381:
virtue of their different isotopic constitution, and can thus be used to determine the oral and intravenous pharmacokinetics from the same dose administration. This technique eliminates pharmacokinetic issues with non-equivalent clearance as well as enabling the intravenous dose to be administered with a minimum of toxicology and formulation. The technique was first applied using stable-isotopes such as C and mass-spectrometry to distinguish the isotopes by mass difference. More recently, C labelled drugs are administered intravenously and accelerator mass spectrometry (AMS) used to measure the isotopically labelled drug along with mass spectrometry for the unlabelled drug.
132:, which covers the intake of nutrients and non-drug dietary ingredients, the concept of bioavailability lacks the well-defined standards associated with the pharmaceutical industry. The pharmacological definition cannot apply to these substances because utilization and absorption is a function of the nutritional status and physiological state of the subject, resulting in even greater differences from individual to individual (inter-individual variation). Therefore, bioavailability for dietary supplements can be defined as the proportion of the administered substance capable of being absorbed and available for use or storage.
620:< 100%). Various physiological factors reduce the availability of drugs prior to their entry into the systemic circulation. Whether a drug is taken with or without food will also affect absorption, other drugs taken concurrently may alter absorption and first-pass metabolism, intestinal motility alters the dissolution of the drug and may affect the degree of chemical degradation of the drug by intestinal microflora. Disease states affecting liver metabolism or gastrointestinal function will also have an effect.
88:. To ensure that the drug taker who has poor absorption is dosed appropriately, the bottom value of the deviation range is employed to represent real bioavailability and to calculate the drug dose needed for the drug taker to achieve systemic concentrations similar to the intravenous formulation. To dose without knowing the drug taker's absorption rate, the bottom value of the deviation range is used in order to ensure the intended efficacy, unless the drug is associated with a narrow
376:; that is, a route of administration that guarantees all of the administered drug reaches systemic circulation. Such studies come at considerable cost, not least of which is the necessity to conduct preclinical toxicity tests to ensure adequate safety, as well as potential problems due to solubility limitations. These limitations may be overcome, however, by administering a very low dose (typically a few micrograms) of an
166:
156:
and precipitation with calcium phosphates at high soil pH. Toxic materials in soil, such as lead from paint may be rendered unavailable to animals ingesting contaminated soil by supplying phosphorus fertilizers in excess. Organic pollutants such as solvents or pesticides may be rendered unavailable
151:
Bioavailability is the measure by which various substances in the environment may enter into living organisms. It is commonly a limiting factor in the production of crops (due to solubility limitation or absorption of plant nutrients to soil colloids) and in the removal of toxic substances from the
384:
There is no regulatory requirement to define the intravenous pharmacokinetics or absolute bioavailability however regulatory authorities do sometimes ask for absolute bioavailability information of the extravascular route in cases in which the bioavailability is apparently low or variable and there
196:
administration), with the bioavailability of the same drug following intravenous administration. It is the fraction of exposure to a drug (AUC) through non-intravenous administration compared with the corresponding intravenous administration of the same drug. The comparison must be dose normalized
380:
concomitantly with a therapeutic non-isotopically labelled oral dose (the isotopically labelled intravenous dose is sufficiently low so as not to perturb the systemic drug concentrations achieved from the non-labelled oral dose). The intravenous and oral concentrations can then be deconvoluted by
420:) of a formulation (A) of a certain drug when compared with another formulation (B) of the same drug, usually an established standard, or through administration via a different route. When the standard consists of intravenously administered drug, this is known as absolute bioavailability (see
607:
While the mechanisms by which a formulation affects bioavailability and bioequivalence have been extensively studied in drugs, formulation factors that influence bioavailability and bioequivalence in nutritional supplements are largely unknown. As a result, in nutritional sciences, relative
355:
152:
food chain by microorganisms (due to sorption to or partitioning of otherwise degradable substances into inaccessible phases in the environment). A noteworthy example for agriculture is plant phosphorus deficiency induced by precipitation with iron and aluminum phosphates at low
543:
371:
Although knowing the true extent of systemic absorption (referred to as absolute bioavailability) is clearly useful, in practice it is not determined as frequently as one may think. The reason for this is that its assessment requires an
1652:
Schuppan, D.; Molz, K. H.; Staib, A. H.; Rietbrock, N. (1981). "Bioavailability of theophylline from a sustained-release aminophylline formulation (Euphyllin retard tablets) – plasma levels after single and multiple oral doses".
230:
1114:
Bioavailability is the major factor affecting dietary requirements (Sandstrom, 1997). Flesh foods facilitate bioavailability, although indigestible Zn-binding ligands decrease bioavailability (Mills, 1985).
430:
863:
99:, herbs and other nutrients in which the route of administration is nearly always oral, bioavailability generally designates simply the quantity or fraction of the ingested dose that is absorbed.
1090:
SANDSTEAD, HAROLD H.; AU, WILLIAM (2007). "Zinc**Dr. Carl-Gustaf
Elinder was the author of this chapter in the 2nd edition of the Handbook on Toxicology of Metals; his text provided guidance.".
1358:
O'Loughlin, Edward J.; Traina, Samuel J.; Sims, Gerald K. (2000). "Effects of sorption on the biodegradation of 2-methylpyridine in aqueous suspensions of reference clay minerals".
69:(AUC) for the extravascular formulation to the AUC for the intravascular formulation. AUC is used because AUC is proportional to the dose that has entered the systemic circulation.
208:
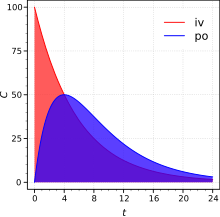
plot for the drug after both intravenous (iv) and extravascular (non-intravenous, i.e., oral) administration. The absolute bioavailability is the dose-corrected area under curve (
608:
bioavailability or bioequivalence is the most common measure of bioavailability, comparing the bioavailability of one formulation of the same dietary ingredient to another.
