4157(90)90008-Z
992:10.1016/j.bbamem.2012.04.017
952:10.1016/0022-2836(70)90279-2
409:solid state, white crystals
1371:Jennifer Kay (2014-12-16).
1241:The Journal of Cell Biology
868:Classics in Total Synthesis
1526:
1177:10.1016/j.bios.2004.10.007
1078:10.1016/j.bmcl.2008.03.038
610:Streptomyces cinnamonensis
1449:
864:; E. J. Sorensen (1996).
553:
519:
459:
366:
346:
311:
67:
59:
35:
30:
21:
1066:Bioorg. Med. Chem. Lett.
695:ion-selective electrodes
547:Monensin A methyl ester
431:3x10 g/dm (20 °C)
1490:Polyketide antibiotics
637:
1147:10.2116/analsci.6.227
635:
1253:10.1083/jcb.96.3.835
37:Preferred IUPAC name
1399:High Plains Journal
1214:2000SeAcB..64..205T
1134:Analytical Sciences
916:2007JMoSt.871...92H
780:10.1155/2013/742149
628:Mechanism of action
426:Solubility in water
18:
711:median lethal dose
638:
586:Infobox references
520:Related compounds
16:
1477:
1476:
1171:(11): 2283–2291.
640:Monensin A is an
594:Chemical compound
592:
591:
542:Related compounds
508:
280:CompTox Dashboard
109:Interactive image
1517:
1510:Tetrahydrofurans
1500:Carboxylic acids
1434:
1427:
1420:
1411:
1404:
1403:
1389:
1383:
1382:
1378:Associated Press
1368:
1362:
1361:
1333:
1327:
1326:
1301:(2–3): 305–308.
1289:
1283:
1282:
1272:
1232:
1226:
1225:
1208:(1–3): 205–215.
1195:
1189:
1188:
1158:
1152:
1151:
1149:
1125:
1119:
1118:
1096:
1090:
1089:
1072:(8): 2585–2589.
1060:
1054:
1053:
1043:
1011:
1005:
1004:
994:
985:(9): 2108–2119.
970:
964:
963:
934:
928:
927:
894:
888:
887:
871:
858:
852:
851:
841:
809:
803:
802:
792:
782:
758:
749:
748:
746:
744:
727:
576:
570:
567:
566:
507:
504:
490:
480:
374:Chemical formula
304:
303:
288:
286:
270:
250:
239:
225:
201:
193:
182:
171:
151:
131:
111:
87:
26:
19:
1525:
1524:
1520:
1519:
1518:
1516:
1515:
1514:
1505:Spiro compounds
1480:
1479:
1478:
1473:
1445:
1438:
1408:
1407:
1391:
1390:
1386:
1370:
1369:
1365:
1335:
1334:
1330:
1291:
1290:
1286:
1234:
1233:
1229:
1197:
1196:
1192:
1160:
1159:
1155:
1127:
1126:
1122:
1098:
1097:
1093:
1062:
1061:
1057:
1013:
1012:
1008:
972:
971:
967:
936:
935:
931:
904:J. Mol. Struct.
896:
895:
891:
884:
862:Nicolaou, K. C.
860:
859:
855:
811:
810:
806:
760:
759:
752:
742:
740:
739:. 3 August 2022
729:
728:
724:
719:
707:
683:
644:related to the
630:
618:total synthesis
595:
588:
583:
582:
581: ?)
572:
568:
564:
560:
543:
527:
515:
469:
428:
390:
386:
382:
376:
362:
359:
354:
353:
342:
339:
338:
335:
329:
328:
325:
319:
318:
307:
289:
282:
273:
253:
240:
228:
174:
154:
134:
114:
101:
90:
77:
63:
55:
54:
12:
11:
5:
1523:
1521:
1513:
1512:
1507:
1502:
1497:
1492:
1482:
1481:
1475:
1474:
1472:
1471:
1466:
1461:
1456:
1450:
1447:
1446:
1439:
1437:
1436:
1429:
1422:
1414:
1406:
1405:
1384:
1363:
1344:(4): 332–340.
1328:
1284:
1247:(3): 835–850.
1227:
1190:
1153:
1140:(2): 227–232.
1120:
1091:
1055:
1026:(2): 225–246.
1006:
965:
946:(3): 533–546.
929:
910:(1–3): 92–97.
899:Brzezinski, B.
889:
882:
853:
824:(2): 175–188.
