90:
249:
and methyl iodide, which in turn affords, through carbonylation, acetyl iodide. Acetyl iodide reacts with acetate salts or acetic acid to give the anhydride. Rhodium iodides and lithium salts are employed as catalysts. Because acetic anhydride hydrolyzes, the conversion is conducted under anhydrous
645:
377:
Zoeller, J. R.; Agreda, V. H.; Cook, S. L.; Lafferty, N. L.; Polichnowski, S. W.; Pond, D. M. (1992). "Eastman
Chemical Company Acetic Anhydride Process".
287:
435:
478:
213:
in a process that is similar to the
Monsanto acetic acid synthesis. Methyl acetate is used in place of methanol as a source of methyl iodide.
493:
585:
741:
89:
620:
615:
580:
408:
630:
625:
610:
513:
635:
330:
428:
312:
125:
756:
686:
325:
Hartwig, J. F. Organotransition Metal
Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010.
691:
73:
of 150–200 °C and gives a selectivity greater than 99%. It was developed in 1960 by the German chemical company
503:
575:
539:
444:
421:
109:
720:
605:
640:
191:
152:
595:
458:
379:
351:
715:
534:
508:
463:
129:
751:
529:
483:
343:
187:
113:
761:
710:
681:
671:
600:
409:
https://web.archive.org/web/20050412040850/http://www.uyseg.org/catalysis/ethacid/ethacid2.htm
326:
306:
183:
66:
17:
746:
570:
473:
468:
388:
359:
270:
202:
78:
549:
544:
246:
164:
148:
666:
565:
488:
242:
210:
47:
735:
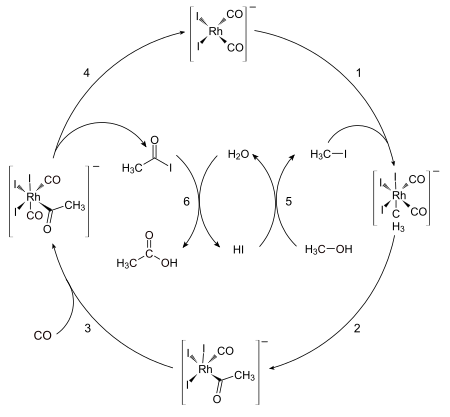
661:
392:
206:
172:
168:
156:
117:
39:
590:
176:
70:
35:
363:
274:
702:
498:
98:
413:
137:
62:
43:
51:
144:
140:
133:
102:
167:
involves two non-organometallic steps: conversion of methanol to
265:
Hosea Cheung, Robin S. Tanke, G. Paul
Torrence "Acetic Acid" in
74:
417:
151:
to form the six-coordinate dicarbonyl complex, which undergoes
250:
conditions in contrast to the
Monsanto acetic acid synthesis.
88:
55:
46:. The Monsanto process has largely been supplanted by the
646:
Arene complexes of univalent gallium, indium, and thallium
58:, which is more economical and environmentally friendly.
147:
complex . This five-coordinate complex then reacts with
344:"The Cativa Process for the Manufacture of Acetic Acid"
700:
654:
558:
522:
451:
128:species . This anion rapidly transforms, via the
81:in 1966, which introduced a new catalyst system.
34:is an industrial method for the manufacture of
267:Ullmann's Encyclopedia of Industrial Chemistry
186:with respect to methyl iodide and . Hence the
429:
8:
93:The catalytic cycle of the Monsanto process
530:Oxidative addition / reductive elimination
436:
422:
414:
294:. Archived from the original on 2014-08-11
198:Tennessee Eastman acetic anhydride process
288:"Production method: The Monsanto process"
27:Method for the manufacture of acetic acid
479:Polyhedral skeletal electron pair theory
258:
304:
7:
586:Transition metal fullerene complexes
190:of methyl iodide is proposed as the
621:Transition metal carbyne complexes
616:Transition metal carbene complexes
581:Transition metal indenyl complexes
182:The reaction has been shown to be
25:
631:Transition metal alkyne complexes
626:Transition metal alkene complexes
636:Transition-metal allyl complexes
143:, affording the pentacoordinate
611:Transition metal acyl complexes
18:Monsanto acetic acid synthesis
1:
269:, 2002, Wiley-VCH, Weinheim.
108:- (top of scheme). The first
393:10.1016/0920-5861(92)80188-S
54:-based process developed by
687:Shell higher olefin process
494:Dewar–Chatt–Duncanson model
292:www.greener-industry.org.uk
245:converts methyl acetate to
61:This process operates at a
778:
576:Cyclopentadienyl complexes
540:β-hydride elimination
514:Metal–ligand multiple bond
364:10.1595/003214000X44394105
171:and the hydrolysis of the
641:Transition metal carbides
311:: CS1 maint: unfit URL (
742:Organometallic chemistry
445:Organometallic chemistry
275:10.1002/14356007.a01_045
606:Half sandwich compounds
721:Bioinorganic chemistry
101:active species is the
94:
692:Ziegler–Natta process
596:Metal tetranorbornyls
342:Jones, J. H. (2000).
192:rate-determining step
179:and hydrogen iodide.
153:reductive elimination
136:group to an adjacent
92:
701:Related branches of
459:Crystal field theory
352:Platinum Metals Rev.