1919:
197:(e.g., account for different doses or varying weights of the subjects); consequently, the amount absorbed is corrected by dividing the corresponding dose administered.
368:
than one. If we compare the two different dosage forms having same active ingredients and compare the two drug bioavailability is called comparative bioavailability.
788:, inter-individual variation is a critical measurement used to assess the bioavailability differences from patient to patient in order to ensure predictable dosing.
112:
Bioavailability is a term used to describe the percentage of an administered dose of a xenobiotic that reaches the systemic circulation. It is denoted by the letter
1798:
784:
Each of these factors may vary from patient to patient (inter-individual variation), and indeed in the same patient over time (intra-individual variation). In
389:
and the pharmacokinetics at therapeutic doses. In all such cases, to conduct an absolute bioavailability study requires that the drug be given intravenously.
1234:"Bioavailability of Nutrients: A Practical Approach to In Vitro Demonstration of the Availability of Nutrients in Multivitamin-Mineral Combination Products"
1528:
Lappin, Graham; Rowland, Malcolm; Garner, R. Colin (2006). "The use of isotopes in the determination of absolute bioavailability of drugs in humans".
1343:
Sims, G.K.; Radosevich, M.; He, X.-T.; Traina, S. J. (1991). "The effects of sorption on the bioavailability of pesticides". In Betts, W. B. (ed.).
140:
1952:
802:
1767:
1741:
1712:
1693:
1273:
Hinsinger, Philippe (2001). "Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review".
1175:
1143:
1107:
1033:
1000:
960:
927:
902:
350:{\displaystyle F_{\mathrm {abs} }=100\cdot {\frac {AUC_{\mathrm {po} }\cdot D_{\mathrm {iv} }}{AUC_{\mathrm {iv} }\cdot D_{\mathrm {po} }}}}
169:
Absolute bioavailability is a ratio of areas under the curves. IV, intravenous; PO, oral route. C is plasma concentration (arbitrary units).
157:
to microorganisms and thus persist in the environment when they are adsorbed to soil minerals or partition into hydrophobic organic matter.
2044:
2009:
1574:
Lappin, Graham; Stevens, Lloyd (2008). "Biomedical accelerator mass spectrometry: Recent applications in metabolism and pharmacokinetics".
538:{\displaystyle F_{\mathrm {rel} }=100\cdot {\frac {AUC_{\mathrm {A} }\cdot D_{\mathrm {B} }}{AUC_{\mathrm {B} }\cdot D_{\mathrm {A} }}}}
66:
2738:
1406:
392:
Intravenous administration of a developmental drug can provide valuable information on the fundamental pharmacokinetic parameters of
1791:
843:
is 111%, since the drug is completely absorbed and first-pass metabolism in the lung after intravenous administration is bypassed.
2768:
2728:
858:
2778:
2668:
2161:
2196:
2723:
2419:
2330:
1617:
Hoag, Stephen W.; Hussain, Ajaz S. (2001). "The Impact of
Formulation on Bioavailability: Summary of Workshop Discussion".
2648:
2273:
2773:
2201:
1784:
708:
2391:
616:
The absolute bioavailability of a drug, when administered by an extravascular route, is usually less than one (i.e.,
2126:
1945:
1874:
632:
1308:
Ma, Qi-Ying; Traina, Samuel J.; Logan, Terry J.; Ryan, James A. (1993). "In situ lead immobilization by apatite".
2638:
2386:
2380:
759:
39:
2703:
2405:
173:
Absolute bioavailability compares the bioavailability of the active drug in systemic circulation following non-
2454:
2216:
2211:
2121:
2039:
812:
643:
177:
58:
2590:
2351:
2326:
2261:
2150:
2034:
1859:
393:
189:
77:
1728:"Metrics to characterize concentration-time profiles in single- and multiple-dose bioequivalence studies"
2698:
2490:
2292:
1969:
1938:
1879:
744:(decreased rate of metabolism), e.g., grapefruit juice inhibits CYP3A → higher nifedipine concentrations
697:
62:
2733:
2595:
2505:
2346:
2226:
1994:
1393:
Sims, Gerald K.; Cupples, Alison M. (1999). "Factors controlling degradation of pesticides in soil".
1317:
401:
47:
556:) between two drug products. For FDA approval, a generic manufacturer must demonstrate that the 90%
2643:
2531:
2500:
2146:
2106:
2029:
1894:
1884:
1808:
557:
129:
96:
2613:
2563:
2464:
2277:
2256:
2156:
2077:
1989:
1599:
1553:
1461:
1375:
1290:
89:
81:
61:
other than intravenous, its bioavailability is lower due to intestinal epithelium absorption and
752:
Age: In general, drugs are metabolized more slowly in fetal, neonatal, and geriatric populations
360:
Therefore, a drug given by the intravenous route will have an absolute bioavailability of 100% (
2608:
2469:
2459:
2287:
2281:
2116:
1869:
1763:
1737:
1708:
1689:
1662:
1634:
1591:
1545:
1510:
1453:
1255:
1214:
1171:
1139:
1103:
1072:
1029:
996:
956:
923:
898:
377:
1727:
571:) of its product to that of the "brand name drug" is within the limits of 80% to 125%. Where
2653:
2541:
2536:
2336:
2306:
2092:
2072:
2054:
1904:
1899:
1864:
1849:
1626:
1583:
1537:
1500:
1492:
1443:
1433:
1402:
1367:
1325:
1282:
1245:
1204:
1164:
1131:
1095:
1062:
1021:
988:
948:
890:
741:
676:
386:
1150:
Bioavailability strictly refers to both the uptake and metabolic utilization of a nutrient.