804:
750:
721:
720:
718:
715:
706:
703:
682:
679:
629:
626:
607:isolated from
593:
590:
589:
584:
562:
561:
557:standard state
554:
551:
550:
544:
541:
538:
537:
528:
525:
522:
521:
517:
516:
514:
513:
501:
499:
493:
492:
470:
465:
462:
461:
457:
456:
439:
433:
432:
429:
424:
421:
420:
417:
411:
410:
407:
403:
402:
399:
393:
392:
388:
384:
380:
377:
372:
369:
368:
364:
363:
361:
360:
357:
349:
348:
347:
344:
343:
341:
340:
336:
333:
332:
330:
326:
323:
322:
314:
313:
312:
309:
308:
306:
305:
292:
290:
278:
275:
274:
272:
271:
263:
261:
255:
254:
252:
251:
243:
241:
233:
230:
229:
227:
226:
218:
216:
210:
209:
203:
195:
194:
184:
176:
175:
173:
172:
164:
162:
156:
155:
153:
152:
144:
142:
136:
135:
133:
132:
124:
122:
116:
115:
113:
112:
104:
102:
95:
92:
91:
89:
88:
80:
78:
73:
70:
69:
65:
64:
61:
57:
56:
40:
39:
33:
32:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1522:
1511:
1508:
1506:
1503:
1501:
1498:
1496:
1493:
1491:
1488:
1487:
1485:
1470:
1467:
1465:
1462:
1460:
1457:
1455:
1452:
1451:
1448:
1443:
1435:
1430:
1428:
1423:
1421:
1416:
1415:
1412:
1401:
1400:
1395:
1388:
1385:
1380:
1379:
1374:
1367:
1364:
1359:
1355:
1351:
1347:
1343:
1339:
1332:
1329:
1324:
1320:
1316:
1312:
1308:
1304:
1300:
1296:
1288:
1285:
1280:
1276:
1271:
1266:
1262:
1258:
1254:
1250:
1246:
1242:
1238:
1231:
1228:
1223:
1219:
1215:
1211:
1207:
1203:
1202:
1194:
1191:
1186:
1182:
1178:
1174:
1170:
1166:
1165:
1157:
1154:
1148:
1143:
1139:
1135:
1131:
1124:
1121:
1116:
1112:
1108:
1104:
1103:
1095:
1092:
1087:
1083:
1079:
1075:
1071:
1068:
1067:
1059:
1056:
1051:
1047:
1042:
1037:
1033:
1029:
1025:
1021:
1017:
1010:
1007:
1002:
998:
993:
988:
984:
980:
976:
969:
966:
961:
957:
953:
949:
945:
942:
941:
940:J. Mol. Biol.
933:
930:
925:
921:
917:
913:
909:
906:
905:
900:
893:
890:
885:
883:3-527-29284-5
879:
875:
870:
869:
863:
857:
854:
849:
845:
840:
835:
831:
827:
823:
819:
815:
808:
805:
800:
796:
791:
786:
781:
776:
772:
768:
764:
757:
755:
751:
738:
737:
736:Health Canada
732:
726:
723:
716:
714:
712:
704:
702:
700:
696:
692:
688:
680:
678:
676:
672:
671:antibacterial
668:
664:
660:
659:intracellular
656:
651:
647:
643:
634:
627:
625:
623:
619:
614:
612:
611:
606:
603:
599:
587:
580:
575:
558:
552:
548:
545:
540:
539:
536:
532:
529:
524:
523:
518:
512:
503:
502:
500:
498:
495:
494:
489:
484:
479:
474:
471:
468:
464:
463:
460:Pharmacology
458:
455:
451:
450:diethyl ether
447:
443:
440:
438:
435:
434:
430:
427:
423:
422:
418:
416:
415:Melting point
413:
412:
408:
405:
404:
400:
398:
395:
394:
378:
375:
371:
370:
365:
356:
352:
345:
331:
321:
317:
310:
302:
298:
297:DTXSID4048561
294:
293:
291:
281:
277:
276:
269:
265:
264:
262:
260:
257:
256:
249:
245:
244:
242:
236:
232:
231:
224:
220:
219:
217:
215:
212:
211:
208:
207:(antibiotics)
204:
202:
197:
196:
192:
188:
185:
183:
181:ECHA InfoCard
178:
177:
170:
166:
165:
163:
161:
158:
157:
150:
146:
145:
143:
141:
138:
137:
130:
126:
125:
123:
121:
118:
117:
110:
106:
105:
103:
99:
94:
93:
86:
82:
81:
79:
76:
72:
71:
66:
62:Monensic acid
58:
52:
48:
44:
38:
34:
29:
25:
20:
1458:
1397:
1387:
1376:
1366:
1341:
1337:
1331:
1298:
1294:
1287:
1244:
1240:
1230:
1205:
1199:
1193:
1168:
1162:
1156:
1137:
1133:
1123:
1106:
1100:
1094:
1069:
1064:
1058:
1023:
1019:
1009:
982:
978:
968:
943:
938:
932:
907:
902:
892:
867:
856:
821:
817:
807:
770:
766:
741:. Retrieved
734:
725:
708:
684:
667:antimalarial
646:crown ethers
639:
615:
608:
597:
596:
497:Legal status
149:ChEMBL256105
68:Identifiers
60:Other names
50:
46:
42:
1454:Valinomycin
701:transport.
