570:
152:
617:
425:
260:
217:
360:
83:, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell
236:, through the activity of a GalNAc transferase enzyme. This precursor is necessary so that the sugar can be transported to where it will be added to the protein. The specific residue onto which GalNAc will be attached is not defined, because there are numerous enzymes that can add the sugar and each one will favour different residues. However, there are often proline (Pro) residues near the threonine or serine.
385:. O-GlcNAcylation and phosphorylation can occur on the same threonine and serine residues, suggesting a complex relationship between these modifications that can affect many functions of the cell. The modification affects processes like the cells response to cellular stress, the cell cycle, protein stability and protein turnover. It may be implicated in neurodegenerative diseases like
441:-mannose donor molecule onto the serine or threonine residue of a protein. Most other O-glycosylation processes use a sugar nucleotide as a donor molecule. A further difference from other O-glycosylations is that the process is initiated in the endoplasmic reticulum of the cell, rather than the Golgi apparatus. However, further addition of sugars occurs in the Golgi.
370:-GlcNAcylation differs from other O-glycosylation processes because there are usually no sugars added onto the core structure and because the sugar can be attached or removed from a protein several times. This addition and removal occurs in cycles and is performed by two very specific enzymes. O-GlcNAc is added by
639:
Because both galactose and glucose sugars can be added to the ceramide lipid, we have two groups of glycosphingolipids. Galactosphingolipids are generally very simple in structure and the core galactose is not usually modified. Glucosphingolipids, however, are often modified and can become a lot more
239:
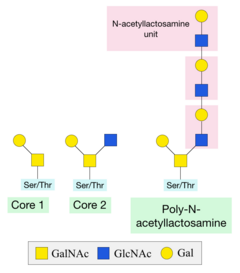
Once this initial sugar has been added, other glycosyltransferases can catalyse the addition of additional sugars. Two of the most common structures formed are Core 1 and Core 2. Core 1 is formed by the addition of a galactose sugar onto the initial GalNAc. Core 2 consists of a Core 1 structure with
643:
Biosynthesis of galacto- and glucosphingolipids occurs differently. Glucose is added onto ceramide from its precursor in the endoplasmic reticulum, before further modifications occur in the Golgi apparatus. Galactose, on the other hand, is added to ceramide already in the Golgi apparatus, where the
550:
Similarly to O-fucosylation, O-glucosylation is an unusual O-linked modification as it occurs in the endoplasmic reticulum, catalysed by O-glucosyltransferases, and also requires a defined sequence in order to be added to the protein. O-glucose is often attached to serine residues between the first
541:
is an important protein in development, with several EGF domains that are O-fucosylated. Changes in the elaboration of the core fucose determine what interactions the protein can form, and therefore which genes will be transcribed during development. O-fucosylation might also play a role in protein
311:
are a group of heavily O-glycosylated proteins that line the gastrointestinal and respiratory tracts to protect these regions from infection. Mucins are negatively charged, which allows them to interact with water and prevent it from evaporating. This is important in their protective function as it
356:-GalNAc modifications which usually occur on proteins that will be secreted. O-GlcNAc modifications were only recently discovered, but the number of proteins with known O-GlcNAc modifications is increasing rapidly. It is the first example of glycosylation that does not occur on secretory proteins.
255:
and play a key role in the immune system. Addition of fucose sugars by fucosyltransferases forms Lewis epitopes and the scaffold for blood group determinants. Addition of a fucose alone creates the H-antigen, present in people with blood type O. By adding a galactose onto this structure, the
304:
on their cell surface to allow this interaction to occur. P-selectin glycoprotein ligand-1 (PSGL-1) is such a ligand, and contains a lot of O-glycans that are necessary for its function. O-glycans near the membrane maintain the elongated structure and a terminal sLe epitope is necessary for
464:
can be added to this structure in a complex modification that forms a long sugar chain. This is required to stabilise the interaction between α-dystroglycan and the extracellular basement membrane. Without these modifications, the glycoprotein cannot anchor the cell which leads to
699:. O-glycan structures, and especially the terminal Lewis epitopes, are important in allowing tumor cells to invade new tissues during metastasis. Understanding these changes in O-glycosylation of cancer cells can lead to new diagnostic approaches and therapeutic opportunities.
408:
of cancer cells to favour their growth. Because both O-GlcNAcylation and phosphorylation can affect specific residues and therefore both have important functions in regulating signalling pathways, both of these processes provide interesting targets for cancer therapy.
190:-GalNAc structure can be modified by the addition of other sugars, or other compounds such as methyl and acetyl groups. These modifications produce 8 core structures known to date. Different cells have different enzymes that can add further sugars, known as
293:, where they help increase rigidity of the region close to the membrane so that the protein extends away from the surface. For example, the low-density lipoprotein receptor (LDL) is projected from the cell surface by a region rigidified by O-glycans.
428:
O-Mannose sugars attached to serine and threonine residues on α-dystroglycan separate the two domains of the protein. Addition of
Ribitol-P, xylose and glucuronic acid forms a long sugar that can stabilise the interaction with the basement
607:
attaches to a serine or threonine residue through GalNAc, and is extended with two galactose sugars, followed by repeating units of glucuronic acid (GlcA) and GlcNAc. Type II keratan sulphate is especially common in cartilage.
588:(ECM), and are important for the strength and flexibility of cartilage and tendons. Absence of proteoglycans is associated with heart and respiratory failure, defects in skeletal development and increased tumor metastasis.
1570:"Studies on the glycosylation of hydroxylysine residues during collagen biosynthesis and the subcellular localization of collagen galactosyltransferase and collagen glucosyltransferase in tendon and cartilage cells"
526:
Several different enzymes catalyse the elongation of the core fucose, meaning that different sugars can be added to the initial fucose on the protein. Along with O-glucosylation, O-fucosylation is mainly found on
503:
While this O-galactosylation is necessary for correct function in all collagens, it is especially common in collagen types IV and V. In some cases, a glucose sugar can be added to the core galactose.