885:
Hebert, Mary F. (2013). "Impact of
Pregnancy on Maternal Pharmacokinetics of Medications".
2693:
2568:
2396:
2341:
2049:
1999:
684:
201:
416:
In pharmacology, relative bioavailability measures the bioavailability (estimated as the
1321:
2683:
2663:
2582:
2424:
2166:
2111:
1889:
1854:
1505:
1480:
1099:
1025:
992:
894:
785:
701:
549:
364:= 1), whereas drugs given by other routes usually have an absolute bioavailability of
2762:
2688:
2175:
2082:
1914:
1839:
1829:
1465:
1135:
952:
1707:. Drugs and the Pharmaceutical Sciences. Vol. 48. New York, NY: Marcel Dekker.
1603:
1379:
17:
2658:
2618:
2519:
2443:
2321:
2004:
1961:
1909:
1557:
1294:
1193:"Factors Influencing the Measurement of Bioavailability, Taking Calcium as a Model"
1051:"Factors Influencing the Measurement of Bioavailability, Taking Calcium as a Model"
854:
836:
136:
31:
1481:"Bioavailability and bioequivalence in drug development: BABE in drug development"
1731:
642:
The drug formulation (immediate release, excipients used, manufacturing methods,
2743:
2230:
2206:
1844:
1776:
1723:
185:
174:
54:
1736:. Statistics in Practice. Chichester, UK: John Wiley and Sons. pp. 17–36.
2623:
2555:
2447:
2222:
1834:
1587:
1422:"Defining and unpacking the core concepts of pharmacology A global initiative"
1286:
636:
628:
193:
57:, its bioavailability is 100%. However, when a medication is administered via
43:
1630:
1541:
1250:
1233:
1209:
1192:
1067:
1050:
200:
In pharmacology, in order to determine absolute bioavailability of a drug, a
65:. Thereby, mathematically, bioavailability equals the ratio of comparing the
2401:
755:
720:
659:
1638:
1595:
1549:
1514:
1457:
1371:
1259:
1218:
1076:
1666:
2523:
2495:
2482:
2251:
2171:
1686:
1407:
10.1002/(SICI)1096-9063(199905)55:5<598::AID-PS962>3.0.CO;2-N
797:
688:
1329:
1984:
1655:
International
Journal of Clinical Pharmacology, Therapy, and Toxicology
1448:
771:
732:
680:
669:
650:
216:
intravenous. The formula for calculating the absolute bioavailability,
153:
139:
and nutrition sciences, bioavailability is measured by calculating the
1496:
1438:
1421:
807:
736:
728:
724:
713:
181:
1762:. Biostatistics Series. Vol. 27 (3rd ed.). FL: CRC Press.
1733:
Bioequivalence
Studies in Drug Development: Methods and Applications
853: Reference listed drug products (i.e., innovator's) as well as
165:
1688:(4 ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
590:
refers to the maximum concentration of the drug in the blood. When
1703:
Welling, Peter G.; Tse, Francis L. S.; Dighe, Shrikant V. (1991).
775:
164:
1760:
Design and
Analysis of Bioavailability and Bioequivalence Studies
2376:
2269:
2265:
2067:
1819:
85:
73:
1934:
1930:
1780:
575:
refers to the concentration of the drug in the blood over time
548:
Relative bioavailability is one of the measures used to assess
646:– delayed release, extended release, sustained release, etc.)
597:
is given, it refers to the time it takes for a drug to reach
220:, of a drug administered orally (po) is given below (where
67:
area under the plasma drug concentration curve versus time
1485:
983:
Davis, Jennifer L. (2018). "Pharmacologic
Principles".
978:
976:
974:
972:
719:
Enzyme induction (increased rate of metabolism), e.g.,
1569:
1567:
433:
233:
943:
Flynn, Edward (2007). "Pharmacokinetic
Parameters".
649:
Whether the formulation is administered in a fed or
2716:
2676:
2632:
2581:
2554:
2518:
2481:
2442:
2433:
2369:
2314:
2305:
2244:
2189:
2138:
2100:
2091:
2022:
1977:
1968:
1758:Chow, Shein-Chung; Liu, Jen-pei (15 October 2008).
831: One of the few exceptions where a drug shows
623:Other factors may include, but are not limited to:
1576:Expert Opinion on Drug Metabolism & Toxicology
1530:Expert Opinion on Drug Metabolism & Toxicology
1163:
537:
349:
1345:Biodegradation of Natural and Synthetic Materials
1126:Solomons, N.W. (2003). "ZINC | Physiology".
53:By definition, when a medication is administered
1016:Johanson, G. (2010). "Modeling of Disposition".
945:xPharm: The Comprehensive Pharmacology Reference
560:for the ratio of the mean responses (usually of
1166:Applied Biopharmaceutics & Pharmacokinetics
749:Individual variation in metabolic differences
143:(AUC) of the drug concentration time profile.
1946:
1792:
857:products that have been approved based on an
421:
8:
1920:Quantitative structure–activity relationship
1128:Encyclopedia of Food Sciences and Nutrition
716:induction/inhibition by other drugs/foods:
412:Relative bioavailability and bioequivalence
42:and is the fraction (%) of an administered
2713:
2673:
2578:
2551:
2515:
2478:
2439:
2366:
2311:
2241:
2186:
2135:
2097:
2019:
1974:
1953:
1939:
1931:
1799:
1785:
1777:
1504:
1447:
1437:
1249:
1208:
1066:
525:
524:
510:
509:
490:
489:
475:
474:
461:
439:
438:
432:
334:
333:
316:
315:
293:
292:
275:
274:
261:
239:
238:
232:
1170:(4th ed.). New York: McGraw-Hill.