687:coccidiosis
531:antibiotics
467:ATCvet code
406:Appearance
367:Properties
187:100.037.398
129:CHEBI:27617
17:Monensin A
1495:Ionophores
1484:Categories
717:References
663:antibiotic
655:antiporter
605:antibiotic
535:ionophores
437:Solubility
397:Molar mass
268:906O0YJ6ZP
160:ChemSpider
96:3D model (
85:17090-79-8
75:CAS Number
1469:Ionomycin
1464:Nigericin
1350:0171-9335
1315:0006-3002
1261:0021-9525
675:membranes
642:ionophore
602:polyether
1459:Monensin
1185:15797327
1109:: 8–15.
1086:18375122
1001:22564680
848:12692092
799:23509771
773:: 1–14.
743:25 March
705:Toxicity
598:Monensin
526:Related
483:QP51BB03
473:QA16QA06
200:E number
1358:8980903
1323:8443249
1279:6682112
1270:2112386
1210:Bibcode
1050:2160275
1041:7148783
960:5453344
912:Bibcode
790:3586448
650:cations
624:et al.
579:what is
577: (
509::
485: (
481:)
475: (
454:benzene
446:acetone
442:ethanol
391:
235:PubChem
1356:
1348:
1321:
1313:
1277:
1267:
1259:
1183:
1084:
1048:
1038:
999:
958:
880:
876:–187.
846:
839:153145
836:
797:
787:
574:verify
571:
511:℞-only
351:SMILES
248:441145
223:D08228
169:389937
140:ChEMBL
31:Names
1442:TC 2B
699:Golgi
691:ester
622:Kishi
600:is a
316:InChI
205:E714
120:ChEBI
98:JSmol
1354:PMID
1346:ISSN
1319:PMID
1311:ISSN
1299:1166
1275:PMID
1257:ISSN
1181:PMID
1082:PMID
1046:PMID
1024:1031
997:PMID
983:1818
956:PMID
878:ISBN
844:PMID
795:PMID
771:2013
745:2024
681:Uses
259:UNII
214:KEGG
1303:doi
1265:PMC
1249:doi
1218:doi
1173:doi
1142:doi
1111:doi
1074:doi
1036:PMC
1028:doi
987:doi
948:doi
920:doi
908:871
874:185
834:PMC
826:doi
785:PMC
775:doi
488:WHO
478:WHO
285:EPA
238:CID
1486::
1396:.
1375:.
1352:.
1342:71
1340:.
1317:.
1309:.
1297:.
1273:.
1263:.
1255:.
1245:96
1243:.
1239:.
1216:.
1206:64
1204:.
1179:.
1169:20
1167:.
1136:.
1132:.
1107:16
1105:.
1080:.
1070:18
1044:.
1034:.
1022:.
1018:.
995:.
981:.
977:.
954:.
944:49
918:.
842:.
832:.
822:16
820:.
816:.
793:.
783:.
769:.
765:.
753:^
733:.
677:.
665:,
549:,
533:,
506:CA
491:)
452:,
448:,
444:,
389:11
385:62
381:36
49:,4
45:,3
41:(2
1444:)
1433:e
1426:t
1419:v
1402:.
1381:.
1360:.
1325:.
1305::
1281:.
1251::
1224:.
1220::
1212::
1187:.
1175::
1150:.
1144::
1138:6
1117:.
1113::
1088:.
1076::
1052:.
1030::
1003:.
989::
962:.
950::
926:.
922::
914::
886:.
850:.
828::
801:.
777::
747:.
569:N
387:O
383:H
379:C
287:)
283:(
100:)
51:S
47:R
43:S
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.