1871:"Site-specific O-glucosylation of the epidermal growth factor-like (EGF) repeats of notch: efficiency of glycosylation is affected by proper folding and amino acid sequence of individual EGF repeats"
692:
may be affected by O-glycosylation. Tau, the protein that accumulates to cause neurodegeneration in
Alzheimer's, contains O-GlcNAc modifications which may be implicated in disease progression.
515:
sugars to serine and threonine residues is an unusual form of O-glycosylation that occurs in the endoplasmic reticulum and is catalysed by two fucosyltransferases. These were discovered in
686:
contain highly O-glycosylated regions between individual domains to maintain their structure, allow interactions with foreign antigens and protect the region from proteolytic cleavage.
584:(GAGs), attached to the oxygen of serine and threonine residues. GAGs consist of long chains of repeating sugar units. Proteoglycans are usually found on the cell surface and in the
500:
to the hydroxyl group is initiated in the endoplasmic reticulum, but occurs predominantly in the Golgi apparatus and only on hydroxylysine residues in a specific sequence.
573:
Structures of heparan sulphate and keratan sulphate, formed by the addition of xylose or GalNAc sugars, respectively, onto serine and threonine residues of proteins.
2155:
263:
PSGL-1 has several O-glycans to extend the ligand away from the cell surface. An sLe epitope allows interactions with the receptor for leukocyte localisation.
87:
and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including
378:(OGA). Because there are only two enzymes that affect this specific modification, they are very tightly regulated and depend on a lot of other factors.
591:
Different types of proteoglycans exist, depending on the sugar that is linked to the oxygen atom of the residue in the protein. For example, the GAG
2636:
452:. O-Man sugars separate two domains of the protein, required to connect the extracellular and intracellular regions to anchor the cell in position.
448:, however it occurs in all domains of life; eukaryotes, (eu)bacteria and archae(bacteri)a. The best characterised O-mannosylated human protein is
535:
residues in the protein sequence. Once the core O-fucose has been added, it is often elongated by addition of GlcNAc, galactose and sialic acid.
632:, which are important for the localisation of receptors in membranes. Incorrect breakdown of these lipids leads to a group of diseases known as
962:"Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation"
1985:
1145:
2019:
1673:"The O-linked fucose glycosylation pathway. Evidence for protein-specific elongation of o-linked fucose in Chinese hamster ovary cells"
569:
223:-acetylgalactosamine (GalNAc) can be added to the H-antigen to form the A-antigen. Galactose (Gal) can be added to form the B-antigen.
1053:
352:(O-GlcNAc) to serine and threonine residues usually occurs on cytoplasmic and nuclear proteins that remain in the cell, compared to
603:-acetyllactosamine repeating sugar units added onto the xylose. This process is unusual and requires specific xylosyltransferases.
312:
lubricates the tracts so bacteria cannot bind and infect the body. Changes in mucins are important in numerous diseases, including
616:
151:
2150:
660:. Glycogenin is a glycosyltransferase that initiates the conversion of glucose to glycogen, present in muscle and liver cells.
60:
248:-acetyllactosamine structure can be formed by the alternating addition of GlcNAc and galactose sugars onto the GalNAc sugar.
2553:
466:
296:
In order for leukocytes of the immune system to move into infected cells, they have to interact with these cells through
256:
B-antigen of blood group B is created. Alternatively, adding a GalNAc sugar will create the A-antigen for blood group A.
854:"A general protein O-glycosylation system within the Burkholderia cepacia complex is involved in motility and virulence"
1822:"Fringe benefits: functional and structural impacts of O-glycosylation on the extracellular domain of Notch receptors"
555:
VII and IX. O-glucosylation also appears to be necessary for the proper folding of EGF domains in the Notch protein.
1619:"A novel functional role of collagen glycosylation: interaction with the endocytic collagen receptor uparap/ENDO180"
424:
2060:
668:
All forms of O-glycosylation are abundant throughout the body and play important roles in many cellular functions.
317:
1088:"Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds"
381:
Because O-GlcNAc can be added and removed, it is known as a dynamic modification and has a lot of similarities to
320:. Absence of O-glycans on mucin proteins changes their 3D shape dramatically and often prevents correct function.
259:
2424:
2346:
2101:
675:, and allow the generation of an immune response if we detect foreign organs. Understanding them is important in
401:
1287:"Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease"
2444:
2165:
2106:
2012:
216:
1714:"Protein O-fucosylation in Plasmodium falciparum ensures efficient infection of mosquito and vertebrate hosts"
496:. Because of this addition of an oxygen, hydroxylysine can then be modified by O-glycosylation. Addition of a
2439:
767:
Van den Steen P, Rudd PM, Dwek RA, Opdenakker G (1998). "Concepts and principles of O-linked glycosylation".
531:(EGF) domains found in proteins. O-fucosylation on EGF domains occurs between the second and third conserved
363:
O-GlcNAc is added to the protein by O-GlcNAc transferase and is removed by O-GlcNAcase in a continuous cycle.
2538:
2287:
2282:
2268:
2243:
809:
Hounsell EF, Davies MJ, Renouf DV (February 1996). "O-linked protein glycosylation structure and function".
528:
297:
2573:
2507:
2388:
2093:
713:
359:
2578:
2543:
2499:
2263:
2122:
1617:
Jürgensen HJ, Madsen DH, Ingvarsen S, Melander MC, Gårdsvoll H, Patthy L, et al. (September 2011).