920:The Textbook of Pharmaceutical Medicine
877:
1722:Hauschke, Dieter; Steinijans, Volker;
1360:Environmental Toxicology and Chemistry
1310:Environmental Science & Technology
887:Clinical Pharmacology During Pregnancy
839:. If administered as an oral solution
803:Biopharmaceutics Classification System
1347:. London: Springer. pp. 119–137.
675:Interactions with other foods (e.g.,
668:Interactions with other drugs (e.g.,
665:Interactions with other drugs/foods:
385:is a proven relationship between the
7:
1684:Rowland, Malcolm; Tozer, N. (2010).
1092:Handbook on the Toxicology of Metals
147:In environmental sciences or science
922:(6th ed.). Jersey: BMJ Books.
612:Factors influencing bioavailability
2739:Minimum bactericidal concentration
1026:10.1016/b978-0-08-046884-6.00108-1
993:10.1016/b978-0-323-44329-6.00002-4
918:Griffin, J. P. (7 December 2009).
895:10.1016/b978-0-12-386007-1.00003-9
526:
511:
491:
476:
446:
443:
440:
338:
335:
320:
317:
297:
294:
279:
276:
246:
243:
240:
25:
627:Physical properties of the drug (
206:plasma drug concentration vs time
184:, buccal, ocular, nasal, rectal,
116:(or, if expressed in percent, by
2729:Minimum inhibitory concentration
1130:. Elsevier. pp. 6272–6277.
1100:10.1016/b978-012369413-3/50102-6
953:10.1016/b978-008055232-3.60034-0
859:Abbreviated New Drug Application
72:Bioavailability of a drug is an
2669:WHO list of essential medicines
2162:Non-specific effect of vaccines
1479:Chow, Shein-Chung (July 2014).
1426:British Journal of Pharmacology
1162:Shargel, L.; Yu, A. B. (1999).
564:and the maximum concentration,
204:study must be done to obtain a
2724:Antimicrobial pharmacodynamics
1136:10.1016/b0-12-227055-x/01309-2
1094:. Elsevier. pp. 925–947.
1020:. Elsevier. pp. 153–177.
1:
2649:Functional analog (chemistry)
1705:Pharmaceutical Bioequivalence
1232:Srinivasan, V. Srini (2001).
987:. Elsevier. pp. 79–137.
212:) non-intravenous divided by
2202:Hill equation (biochemistry)
889:. Elsevier. pp. 17–39.
696:Transporters: Substrate of
27:Pharmacological measurement
2795:
2717:Antimicrobial pharmacology
2197:Dose–response relationship
2127:Desensitization (medicine)
1191:Heaney, Robert P. (2001).
1049:Heaney, Robert P. (2001).
947:. Elsevier. pp. 1–3.
378:isotopically labelled drug
2639:Coinduction (anesthetics)
1815:
1588:10.1517/17425255.4.8.1021
760:enterohepatic circulation
2704:Multiple drug resistance
2677:Tolerance and resistance
2045:Physiological antagonist
1619:The Journal of Nutrition
1542:10.1517/17425255.2.3.419
1420:Guilding, Clare (2023).
1238:The Journal of Nutrition
1197:The Journal of Nutrition
1055:The Journal of Nutrition
1018:Comprehensive Toxicology
985:Equine Internal Medicine
161:Absolute bioavailability
2769:Pharmacokinetic metrics
2455:Neuropsychopharmacology
2217:Cheng-Prussoff Equation
2212:Del Castillo Katz model
2139:Other effects of ligand
2122:Receptor (biochemistry)
2040:Irreversible antagonist
1875:Lipinski's rule of five
1625:(4 Suppl): 1389–1391S.
1287:10.1023/A:1013351617532
1244:(4 Suppl): 1349–1350S.
1203:(4 Suppl): 1344–1348S.
224:is dose administered).
2779:Life sciences industry
2591:Classical pharmacology
2352:Plasma protein binding
2327:Volume of distribution
2035:Competitive antagonist
1631:10.1093/jn/131.4.1389S
1372:10.1002/etc.5620190904
1251:10.1093/jn/131.4.1349S
1210:10.1093/jn/131.4.1344S
1068:10.1093/jn/131.4.1344S
756:Phenotypic differences
709:gastrointestinal tract
539:
394:volume of distribution
351:
170:
124:In nutritional science
78:population variability
2699:Antibiotic resistance
2491:Clinical pharmacology
2010:Physiological agonist
1970:Ligand (biochemistry)
1880:Lipophilic efficiency
656:Gastric emptying rate
540:
374:intravenous reference
352:
168:
63:first-pass metabolism
2596:Reverse pharmacology
2506:Pharmacoepidemiology
2347:Biological half-life
2227:Ligand binding assay
2101:Activity at receptor
1995:Irreversible agonist
813:Lipinski's Rule of 5
774:insufficiency, poor
672:, alcohol, nicotine)
431:
231:
48:systemic circulation
38:is a subcategory of
18:Oral bioavailability
2774:Medicinal chemistry
2644:Combination therapy
2532:Pharmacoinformatics
2501:Medicinal chemistry
2107:Mechanism of action
1895:New chemical entity
1885:Mechanism of action
1809:medicinal chemistry
1330:10.1021/es00046a007
1322:1993EnST...27.1803M
861:are given in FDA's
700:transporters (e.g.