77:and improved by the
716:Inorganic chemistry
535:Migratory insertion
509:Agostic interaction
464:Ligand field theory
757:Chemical processes
601:Sandwich compounds
559:Types of compounds
484:Isolobal principle
188:oxidative addition
114:oxidative addition
95:
729:
728:
711:Organic chemistry
682:Olefin metathesis
672:Grignard reaction
571:Grignard reagents
16:(Redirected from
769:
677:Monsanto process
474:d electron count
469:18-electron rule
438:
431:
424:
415:
397:
396:
374:
368:
367:
348:
339:
333:
323:
317:
316:
310:
302:
300:
299:
284:
278:
263:
241:In this process
203:Acetic anhydride
79:Monsanto Company
56:BP Chemicals Ltd
32:Monsanto process
21:
777:
776:
772:
771:
770:
768:
767:
766:
732:
731:
730:
725:
696:
650:
566:Gilman reagents
554:
550:Carbometalation
545:Transmetalation
518:
447:
442:
405:
400:
380:Catalysis Today
376:
375:
371:
346:
341:
340:
336:
324:
320:
303:
297:
295:
286:
285:
281:
264:
260:
256:
247:lithium acetate
236:
232:
228:
224:
220:
205:is produced by
200:
165:catalytic cycle
162:
149:carbon monoxide
87:
85:Catalytic cycle
28:
23:
22:
15:
12:
11:
5:
775:
773:
765:
764:
759:
754:
749:
744:
734:
733:
727:
726:
724:
723:
718:
713:
707:
705:
698:
697:
695:
694:
689:
684:
679:
674:
669:
667:Cativa process
664:
658:
656:
652:
651:
649:
648:
643:
638:
633:
628:
623:
618:
613:
608:
603:
598:
593:
588:
583:
578:
573:
568:
562:
560:
556:
555:
553:
552:
547:
542:
537:
532:
526:
524:
520:
519:
517:
516:
511:
506:
501:
496:
491:
486:
481:
476:
471:
466:
461:
455:
453:
449:
448:
443:
441:
440:
433:
426:
418:
412:
411:
404:
403:External links
401:
399:
398:
369:
334:
318:
279:
257:
255:
252:
243:lithium iodide
239:
238:
234:
230:
226:
222:
218:
211:methyl acetate
199:
196:
160:
126:hexacoordinate
124:- to form the
110:organometallic
86:
83:
48:Cativa process
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
774:
763:
760:
758:
755:
753:
750:
748:
745:
743:
740:
739:
737:
722:
719:
717:
714:
712:
709:
708:
706:
704:
699:
693:
690:
688:
685:
683:
680:
678:
675:
673:
670:
668:
665:
663:
662:Carbonylation
660:
659:
657:
653:
647:
644:
642:
639:
637:
634:
632:
629:
627:
624:
622:
619:
617:
614:
612:
609:
607:
604:
602:
599:
597:
594:
592:
589:
587:
584:
582:
579:
577:
574:
572:
569:
567:
564:
563:
561:
557:
551:
548:
546:
543:
541:
538:
536:
533:
531:
528:
527:
525:
521:
515:
512:
510:
507:
505:
502:
500:
497:
495:
492:
490:
489:π backbonding
487:
485:
482:
480:
477:
475:
472:
470:
467:
465:
462:
460:
457:
456:
454:
450:
446:
439:
434:
432:
427:
425:
420:
419:
416:
410:
407:
406:
402:
394:
390:
386:
382:
381:
373:
370:
365:
361:
358:(3): 94–105.
357:
354:
353:
345:
338:
335:
332:
328:
322:
319:
314:
308:
293:
289:
283:
280:
276:
272:
268:
262:
259:
253:
251:
248:
244:
229:+ CO → (CH
216:
215:
214:
212:
208:
207:carbonylation
204:
197:
195:
193:
189:
185:
180:
178:
174:
173:acetyl iodide
170:
169:methyl iodide
166:
158:
157:acetyl iodide
154:
150:
146:
142:
139:
135:
131:
127:
123:
119:
118:methyl iodide
115:
111:
107:
104:
100:
99:catalytically
91:
84:
82:
80:
76:
72:
68:
64:
59:
57:
53:
49:
45:
41:
40:carbonylation
38:by catalytic
37:
33:
19:
676:
655:Applications
591:Metallocenes
387:(1): 73–91.
384:
378:
372:
355:
350:
337:
321:
296:. Retrieved
291:
282:
266:
261:
240:
201:
181:
163:C(O)I). The
121:
112:step is the
105:
96:
60:
50:, a similar
31:
29:
504:spin states
184:first-order
177:acetic acid
155:to release
71:temperature
36:acetic acid
736:Categories
452:Principles
331:189138953X
298:2014-08-27
254:References
752:Catalysis
703:chemistry
523:Reactions
499:Hapticity
130:migration
65:of 30–60
762:Monsanto
307:cite web
138:carbonyl
63:pressure
44:methanol
747:Rhodium
52:iridium
329:
145:acetyl
141:ligand
134:methyl
69:and a
347:(PDF)
132:of a
103:anion
327:ISBN
313:link
97:The
75:BASF
30:The
389:doi
360:doi
271:doi
233:CO)
209:of
175:to
159:(CH
122:cis
120:to
116:of
106:cis
67:atm
42:of
738::
385:13
383:.
356:44
349:.
309:}}
305:{{
290:.
225:CH
221:CO
217:CH
194:.
437:e
430:t
423:v
395:.
391::
366:.
362::
315:)
301:.
277:.
273::
237:O
235:2
231:3
227:3
223:2
219:3
161:3
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.