233:
168:
68:
2517:
2222:
2198:
2160:
1725:
1524:
1238:
914:
585:
371:
191:
2462:
2081:
2005:
656:, rather than on serine or threonine residues, is the addition of glucose to a tyrosine residue in
346:
199:
2585:
2258:
2227:
2203:
2190:
1225:
Lazarus MB, Jiang J, Kapuria V, Bhuiyan T, Janetzko J, Zandberg WF, et al. (December 2013).
883:
834:
629:
581:
1441:
Lommel M, Strahl S (August 2009). "Protein O-mannosylation: conserved from bacteria to humans".
1712:
Lopaticki S, Yang AS, John A, Scott NE, Lingford JP, O'Neill MT, et al. (September 2017).
901:
Vik A, Aas FE, Anonsen JH, Bilsborough S, Schneider A, Egge-Jacobsen W, Koomey M (March 2009).
2457:
2253:
2097:
2055:
2050:
1981:
1958:
1902:
1851:
1802:
1751:
1694:
1650:
1599:
1550:
1493:
1458:
1416:
1365:
1316:
1264:
1207:
1141:
1109:
1049:
993:
942:
875:
852:
Lithgow KV, Scott NE, Iwashkiw JA, Thomson EL, Foster LJ, Feldman MF, Dennis JJ (April 2014).
826:
784:
676:
2480:
1948:
1938:
1892:
1882:
1841:
1833:
1792:
1782:
1769:
Khurana S, Coffey MJ, John A, Uboldi AD, Huynh MH, Stewart RJ, et al. (February 2019).
1741:
1733:
1684:
1640:
1630:
1589:
1581:
1540:
1532:
1485:
1450:
1406:
1396:
1355:
1347:
1306:
1298:
1254:
1246:
1197:
1189:
1099:
983:
973:
932:
922:
865:
818:
776:
633:
628:
lipids in a different form of O-glycosylation, as it does not occur on proteins. This forms
604:
592:
552:
903:"Broad spectrum O-linked protein glycosylation in the human pathogen Neisseria gonorrhoeae"
2434:
2316:
1302:
683:
461:
382:
175:
72:
1729:
1528:
1242:
960:
Iwashkiw JA, Seper A, Weber BS, Scott NE, Vinogradov E, Stratilo C, et al. (2012).
918:
2511:
2429:
2419:
2368:
2356:
2329:
1953:
1926:
1897:
1870:
1846:
1821:
1797:
1770:
1746:
1713:
1645:
1618:
1594:
1569:
1545:
1513:"Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE"
1512:
1511:
Inamori K, Yoshida-Moriguchi T, Hara Y, Anderson ME, Yu L, Campbell KP (January 2012).
1411:
1384:
1360:
1335:
1311:
1286:
1259:
1226:
1202:
1177:
988:
961:
937:
902:
689:
538:
390:
386:
96:
1489:
279:
circulation during an immune response, fertilisation, and protection against invading
2630:
2383:
2070:
708:
636:, which are often characterised by neurodegeneration and developmental disabilities.
577:
564:
493:
290:
1997:
1476:
Strahl-Bolsinger S, Gentzsch M, Tanner W (January 1999). "Protein O-mannosylation".
887:
838:
2363:
2333:
2138:
2110:
1048:(3rd ed.). Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
672:
449:
183:
213:. These sugars can also be modified by the addition of sulfates or acetyl groups.
1351:
978:
194:, and structures therefore change from cell to cell. Common sugars added include
2489:
2351:
375:
210:
1737:
907:
Proceedings of the
National Academy of Sciences of the United States of America
2590:
2312:
2233:
2214:
2146:
2089:
2032:
2028:
1837:
1104:
1087:
780:
657:
405:
108:
100:
84:
80:
1787:
1689:
1672:
2485:
2292:
2036:
1887:
1869:
Takeuchi H, Kantharia J, Sethi MK, Bakker H, Haltiwanger RS (October 2012).
1635:
1536:
1454:
1401:
1250:
927:
644:
galactosphingolipid formed is often sulfated by addition of sulfate groups.
497:
276:
195:
76:
64:
49:
1962:
1906:
1855:
1806:
1755:
1654:
1554:
1462:
1420:
1369:
1320:
1268:
1211:
1113:
997:
946:
879:
1698:
1603:
1497:
830:
788:
653:
625:
532:
489:
394:
340:
280:
92:
2612:
1193:
17:
2548:
2533:
1943:
822:
453:
434:
301:
252:
179:
104:
1585:
870:
853:
551:
and second conserved cysteine residues of EGF domains, for example in
696:
596:
512:
485:
457:
313:
206:
88:
45:
41:
1165:. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
624:
Galactose or glucose sugars can be attached to a hydroxyl group of
615:
580:
consist of a protein with one or more sugar side chains, known as
568:
445:
444:
Until recently, it was believed that the process is restricted to
423:
358:
308:
275:-GalNAc sugars are important in a variety of processes, including
258:
215:
150:
37:
178:, after the protein has been folded. The process is performed by
1336:"Protein O-GlcNAcylation in diabetes and diabetic complications"
2001:
620:
Structure of ceramide, galactosylceramide and glucosylceramide.
1227:"HCF-1 is cleaved in the active site of O-GlcNAc transferase"
251:
Terminal sugars on O-glycans are important in recognition by
186:(GALNTs), of which there are 20 different types. The initial
1178:"Protein O-GlcNAcylation: emerging mechanisms and functions"
99:. O-glycosylation occurs in all domains of life, including
232:
GalNAc is added onto a serine or threonine residue from a
1285:
Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O (2011).
652:
One of the first and only examples of O-glycosylation on
1140:(3rd ed.). New York: Oxford University Press Inc.