558:confidence interval
130:nutritional science
97:dietary supplements
2614:Immunopharmacology
2564:Pharmacotoxicology
2465:Psychopharmacology
2257:Intrinsic activity
2157:Pleiotropy (drugs)
2078:Agonist-antagonist
1990:Endogenous agonist
535:
347:
171:
90:therapeutic window
2756:
2755:
2752:
2751:
2712:
2711:
2609:Photopharmacology
2604:
2603:
2577:
2576:
2550:
2549:
2514:
2513:
2477:
2476:
2470:Electrophysiology
2460:Neuropharmacology
2415:
2414:
2365:
2364:
2301:
2300:
2288:Therapeutic index
2240:
2239:
2185:
2184:
2134:
2133:
2063:
2062:
2018:
2017:
1928:
1927:
1870:Ligand efficiency
1769:978-1-58488-668-6
1743:978-0-470-09475-4
1714:978-0-8247-8484-3
1695:978-0-7817-5009-7
1497:10.1002/wics.1310
1439:10.1111/bph.16222
1395:Pesticide Science
1177:978-0-8385-0278-5
1145:978-0-12-227055-0
1109:978-0-12-369413-3
1035:978-0-08-046884-6
1002:978-0-323-44329-6
962:978-0-08-055232-3
929:978-1-4051-8035-1
904:978-0-12-386007-1
742:Enzyme inhibition
533:
345:
46:that reaches the
16:(Redirected from
2786:
2714:
2674:
2654:Polypharmacology
2579:
2552:
2542:Pharmacogenomics
2537:Pharmacogenetics
2516:
2479:
2440:
2367:
2337:Rate of infusion
2312:
2307:Pharmacokinetics
2242:
2187:
2136:
2098:
2093:Pharmacodynamics
2073:Neurotransmitter
2055:Enzyme inhibitor
2020:
1975:
1955:
1948:
1941:
1932:
1905:Pharmacokinetics
1900:Pharmacodynamics
1865:Enzyme inhibitor
1850:Drug development
1801:
1794:
1787:
1778:
1773:
1754:
1752:
1750:
1718:
1699:
1671:
1670:
1649:
1643:
1642:
1614:
1608:
1607:
1582:(8): 1021–1033.
1571:
1562:
1561:
1525:
1519:
1518:
1508:
1476:
1470:
1469:
1451:
1441:
1417:
1411:
1410:
1390:
1384:
1383:
1366:(9): 2168–2174.
1355:
1349:
1348:
1340:
1334:
1333:
1316:(9): 1803–1810.
1305:
1299:
1298:
1270:
1264:
1263:
1253:
1229:
1223:
1222:
1212:
1188:
1182:
1181:
1169:
1159:
1153:
1152:
1123:
1117:
1116:
1087:
1081:
1080:
1070:
1046:
1040:
1039:
1013:
1007:
1006:
980:
967:
966:
940:
934:
933:
915:
909:
908:
882:
848:
835:of over 100% is
826:
677:grapefruit juice
644:modified release
544:
542:
541:
536:
534:
532:
531:
530:
529:
516:
515:
514:
497:
496:
495:
494:
481:
480:
479:
462:
451:
450:
449:
387:pharmacodynamics
356:
354:
353:
348:
346:
344:
343:
342:
341:
325:
324:
323:
303:
302:
301:
300:
284:
283:
282:
262:
251:
250:
249:
141:area under curve
21:
2794:
2793:
2789:
2788:
2787:
2785:
2784:
2783:
2759:
2758:
2757:
2748:
2708:
2694:Drug resistance
2672:
2628:
2600:
2573:
2569:Neurotoxicology
2546:
2510:
2473:
2435:
2429:
2411:
2361:
2357:Bioavailability
2342:Onset of action
2297:
2236:
2181:
2130:
2087:
2059:
2050:Inverse agonist
2014:
2000:Partial agonist
1964:
1959:
1929:
1924:
1825:Bioavailability
1811:
1805:
1770:
1757:
1748:
1746:
1744:
1721:
1715:
1702:
1696:
1683:
1680:
1675:
1674:
1651:
1650:
1646:
1616:
1615:
1611:
1573:
1572:
1565:
1527:
1526:
1522:
1478:
1477:
1473:
1419:
1418:
1414:
1392:
1391:
1387:
1357:
1356:
1352:
1342:
1341:
1337:
1307:
1306:
1302:
1272:
1271:
1267:
1231:
1230:
1226:
1190:
1189:
1185:
1178:
1161:
1160:
1156:
1146:
1125:
1124:
1120:
1110:
1089:
1088:
1084:
1061:(4): 1344S–8S.
