195:
187:
47:
156:
33:
40:
163:
149:
203:
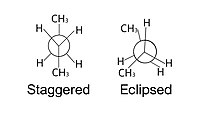
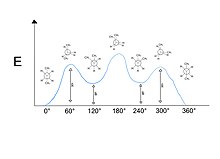
A staggered projection appears to have the surrounding species equidistant from each other. This kind of conformation tends to experience both anti and gauche interactions. Anti interactions refer to the molecules (usually of the same type) sitting exactly opposite of each other at 180° on the Newman
182:
Because of the free rotation around single bonds, there are various conformations for a single molecule. Up to six unique conformations may be drawn for any given chemical bond. Each conformation is drawn by rotation of either the proximal or distal atom 60 degrees. Of these six conformations, three
207:
An eclipsed projection appears to have the surrounding species almost on top of each other. In reality, these species are in line with each other, but are drawn slightly staggered to help format the projection onto paper. These types of conformations are generally higher in energy due to increased
204:
projection. Gauche interactions refer to molecules (also usually of the same type) being 60° from each other on a Newman projection. Anti interactions experience less steric strain than gauche interactions, but both experience less steric strain than the eclipsed conformation.
74:
is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman projection visualizes the
399:
198:
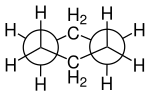
Butane molecule and all of its possible Newman conformations represented on a relative energy diagram. The diagram takes staggered and eclipsed conformations, as well as gauche and anti interactions into
183:
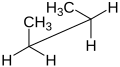
will be in a staggered conformation, while the other three will be in an eclipsed conformation. These six conformations can be represented in a relative energy diagram.
208:
bond strain. However, this strain can be somewhat lower if a hydrogen is eclipsed over a larger species, as opposed to two large species eclipsed over each other.
83:
from front to back, with the front atom represented by the intersection of three lines (a dot) and the back atom as a circle. The front atom is called
194:
430:
76:
186:
115:
46:
99:
340:
155:
190:
Butane molecule represented on a staggered and eclipsed Newman projection down a carbon-carbon bond
111:
103:
32:
310:
222:
217:
134:
39:
356:
302:
162:
348:
292:
227:
119:
148:
130:
123:
344:
92:
424:
80:
314:
331:
Newman, MS (1955). "A notation for the study of certain stereochemical problems".
255:
138:
107:
360:
306:
297:
280:
352:
23:
281:"Basic terminology of stereochemistry (IUPAC Recommendations 1996)"
193:
185:
16:
Method of representing the conformation of a single molecular bond
91:. This type of representation clearly illustrates the specific
118:
from an oblique angle, or a wedge-and-dash style, such as a
102:, who introduced it in 1952 as a partial replacement for
106:, which are unable to represent conformations and thus
110:
properly. This diagram style is an alternative to a
122:. These other styles can indicate the bonding and
98:This projection is named after American chemist
375:Record. Chem. Progr. (Kresge-Hooker Sci. Lib.)
129:A Newman projection can also be used to study
8:
296:
126:, but not as much conformational detail.
95:between the proximal and distal atoms.
239:
249:
247:
245:
243:
7:
394:
392:
390:
326:
324:
14:
26:in syn-clinal (-sc) conformation
161:
154:
147:
87:, while the back atom is called
45:
38:
31:
254:Valqui, Melissa (2021-07-26).
1:
333:Journal of Chemical Education
175:
172:
169:
63:
58:
53:
400:"3.4.1. Newman Projections"
449:
285:Pure and Applied Chemistry
21:
298:10.1351/pac199668122193
279:Moss, GP (1996-01-01).
200:
191:
197:
189:
100:Melvin Spencer Newman
404:Chemistry LibreTexts
256:"Newman Projections"
170:Bond-line structure
345:1955JChEd..32..344N
112:sawhorse projection
104:Fischer projections
223:Fischer projection
218:Haworth projection
201:
192:
173:Newman projection
135:chair conformation
116:carbon–carbon bond
353:10.1021/ed032p344
291:(12): 2193–2222.
180:
179:
72:Newman projection
68:
67:
438:
415:
414:
412:
411:
396:
385:
371:
365:
364:
328:
319:
318:
300:
276:
270:
269:
267:
266:
251:
228:Natta projection
165:
158:
151:
144:
143:
131:cyclic molecules
120:Natta projection
114:, which views a
49:
42:
35:
19:
18:
448:
447:
441:
440:
439:
437:
436:
435:
431:Stereochemistry
421:
420:
419:
418:
409:
407:
398:
397:
388:
372:
368:
330:
329:
322:
278:
277:
273:
264:
262:
253:
252:
241:
236:
214:
124:stereochemistry
60:
55:
17:
12:
11:
5:
446:
445:
442:
434:
433:
423:
422:
417:
416:
386:
366:
320:
271:
238:
237:
235:
232:
231:
230:
225:
220:
213:
210:
178:
177:
174:
171:
167:
166:
159:
152:
133:, such as the
93:dihedral angle
66:
65:
62:
57:
51:
50:
43:
36:
28:
27:
15:
13:
10:
9:
6:
4:
3:
2:
444:
443:
432:
429:
428:
426:
405:
401:
395:
393:
391:
387:
384:
380:
378:
370:
367:
362:
358:
354:
350:
346:
342:
338:
334:
327:
325:
321:
316:
312:
308:
304:
299:
294:
290:
286:
282:
275:
272:
261:
257:
250:
248:
246:
244:
240:
233:
229:
226:
224:
221:
219:
216:
215:
211:
209:
205:
196:
188:
184:
176:3D structure
168:
164:
160:
157:
153:
150:
146:
145:
142:
140:
136:
132:
127:
125:
121:
117:
113:
109:
105:
101:
96:
94:
90:
86:
82:
81:chemical bond
78:
73:
64:3D structure
52:
48:
44:
41:
37:
34:
30:
29:
25:
20:
408:. Retrieved
406:. 2015-06-16
403:
382:
376:
374:
373:Newman, MS.
369:
336:
332:
288:
284:
274:
263:. Retrieved
259:
206:
202:
181:
128:
97:
88:
84:
77:conformation
71:
69:
22:Molecule of
139:cyclohexane
61:projection
56:projection
410:2022-11-18
339:(7): 344.
265:2022-11-18
234:References
108:conformers
361:0021-9584
307:1365-3075
425:Category
315:98272391
260:ChemTalk
212:See also
199:account.
85:proximal
54:Sawhorse
341:Bibcode
359:
313:
305:
89:distal
59:Newman
24:butane
383:, 111
311:S2CID
79:of a
377:1952
357:ISSN
303:ISSN
349:doi
293:doi
137:of
427::
402:.
389:^
381:13
355:.
347:.
337:32
335:.
323:^
309:.
301:.
289:68
287:.
283:.
258:.
242:^
141::
70:A
413:.
379:,
363:.
351::
343::
317:.
295::
268:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.