63:
that occurs after the protein has been synthesised. In
2230:(amino acid→pyruvate, acetyl CoA, or TCA intermediate)
1478:
Biochimica et
Biophysica Acta (BBA) - General Subjects
769:
Critical
Reviews in Biochemistry and Molecular Biology
469:(CMD), characterised by severe brain malformations.
404:, which is defined as the change that occurs in the
2566:
2526:
2498:
2479:
2408:
2301:
2281:
2242:
2213:
2189:
2180:
2137:
2121:
2080:
2069:
2043:
695:Changes in O-glycosylation are extremely common in
595:is attached to a protein serine residue through a
492:, which often have a hydroxyl group added to form
1568:Harwood R, Grant ME, Jackson DS (November 1975).
159:-GalNAc core structures; Core 1, Core 2 and poly-
27:Molecular process that occurs within living cells
1671:Moloney DJ, Lin AI, Haltiwanger RS (July 1997).
174:(GalNAc) to a serine or threonine occurs in the
1666:
1664:
1385:"O-GlcNAcylation: The Sweet Side of the Cancer"
804:
802:
800:
798:
1436:
1434:
1432:
1430:
599:sugar. The structure is extended with several
400:Additionally, O-GlcNAcylation can enhance the
2013:
1920:
1918:
1916:
1131:
1129:
1127:
1125:
1123:
1039:
1037:
1035:
1033:
1031:
1029:
1027:
762:
760:
758:
756:
754:
752:
8:
1280:
1278:
1081:
1079:
1077:
1075:
1073:
1071:
1069:
1067:
1065:
1025:
1023:
1021:
1019:
1017:
1015:
1013:
1011:
1009:
1007:
750:
748:
746:
744:
742:
740:
738:
736:
734:
732:
671:Lewis epitopes are important in determining
2623:-Glycosites in Eukaryotic Protein Sequences
1383:de Queiroz RM, Carvalho E, Dias WB (2014).
433:O-mannosylation involves the transfer of a
2495:
2412:
2305:
2298:
2210:
2186:
2077:
2020:
2006:
1998:
244:-acetylglucosamine (GlcNAc) sugar. A poly-
1952:
1942:
1896:
1886:
1845:
1796:
1786:
1745:
1688:
1644:
1634:
1593:
1544:
1410:
1400:
1359:
1310:
1258:
1201:
1103:
987:
977:
936:
926:
869:
1820:Rana NA, Haltiwanger RS (October 2011).
1771:"Toxoplasma gondii tachyzoite infection"
2615:: In silico Platform for Prediction of
728:
1927:"Glycosaminoglycans and Proteoglycans"
1182:Nature Reviews. Molecular Cell Biology
289:-GalNAc sugars are common on membrane
1826:Current Opinion in Structural Biology
1303:10.1146/annurev-biochem-060608-102511
393:and has been found to play a role in
7:
2130:Electron acceptors other than oxygen
1980:. Academic Press. pp. 161–181.
1925:Pomin VH, Mulloy B (February 2018).
1875:The Journal of Biological Chemistry
1775:The Journal of Biological Chemistry
1677:The Journal of Biological Chemistry
1623:The Journal of Biological Chemistry
25:
484:O-galactose is commonly found on
1136:E Taylor M, Drickamer K (2011).
305:interactions with the receptor.
2637:Post-translational modification
2151:Substrate-level phosphorylation
61:post-translational modification
163:-acetyllactosamine structures.
1:
2554:Reverse cholesterol transport
1490:10.1016/S0304-4165(98)00131-7
1334:Ma J, Hart GW (August 2013).
1291:Annual Review of Biochemistry
467:congenital muscular dystrophy
52:(Thr) residues in a protein.
2206:(protein→peptide→amino acid)
1352:10.1586/14789450.2013.820536
1176:Yang X, Qian K (July 2017).
1138:Introduction to Glycobiology
979:10.1371/journal.ppat.1002758
1340:Expert Review of Proteomics
2653:
2591:Phospagen system (ATP-PCr)
2061:Primary nutritional groups
1738:10.1038/s41467-017-00571-y
1163:Essentials of Glycobiology
1046:Essentials of glycobiology
562:
338:
318:inflammatory bowel disease
2453:
2425:Anoxygenic photosynthesis
2415:
2379:
2347:Pentose phosphate pathway
2342:
2325:
2308:
2102:Oxidative phosphorylation
1838:10.1016/j.sbi.2011.08.008
781:10.1080/10409239891204198
2445:Entner-Doudoroff pathway
2107:electron transport chain
2094:Pyruvate decarboxylation
1788:10.1074/jbc.RA118.005357
1690:10.1074/jbc.272.30.19046
542:breakdown in the liver.
113:Burkholderia cenocepacia
75:and occasionally in the
2539:Sphingolipid metabolism
2440:DeLey-Doudoroff pathway
2288:carbohydrate catabolism
2283:Carbohydrate metabolism
2269:Purine nucleotide cycle
1888:10.1074/jbc.M112.401315
1636:10.1074/jbc.M111.266692
1574:The Biochemical Journal
1537:10.1126/science.1214115
1402:10.3389/fonc.2014.00132
1251:10.1126/science.1243990
1105:10.1093/glycob/12.4.43R
1086:Spiro RG (April 2002).