1048:
1047:
1043:
1036:
1015:
1014:
1010:
1003:
982:
981:
970:
963:
942:
941:
937:
930:
917:
916:
912:
905:
884:
883:
879:
874:
868:
845:
844:
823:
821:
794:
786:clinical trials
685:cranberry juice
614:
603:
596:
589:
570:
520:
505:
498:
485:
470:
463:
434:
429:
428:
414:
329:
311:
304:
288:
270:
263:
234:
229:
228:
202:pharmacokinetic
163:
149:
126:
110:
108:In pharmacology
105:
82:deviation range
36:bioavailability
28:
23:
22:
15:
12:
11:
5:
2792:
2790:
2782:
2781:
2776:
2771:
2761:
2760:
2754:
2753:
2750:
2749:
2747:
2746:
2741:
2736:
2734:Bacteriostatic
2731:
2726:
2720:
2718:
2710:
2709:
2707:
2706:
2701:
2696:
2691:
2686:
2684:Drug tolerance
2680:
2678:
2671:
2666:
2664:Lists of drugs
2661:
2656:
2651:
2646:
2641:
2636:
2634:
2630:
2629:
2627:
2626:
2621:
2616:
2611:
2605:
2602:
2601:
2599:
2598:
2593:
2587:
2585:
2583:Drug discovery
2575:
2574:
2572:
2571:
2566:
2560:
2558:
2548:
2547:
2545:
2544:
2539:
2534:
2528:
2526:
2512:
2511:
2509:
2508:
2503:
2498:
2493:
2487:
2485:
2475:
2474:
2472:
2467:
2462:
2457:
2452:
2450:
2437:
2431:
2430:
2428:
2427:
2425:Bioequivalence
2422:
2416:
2413:
2412:
2410:
2409:
2399:
2394:
2389:
2384:
2373:
2371:
2363:
2362:
2360:
2359:
2354:
2349:
2344:
2339:
2334:
2324:
2318:
2316:
2309:
2303:
2302:
2299:
2298:
2296:
2295:
2290:
2285:
2259:
2254:
2248:
2246:
2238:
2237:
2235:
2234:
2219:
2214:
2209:
2204:
2199:
2193:
2191:
2183:
2182:
2180:
2179:
2169:
2167:Adverse effect
2164:
2159:
2154:
2142:
2140:
2132:
2131:
2129:
2124:
2119:
2114:
2112:Mode of action
2109:
2104:
2102:
2095:
2089:
2088:
2086:
2085:
2080:
2075:
2070:
2064:
2061:
2060:
2058:
2057:
2052:
2047:
2042:
2037:
2032:
2026:
2024:
2016:
2015:
2013:
2012:
2007:
2002:
1997:
1992:
1987:
1981:
1979:
1972:
1966:
1965:
1960:
1958:
1957:
1950:
1943:
1935:
1926:
1925:
1923:
1922:
1917:
1912:
1907:
1902:
1897:
1892:
1890:Mode of action
1887:
1882:
1877:
1872:
1867:
1862:
1860:Drug targeting
1857:
1855:Drug discovery
1852:
1847:
1842:
1837:
1832:
1827:
1822:
1816:
1813:
1812:
1806:
1804:
1803:
1796:
1789:
1781:
1775:
1774:
1768:
1755:
1742:
1719:
1713:
1700:
1694:
1679:
1676:
1673:
1672:
1661:(5): 223–227.
1644:
1609:
1563:
1536:(3): 419–427.
1520:
1491:(4): 304–312.
1471:
1432:(9): 375–392.
1412:
1401:(5): 598–601.
1385:
1350:
1335:
1300:
1281:(2): 173–195.
1275:Plant and Soil
1265:
1224:
1183:
1176:
1154:
1144:
1118:
1108:
1082:
1041:
1034:
1008:
1001:
968:
961:
935:
928:
910:
903:
876:
875:
873:
870:
820:
817:
816:
815:
810:
805:
800:
793:
790:
782:
781:
780:
779:
767:Disease state
765:
764:
763:
762:, diet, gender
753:
747:
746:
745:
739:
711:
707:Health of the
705:
702:P-glycoprotein
694:
693:
692:
673:
663:
657:
654:
647:
640:
629:hydrophobicity
613:
610:
601:
594:
587:
568:
550:bioequivalence
546:
545:
528:
523:
519:
513:
508:
504:
501:
493:
488:
484:
478:
473:
469:
466:
460:
457:
454:
448:
445:
442:
437:
413:
410:
358:
357:
340:
337:
332:
328:
322:
319:
314:
310:
307:
299:
296:
291:
287:
281:
278:
273:
269:
266:
260:
257:
254:
248:
245:
242:
237:
178:administration
162:
159:
148:
145:
125:
122:
109:
106:
104:
101:
80:into account,
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2791:
2780:
2777:
2775:
2772:
2770:
2767:
2766:
2764:
2745:
2742:
2740:
2737:
2735:
2732:
2730:
2727:
2725:
2722:
2721:
2719:
2715:
2705:
2702:
2700:
2697:
2695:
2692:
2690:
2689:Tachyphylaxis
2687:
2685:
2682:
2681:
2679:
2675:
2670:
2667:
2665:
2662:
2660:
2657:
2655:
2652:
2650:
2647:
2645:
2642:
2640:
2637:
2635:
2631:
2625:
2622:
2620:
2617:
2615:
2612:
2610:
2607:
2606:
2597:
2594:
2592:
2589:
2588:
2586:
2584:
2580:
2570:
2567:
2565:
2562:
2561:
2559:
2557:
2553:
2543:
2540:
2538:
2535:
2533:
2530:
2529:
2527:
2525:
2521:
2517:
2507:
2504:
2502:
2499:
2497:
2494:
2492:
2489:
2488:
2486:
2484:
2480:
2471:
2468:
2466:
2463:
2461:
2458:
2456:
2453:
2451:
2449:
2445:
2441:
2438:
2432:
2426:
2423:
2421:
2418:
2417:
2407:
2403:
2400:
2398:
2395:
2393:
2390:
2388:
2385:
2382:
2378:
2375:
2374:
2372:
2368:
2358:
2355:
2353:
2350:
2348:
2345:
2343:
2340:
2338:
2335:
2332:
2328:
2325:
2323:
2320:
2319:
2317:
2313:
2310:
2308:
2304:
2294:
2291:
2289:
2286:
2283:
2279:
2275:
2271:
2267:
2263:
2260:
2258:
2255:
2253:
2250:
2249:
2247:
2243:
2232:
2228:
2224:
2220:
2218:
2215:
2213:
2210:
2208:
2205:
2203:
2200:
2198:
2195:
2194:
2192:
2188:
2177:
2176:Neurotoxicity
2173:
2170:
2168:
2165:
2163:
2160:
2158:
2155:
2152:
2148:
2145:Selectivity (
2144:
2143:
2141:
2137:
2128:
2125:
2123:
2120:
2118:
2115:
2113:
2110:
2108:
2105:
2103:
2099:
2096:
2094:
2090:
2084:
2083:Pharmacophore
2081:
2079:
2076:
2074:
2071:
2069:
2066:
2065:
2056:
2053:
2051:
2048:
2046:
2043:
2041:
2038:
2036:
2033:
2031:
2028:
2027:
2025:
2021:
2011:
2008:
2006:
2003:
2001:
1998:
1996:
1993:
1991:
1988:
1986:
1983:
1982:
1980:
1976:
1973:
1971:
1967:
1963:
1956:
1951:
1949:
1944:
1942:
1937:
1936:
1933:
1921:
1918:
1916:
1915:Pharmacophore
1913:
1911:
1908:
1906:
1903:
1901:
1898:
1896:
1893:
1891:
1888:
1886:
1883:
1881:
1878:
1876:
1873:
1871:
1868:
1866:
1863:
1861:
1858:
1856:
1853:
1851:
1848:
1846:
1843:
1841:
1840:Drug delivery
1838:
1836:
1833:
1831:
1830:Chemogenomics
1828:
1826:
1823:
1821:
1818:
1817:
1814:
1810:
1802:
1797:
1795:
1790:
1788:
1783:
1782:
1779:
1771:
1765:
1761:
1756:
1745:
1739:
1735:
1734:
1729:
1725:
1720:
1716:
1710:
1706:
1701:
1697:
1691:
1687:
1682:
1681:
1677:
1668:
1664:
1660:
1656:
1648:
1645:
1640:
1636:
1632:
1628:
1624:
1620:
1613:
1610:
1605:
1601:
1597:
1593:
1589:
1585:
1581:
1577:
1570:
1568:
1564:
1559:
1555:
1551:
1547:
1543:
1539:
1535:
1531:
1524:
1521:
1516:
1512:
1507:
1502:
1498:
1494:
1490:
1486:
1482:
1475:
1472:
1467:
1463:
1459:
1455:
1450:
1445:
1440:
1435:
1431:
1427:
1423:
1416:
1413:
1408:
1404:
1400:
1396:
1389:
1386:
1381:
1377:
1373:
1369:
1365:
1361:
1354:
1351:
1346:
1339:
1336:
1331:
1327:
1323:
1319:
1315:
1311:
1304:
1301:
1296:
1292:
1288:
1284:
1280:
1276:
1269:
1266:
1261:
1257:
1252:
1247:
1243:
1239:
1235:
1228:
1225:
1220:
1216:
1211:
1206:
1202:
1198:
1194:
1187:
1184:
1179:
1173:
1168:
1167:
1158:
1155:
1151:
1147:
1141:
1137:
1133:
1129:
1122:
1119:
1115:
1111:
1105:
1101:
1097:
1093:
1086:
1083:
1078:
1074:
1069:
1064:
1060:
1056:
1052:
1045:
1042:
1037:
1031:
1027:
1023:
1019:
1012:
1009:
1004:
998:
994:
990:
986:
979:
977:
975:
973:
969:
964:
958:
954:
950:
946:
939:
936:
931:
925:
921:
914:
911:
906:
900:
896:
892:
888:
881:
878:
871:
869:
866:
865:
860:
856:
852:
847:
842:
838:
834:
830:
825:
818:
814:
811:
809:
806:
804:
801:
799:
796:
795:
791:
789:
787:
777:
773:
769:
768:
766:
761:
757:
754:
751:
750:
748:
743:
740:
738:
734:
730:
726:
722:
718:
717:
715:
712:
710:
706:
703:
699:
695:
690:
686:
682:
678:
674:
671:
667:
666:
664:
661:
658:
655:
652:
648:
645:
641:
638:
634:
630:
626:
625:
624:
621:
619:
611:
609:
605:
600:
593:
586:
582:
578:
574:
567:
563:
559:
555:
551:
521:
517:
506:
502:
499:
486:
482:
471:
467:
464:
458:
455:
452:
435:
427:
426:
425:
423:
419:
411:
409:
407:
403:
399:
395:
390:
388:
382:
379:
375:
369:
367:
363:
330:
326:
312:
308:
305:
289:
285:
271:
267:
264:
258:
255:
252:
235:
227:
226:
225:
223:
219:
215:
211:
207:
203:
198:
195:
191:
187:
183:
180:(i.e., after
179:
176:
167:
160:
158:
155:
146:
144:
142:
138:
133:
131:
123:
121:
119:
115:
107:
102:
100:
98:
93:
91:
87:
83:
79:
75:
74:average value
70:
68:
64:
60:
56:
55:intravenously
51:
49:
45:
41:
37:
33:
19:
2659:Chemotherapy
2619:Cell biology
2520:Biochemistry
2444:Neuroscience
2392:Distribution
2356:
2322:Loading dose
2005:Superagonist
1962:Pharmacology
1910:Pharmacology
1824:
1759:
1747:. Retrieved
1732:
1724:Pigeot, Iris
1704:
1685:
1658:
1654:
1647:
1622:
1618:
1612:
1579:
1575:
1533:
1529:
1523:
1488:
1484:
1474:
1429:
1425:
1415:
1398:
1394:
1388:
1363:
1359:
1353:
1344:
1338:
1313:
1309:
1303:
1278:
1274:
1268:
1241:
1237:
1227:
1200:
1196:
1186:
1165:
1157:
1149:
1127:
1121:
1113:
1091:
1085:
1058:
1054:
1044:
1017:
1011:
984:
944:
938:
919:
913:
886:
880:
862:
855:generic drug
850:
846:
840:
837:theophylline
832:
828:
824:
822:
783:
622:
617:
615:
606:
598:
591:
584:
580:
576:
572:
565:
561:
553:
547:
417:
415:
405:
397:
391:
383:
373:
370:
365:
361:
359:
221:
217:
213:
209:
205:
199:
190:subcutaneous
172:
150:
137:pharmacology
134:
127:
117:
113:
111:
94:
84:is shown as
71:
52:
35:
32:pharmacology
29:
2744:Bactericide
2420:Compartment
2231:Patch clamp
2207:Schild plot
1845:Drug design
1449:2440/139693
864:Orange Book
691:vegetables)
662:differences
186:transdermal
175:intravenous
103:Definitions
2763:Categories
2624:Physiology
2556:Toxicology
2448:psychology
2397:Metabolism
2387:Absorption
2381:Liberation
2223:Organ bath
2151:Functional
2030:Antagonist
2023:Inhibitory
1978:Excitatory
1835:Drug class
1807:Topics in
872:References
637:solubility
194:sublingual
76:; to take
40:absorption
2406:Clearance
2402:Excretion
2221:Methods (
1466:261062472
721:Phenytoin
660:Circadian
518:⋅
483:⋅
459:⋅
402:clearance
327:⋅
286:⋅
259:⋅
2524:genetics
2496:Pharmacy
2483:Medicine
2293:Affinity
2252:Efficacy
2190:Analysis
2172:Toxicity
1749:21 April
1726:(2007).