928:10.1073/pnas.0809504106
529:epidermal growth factor
121:Acinetobacter baumannii
36:is the attachment of a
2508:Fatty acid degradation
2228:Amino acid degradation
858:Molecular Microbiology
811:Glycoconjugate Journal
621:
574:
430:
374:(OGT) and removed by
364:
264:
224:
164:
143:-acetylgalactosamine (
2544:Eicosanoid metabolism
2500:Fatty acid metabolism
2264:Pyrimidine metabolism
2123:Anaerobic respiration
1718:Nature Communications
1455:10.1093/glycob/cwp066
1389:Frontiers in Oncology
717:-linked glycosylation
664:Clinical significance
619:
572:
517:Plasmodium falciparum
427:
362:
300:. Leukocytes express
262:
219:
154:
117:Neisseria gonorrhoeae
69:endoplasmic reticulum
34:-linked glycosylation
2518:Fatty acid synthesis
2223:Amino acid synthesis
586:extracellular matrix
372:O-GlcNAc transferase
331:-acetylglucosamine (
192:glycosyltransferases
172:-acetylgalactosamine
2082:Aerobic respiration
1730:2017NatCo...8..561L
1529:2012Sci...335...93I
1243:2013Sci...342.1235L
1194:10.1038/nrm.2017.22
919:2009PNAS..106.4447V
111:bacteria including
67:, it occurs in the
2586:Ethanol metabolism
2534:Steroid metabolism
2259:Nucleotide salvage
2191:Protein metabolism
1978:Human Biochemistry
1976:Litwack G (2017).
1944:10.3390/ph11010027
823:10.1007/bf01049675
630:glycosphingolipids
622:
582:glycosaminoglycans
575:
431:
365:
350:-acetylglucosamine
265:
234:precursor molecule
225:
203:-acetylglucosamine
165:
2603:
2602:
2599:
2598:
2562:
2561:
2475:
2474:
2471:
2470:
2458:Xylose metabolism
2404:
2403:
2277:
2276:
2254:Purine metabolism
2199:Protein synthesis
2176:
2175:
2098:Citric acid cycle
2056:Metabolic network
2051:Metabolic pathway
1987:978-0-12-383864-3
1586:10.1042/bj1520291
1147:978-0-19-956911-3
871:10.1111/mmi.12540
682:Hinge regions of
677:organ transplants
546:O-Glucose (O-Glc)
521:Toxoplasma gondii
16:(Redirected from
2644:
2574:Metal metabolism
2496:
2481:Lipid metabolism
2413:
2306:
2299:
2211:
2187:
2113:
2078:
2022:
2015:
2008:
1999:
1992:
1991:
1973:
1967:
1966:
1956:
1946:
1922:
1911:
1910:
1900:
1890:
1881:(41): 33934–44.
1866:
1860:
1859:
1849:
1817:
1811:
1810:
1800:
1790:
1781:(5): 1541–1553.
1766:
1760:
1759:
1749:
1709:
1703:
1702:
1692:
1683:(30): 19046–50.
1668:
1659:
1658:
1648:
1638:
1629:(37): 32736–48.
1614:
1608:
1607:
1597:
1565:
1559:
1558:
1548:
1508:
1502:
1501:
1473:
1467:
1466:
1438:
1425:
1424:
1414:
1404:
1380:
1374:
1373:
1363:
1331:
1325:
1324:
1314:
1282:
1273:
1272:
1262:
1237:(6163): 1235–9.
1222:
1216:
1215:
1205:
1173:
1167:
1166:
1161:Varki A (1999).
1158:
1152:
1151:
1133:
1118:
1117:
1107:
1083:
1060:
1059:
1044:Varki A (2015).
1041:
1002:
1001:
991:
981:
957:
951:
950:
940:
930:
898:
892:
891:
873:
849:
843:
842:
806:
793:
792:
764:
634:sphingolipidoses
605:Keratan sulphate
593:heparan sulphate
553:clotting factors
507:O-Fucose (O-Fuc)
437:from a dolichol-
182:known as GalNAc
127:Common types of
107:and a number of
40:molecule to the
21:
2652:
2651:
2647:
2646:
2645:
2643:
2642:
2641:
2627:
2626:
2609:
2604:
2595:
2579:Iron metabolism
2558:
2522:
2483:
2467:
2449:
2435:Carbon fixation
2400:
2375:
2338:
2321:
2317:Gluconeogenesis
2290:
2285:
2273:
2245:
2238:
2209:
2182:
2172:
2133:
2117:
2105:
2072:
2065:
2039:
2026:
1996:
1995:
1988:
1975:
1974:
1970:
1931:Pharmaceuticals
1924:
1923:
1914:
1868:
1867:
1863:
1819:
1818:
1814:
1768:
1767:
1763:
1711:
1710:
1706:
1670:
1669:
1662:
1616:
1615:
1611:
1567:
1566:
1562:
1510:
1509:
1505:
1475:
1474:
1470:
1440:
1439:
1428:
1382:
1381:
1377:
1333:
1332:
1328:
1284:
1283:
1276:
1224:
1223:
1219:
1175:
1174:
1170:
1160:
1159:
1155:
1148:
1135:
1134:
1121:
1085:
1084:
1063:
1056:
1043:
1042:
1005:
972:(6): e1002758.
959:
958:
954:
913:(11): 4447–52.
900:
899:
895:
851:
850:
846:
808:
807:
796:
766:
765:
730:
725:
705:
684:immunoglobulins
666:
650:
614:
567:
561:
548:
509:
482:
462:glucuronic acid
422:
389:and late-onset
383:phosphorylation
343:
337:
270:
230:
176:Golgi apparatus
149:
133:
73:Golgi apparatus
28:
23:
22:
15:
12:
11:
5:
2650:
2648:
2640:
2639:
2629:
2628:
2625:
2624:
2608:
2607:External links
2605:
2601:
2600:
2597:
2596:
2594:
2593:
2588:
2583:
2582:
2581:
2570:
2568:
2564:
2563:
2560:
2559:
2557:
2556:
2551:
2546:
2541:
2536:
2530:
2528:
2524:
2523:
2521:
2520:
2515:
2512:Beta oxidation
2504:
2502:
2493:
2477:
2476:
2473:
2472:
2469:
2468:
2466:
2465:
2460:
2454:
2451:
2450:
2448:
2447:
2442:
2437:
2432:
2430:Chemosynthesis
2427:
2422:
2420:Photosynthesis
2416:
2410:
2406:
2405:
2402:
2401:
2399:
2398:
2397:
2396:
2391:
2380:
2377:
2376:
2374:
2373:
2372:
2371:
2369:Leloir pathway
2361:
2360:
2359:
2357:Polyol pathway
2349:
2343:
2340:
2339:
2337:
2336:
2330:Glycogenolysis
2326:
2323:
2322:
2320:
2319:
2309:
2303:
2296:
2279:
2278:
2275:
2274:
2272:
2271:
2266:
2261:
2256:
2250:
2248:
2240:
2239:
2237:
2236:
2231:
2225:
2219:
2217:
2208:
2207:
2201:
2195:
2193:
2184:
2178:
2177:
2174:
2173:
2171:
2170:
2169:
2168:
2163:
2158:
2143:
2141:
2135:
2134:
2132:
2131:
2127:
2125:
2119:
2118:
2116:
2115:
2086:
2084:
2075:
2067:
2066:
2064:
2063:
2058:
2053:
2047:
2045:
2041:
2040:
2027:
2025:
2024:
2017:
2010:
2002:
1994:
1993:
1986:
1968:
1912:
1861:
1812:
1761:
1704:
1660:
1609:
1580:(2): 291–302.
1560:
1523:(6064): 93–6.
1503:
1484:(2): 297–307.
1468:
1426:
1375:
1326:
1274:
1217:
1188:(7): 452–465.
1168:
1153:
1146:
1119:
1098:(4): 43R–56R.
1061:
1054:
1003:
966:PLOS Pathogens
952:
893:
844:
794:
775:(3): 151–208.
727:
726:
724:
721:
720:
719:
711:
704:
701:
665:
662:
649:
646:
613:
610:
563:Main article:
560:
557:
547:
544:
508:
505:
481:
471:
450:α-dystroglycan
421:
411:
402:Warburg Effect
339:Main article:
336:
322:
269:
266:
240:an additional
229:
226:
148:
134:
132:
131:-glycosylation
125:
57:-glycosylation
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2649:
2638:
2635:
2634:
2632:
2622:
2618:
2614:
2611:
2610:
2606:
2592:
2589:
2587:
2584:
2580:
2577:
2576:
2575:
2572:
2571:
2569:
2565:
2555:
2552:
2550:
2547:
2545:
2542:
2540:
2537:
2535:
2532:
2531:
2529:
2525:
2519:
2516:
2513:
2509:
2506:
2505:
2503:
2501:
2497:
2494:
2491:
2487:
2482:
2478:
2464:
2463:Radiotrophism
2461:
2459:
2456:
2455:
2452:
2446:
2443:
2441:
2438:
2436:
2433:
2431:
2428:
2426:
2423:
2421:
2418:
2417:
2414:
2411:
2407:
2395:
2392:
2390:
2387:
2386:
2385:
2384:Glycosylation
2382:
2381:
2378:
2370:
2367:
2366:
2365:
2362:
2358:
2355:
2354:
2353:
2350:
2348:
2345:
2344:
2341:
2335:
2331:
2328:
2327:
2324:
2318:
2314:
2311:
2310:
2307:
2304:
2300:
2297:
2294:
2289:
2284:
2280:
2270:
2267:
2265:
2262:
2260:
2257:
2255:
2252:
2251:
2249:
2247:
2241:
2235:
2232:
2229:
2226:
2224:
2221:
2220:
2218:
2216:
2212:
2205:
2202:
2200:
2197:
2196:
2194:
2192:
2188:
2185:
2179:
2167:
2164:
2162:
2159:
2157:
2154:
2153:
2152:
2148:
2145:
2144:
2142:
2140:
2136:
2129:
2128:
2126:
2124:
2120:
2112:
2108:
2103:
2099:
2095:
2091:
2088:
2087:
2085:
2083:
2079:
2076:
2074:
2068:
2062:
2059:
2057:
2054:
2052:
2049:
2048:
2046:
2042:
2038:
2034:
2030:
2023:
2018:
2016:
2011:
2009:
2004:
2003:
2000:
1989:
1983:
1979:
1972:
1969:
1964:
1960:
1955:
1950:
1945:
1940:
1936:
1932:
1928:
1921:
1919:
1917:
1913:
1908:
1904:
1899:
1894:
1889:
1884:
1880:
1876:
1872:
1865:
1862:
1857:
1853:
1848:
1843:
1839:
1835:
1831:
1827:
1823:
1816:
1813:
1808:
1804:
1799:
1794:
1789:
1784:
1780:
1776:
1772:
1765:
1762:
1757:
1753:
1748:
1743:
1739:
1735:
1731:
1727:
1723:
1719:
1715:
1708:
1705:
1700:
1696:
1691:
1686:
1682:
1678:
1674:
1667:
1665:
1661:
1656:
1652:
1647:
1642:
1637:
1632:
1628:
1624:
1620:
1613:
1610:
1605:
1601:
1596:
1591:
1587:
1583:
1579:
1575:
1571:
1564:
1561:
1556:
1552:
1547:
1542:
1538:
1534:
1530:
1526:
1522:
1518:
1514:
1507:
1504:
1499:
1495:
1491:
1487:
1483:
1479:
1472:
1469:
1464:
1460:
1456:
1452:
1449:(8): 816–28.
1448:
1444:
1437:
1435:
1433:
1431:
1427:
1422:
1418:
1413:
1408:
1403:
1398:
1394:
1390:
1386:
1379:
1376:
1371:
1367:
1362:
1357:
1353:
1349:
1346:(4): 365–80.