1639:11285360
1604:95122610
1596:18680438
1550:16863443
1515:25215170
1458:37605852
1380:98654832
1260:11285352
1219:11285351
1077:11285351
798:ADME-Tox
792:See also
778:function
723:induces
689:brassica
670:antacids
135:In both
2434:Related
2377:(L)ADME
2331:Initial
2315:Metrics
2262:Potency
2245:Metrics
2147:Binding
2117:Binding
1985:Agonist
1678:Sources
1667:7251238
1558:2383402
1506:4157693
1318:Bibcode
1295:8562338
772:hepatic
733:CYP2C19
681:pomello
579:= 0 to
154:soil pH
2436:fields
1766:
1740:
1711:
1692:
1665:
1637:
1602:
1594:
1556:
1548:
1513:
1503:
1464:
1456:
1378:
1293:
1258:
1217:
1174:
1142:
1106:
1075:
1032:
999:
959:
926:
901:
849:
827:
808:Caco-2
770:E.g.,
737:CYP3A4
735:, and
729:CYP2C9
725:CYP1A2
714:Enzyme
698:efflux
651:fasted
400:) and
59:routes
2633:Other
2370:LADME
1600:S2CID
1554:S2CID
1462:S2CID
1376:S2CID
1291:S2CID
819:Notes
776:renal
653:state
583:= ∞,
422:above
192:, or
2522:and
2446:and
2282:TD50
2278:LD50
2274:ED50
2270:IC50
2266:EC50
2068:Drug
1820:ADME
1764:ISBN
1751:2011
1738:ISBN
1709:ISBN
1690:ISBN
1663:PMID
1635:PMID
1592:PMID
1546:PMID
1511:PMID
1454:PMID
1256:PMID
1215:PMID
1172:ISBN
1140:ISBN
1104:ISBN
1073:PMID
1030:ISBN
997:ISBN
957:ISBN
924:ISBN
899:ISBN
366:less
182:oral
95:For
44:drug
2379:: (
1627:doi
1623:131
1584:doi
1538:doi
1501:PMC
1493:doi
1444:hdl
1434:doi
1430:180
1403:doi
1368:doi
1326:doi
1283:doi
1279:237
1246:doi
1242:131
1205:doi
1201:131
1132:doi
1096:doi
1063:doi
1059:131
1022:doi
989:doi
949:doi
891:doi
851:OB:
829:TH:
633:pKa
602:max
595:max
588:max
573:AUC
569:max
562:AUC
456:100
424:).
418:AUC
408:).
256:100
214:AUC
210:AUC
128:In
120:).
30:In
2765::
2280:,
2276:,
2272:,
2268:,
2229:,
2225:,
2149:,
1730:.
1659:19
1657:.
1633:.
1621:.
1598:.
1590:.
1578:.
1566:^
1552:.
1544:.
1532:.
1509:.
1499:.
1487:.
1483:.
1460:.
1452:.
1442:.
1428:.
1424:.
1399:55
1397:.
1374:.
1364:19
1362:.
1324:.
1314:27
1312:.
1289:.
1277:.
1254:.
1240:.
1236:.
1213:.
1199:.
1195:.
1148:.
1138:.
1112:.
1102:.
1071:.
1057:.
1053:.
1028:.
995:.
971:^
955:.
897:.
758:,
731:,
727:,
687:,
683:,
679:,
635:,
631:,
604:.
554:BE
406:CL
188:,
92:.
50:.
34:,
2408:)
2404:(
2383:)
2333:)
2329:(
2284:)
2264:(
2233:)
2178:)
2174:(
2153:)
1954:e
1947:t
1940:v
1800:e
1793:t
1786:v
1772:.
1753:.
1717:.
1698:.
1669:.
1641:.
1629::
1606:.
1586::
1580:4
1560:.
1540::
1534:2
1517:.
1495::
1489:6
1468:.
1446::
1436::
1409:.
1405::
1382:.
1370::
1332:.
1328::
1320::
1297:.
1285::
1262:.
1248::
1221:.
1207::
1180:.
1134::
1098::
1079:.
1065::
1038:.
1024::
1005:.
991::
965:.
951::
932:.
907:.
893::
867:.
841:F
833:F
704:)
639:)
618:F
599:C
592:T
585:C
581:t
577:t
566:C
552:(
527:A
522:D
512:B
507:C
503:U
500:A
492:B
487:D
477:A
472:C
468:U
465:A
453:=
447:l
444:e
441:r
436:F
404:(
398:V
396:(
362:f
339:o
336:p
331:D
321:v
318:i
313:C
309:U
306:A
298:v
295:i
290:D
280:o
277:p
272:C
268:U
265:A
253:=
247:s
244:b
241:a
236:F
222:D
218:F
118:F
114:f
86:±
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.