1345:
1341:
1337:
1330:
1327:
1322:
1318:
1313:
1308:
1304:
1300:
1297:(1): 825–58.
1296:
1292:
1288:
1281:
1279:
1275:
1270:
1266:
1261:
1256:
1252:
1248:
1244:
1240:
1236:
1232:
1228:
1221:
1218:
1213:
1209:
1204:
1199:
1195:
1191:
1187:
1183:
1179:
1172:
1169:
1164:
1157:
1154:
1149:
1143:
1139:
1132:
1130:
1128:
1126:
1124:
1120:
1115:
1111:
1106:
1101:
1097:
1093:
1089:
1082:
1080:
1078:
1076:
1074:
1072:
1070:
1068:
1066:
1062:
1057:
1055:9781621821328
1051:
1047:
1040:
1038:
1036:
1034:
1032:
1030:
1028:
1026:
1024:
1022:
1020:
1018:
1016:
1014:
1012:
1010:
1008:
1004:
999:
995:
990:
985:
980:
975:
971:
967:
963:
956:
953:
948:
944:
939:
934:
929:
924:
920:
916:
912:
908:
904:
897:
894:
889:
885:
881:
877:
872:
867:
864:(1): 116–37.
863:
859:
855:
848:
845:
840:
836:
832:
828:
824:
820:
816:
812:
805:
803:
801:
799:
795:
790:
786:
782:
778:
774:
770:
763:
761:
759:
757:
755:
753:
751:
749:
747:
745:
743:
741:
739:
737:
735:
733:
729:
722:
718:
716:
712:
710:
709:Glycosylation
707:
706:
702:
700:
698:
693:
691:
687:
685:
680:
678:
674:
669:
663:
661:
659:
655:
647:
645:
641:
637:
635:
631:
627:
618:
611:
609:
606:
602:
598:
594:
589:
587:
583:
579:
578:Proteoglycans
571:
566:
565:Proteoglycans
559:Proteoglycans
558:
556:
554:
545:
543:
540:
536:
534:
530:
524:
522:
518:
514:
506:
504:
501:
499:
495:
494:hydroxylysine
491:
487:
479:
475:
472:
470:
468:
463:
459:
455:
451:
447:
442:
440:
436:
426:
419:
415:
412:
410:
407:
403:
398:
396:
392:
388:
384:
379:
377:
373:
369:
361:
357:
355:
351:
349:
342:
334:
330:
326:
323:
321:
319:
315:
310:
306:
303:
299:
294:
292:
291:glycoproteins
288:
284:
282:
278:
274:
267:
261:
257:
254:
249:
247:
243:
237:
235:
227:
222:
218:
214:
212:
208:
204:
202:
197:
193:
189:
185:
181:
177:
173:
171:
162:
158:
153:
146:
142:
138:
135:
130:
126:
124:
122:
118:
114:
110:
106:
102:
98:
94:
90:
86:
82:
78:
74:
70:
66:
62:
58:
56:
51:
47:
43:
39:
35:
33:
19:
2620:
2616:
2393:
2364:Galactolysis
2334:Glycogenesis
2139:Fermentation
2111:ATP synthase
1977:
1971:
1934:
1930:
1878:
1874:
1864:
1832:(5): 583–9.
1829:
1825:
1815:
1778:
1774:
1764:
1721:
1717:
1707:
1680:
1676:
1626:
1622:
1612:
1577:
1573:
1563:
1520:
1516:
1506:
1481:
1477:
1471:
1446:
1443:Glycobiology
1442:
1392:
1388:
1378:
1343:
1339:
1329:
1294:
1290:
1234:
1230:
1220:
1185:
1181:
1171:
1162:
1156:
1137:
1095:
1092:Glycobiology
1091:
1045:
969:
965:
955:
910:
906:
896:
861:
857:
847:
817:(1): 19–26.
814:
810:
772:
768:
714:
694:
688:
681:
673:blood groups
670:
667:
651:
642:
638:
623:
600:
590:
576:
549:
537:
525:
520:
516:
511:Addition of
510:
502:
488:residues in
483:
477:
476:-Galactose (
473:
443:
438:
432:
417:
413:
399:
380:
367:
366:
353:
347:
345:Addition of
344:
332:
328:
324:
307:
295:
286:
285:
272:
271:
250:
245:
241:
238:
231:
228:Biosynthesis
220:
200:
187:
184:transferases
169:
167:Addition of
166:
160:
156:
144:
140:
136:
128:
120:
116:
112:
54:
53:
31:
30:
29:
2490:lipogenesis
2352:Fructolysis
2166:Lactic acid
690:Alzheimer's
391:Alzheimer's
387:Parkinson's
376:O-GlcNAcase
211:sialic acid
97:Alzheimer's
81:prokaryotes
2619:-, O- and
2313:Glycolysis
2246:metabolism
2244:Nucleotide
2234:Urea cycle
2215:Amino acid
2204:Catabolism
2147:Glycolysis
2090:Glycolysis
2073:metabolism
2033:catabolism
2029:Metabolism
1724:(1): 561.
723:References
658:glycogenin
648:Glycogenin
416:-Mannose (
406:metabolism
109:pathogenic
101:eukaryotes
85:metabolism
65:eukaryotes
2486:lipolysis
2293:anabolism
2037:anabolism
1937:(1): 17.
640:complex.
498:galactose
429:membrane.
298:receptors
277:leukocyte
268:Functions
196:galactose
77:cytoplasm
50:threonine
48:(Ser) or
2631:Category
2409:Nonhuman
2394:O-linked
2389:N-linked
2181:Specific
1963:29495527
1907:22872643
1856:21924891
1807:30514763
1756:28916755
1655:21768090
1555:22223806
1463:19429925
1421:24918087
1370:23992419
1321:21391816
1269:24311690
1212:28488703
1114:12042244
998:22685409
947:19251655
888:25666819
880:24673753
839:31369853
703:See also
654:tyrosine
626:ceramide
533:cysteine
490:collagen
395:diabetes
341:O-GlcNAc
335:-GlcNAc)
281:microbes
147:-GalNAc)
93:diabetes
44:atom of
18:O-glycan
2613:GlycoEP
2549:Ketosis
2161:Ethanol
2044:General
1954:5874723
1898:3464504
1847:3195399
1798:6364784
1747:5601480
1726:Bibcode
1699:9228088
1646:3173195
1604:1220686
1595:1172471
1546:3702376
1525:Bibcode
1517:Science
1498:9878797
1412:4042083
1395:: 132.
1361:3985334
1312:3294376
1260:3930058
1239:Bibcode
1231:Science
1203:5667541
989:3369928
938:2648892
915:Bibcode
831:8785483
789:9673446
454:Ribitol
435:mannose
302:ligands
253:lectins
180:enzymes
155:Common
105:archaea
2071:Energy
1984:
1961:
1951:
1905:
1895:
1854:
1844:
1805:
1795:
1754:
1744:
1697:
1653:
1643:
1602:
1592:
1553:
1543:
1496:
1461:
1419:
1409:
1368:
1358:
1319:
1309:
1267:
1257:
1210:
1200:
1144:
1112:
1052:
996:
986:
945:
935:
886:
878:
837:
829:
787:
697:cancer
612:Lipids
597:xylose
513:fucose
486:lysine
458:xylose
314:cancer
309:Mucins
207:fucose
89:cancer
46:serine
42:oxygen
2567:Other
2527:Other
2302:Human
2183:paths
884:S2CID
835:S2CID
539:Notch
480:-Gal)
446:fungi
420:-Man)
79:; in
59:is a
38:sugar
2291:and
1982:ISBN
1959:PMID
1903:PMID
1852:PMID
1803:PMID
1752:PMID
1695:PMID
1651:PMID
1600:PMID
1551:PMID
1494:PMID
1482:1426
1459:PMID
1417:PMID
1366:PMID
1317:PMID
1265:PMID
1208:PMID
1142:ISBN
1110:PMID
1050:ISBN
994:PMID
943:PMID
876:PMID
827:PMID
785:PMID
519:and
460:and
316:and
209:and
119:and
95:and
2315:⇄
2156:ABE
1949:PMC
1939:doi
1893:PMC
1883:doi
1879:287
1842:PMC
1834:doi
1793:PMC
1783:doi
1779:294
1742:PMC
1734:doi
1685:doi
1681:272
1641:PMC
1631:doi
1627:286
1590:PMC
1582:doi
1578:152
1541:PMC
1533:doi
1521:335
1486:doi
1451:doi
1407:PMC
1397:doi
1356:PMC
1348:doi
1307:PMC
1299:doi
1255:PMC
1247:doi
1235:342
1198:PMC
1190:doi
1100:doi
984:PMC
974:doi
933:PMC
923:doi
911:106
866:doi
819:doi
777:doi
2633::
2488:,
2332:⇄
2149:→
2109:+
2100:→
2096:→
2092:→
2035:,
2031:,
1957:.
1947:.
1935:11
1933:.
1929:.
1915:^
1901:.
1891:.
1877:.
1873:.
1850:.
1840:.
1830:21
1828:.
1824:.
1801:.
1791:.
1777:.
1773:.
1750:.
1740:.
1732:.
1720:.
1716:.
1693:.
1679:.
1675:.
1663:^
1649:.
1639:.
1625:.
1621:.
1598:.
1588:.
1576:.
1572:.
1549:.
1539:.
1531:.
1519:.
1515:.
1492:.
1480:.
1457:.
1447:19
1445:.
1429:^
1415:.
1405:.
1391:.
1387:.
1364:.
1354:.
1344:10
1342:.
1338:.
1315:.
1305:.
1295:80
1293:.
1289:.
1277:^
1263:.
1253:.
1245:.
1233:.
1229:.
1206:.
1196:.
1186:18
1184:.
1180:.
1122:^
1108:.
1096:12
1094:.
1090:.
1064:^
1006:^
992:.
982:.
968:.
964:.
941:.
931:.
921:.
909:.
905:.
882:.
874:.
862:92
860:.
856:.
833:.
825:.
815:13
813:.
797:^
783:.
773:33
771:.
731:^
679:.
523:.
456:,
397:.
283:.
205:,
198:,
123:.
115:,
103:,
91:,
71:,
2621:C
2617:N
2514:)
2510:(
2492:)
2484:(
2295:)
2286:(
2114:)
2104:(
2021:e
2014:t
2007:v
1990:.
1965:.
1941::
1909:.
1885::
1858:.
1836::
1809:.
1785::
1758:.
1736::
1728::
1722:8
1701:.
1687::
1657:.
1633::
1606:.
1584::
1557:.
1535::
1527::
1500:.
1488::
1465:.
1453::
1423:.
1399::
1393:4
1372:.
1350::
1323:.
1301::
1271:.
1249::
1241::
1214:.
1192::
1150:.
1116:.
1102::
1058:.
1000:.
976::
970:8
949:.
925::
917::
890:.
868::
841:.
821::
791:.
779::
715:N
601:N
478:O
474:O
439:P
418:O
414:O
368:O
354:O
348:N
333:O
329:N
327:-
325:O
287:O
273:O
246:N
242:N
221:N
201:N
188:O
170:N
161:N
157:O
145:O
141:N
139:-
137:O
129:O
55:O
32:O
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.