214:
155:
251:
269:
262:
240:
229:
117:
190:
53:
31:
1199:
1050:
1118:
955:
61:
246:
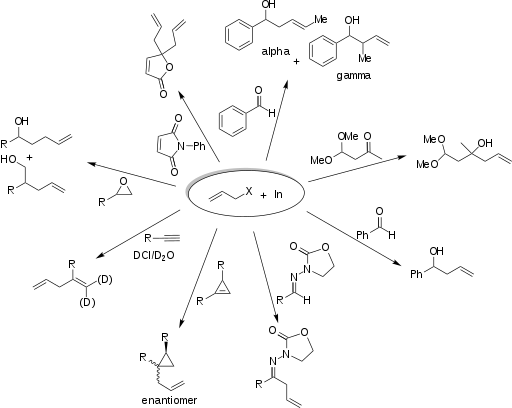
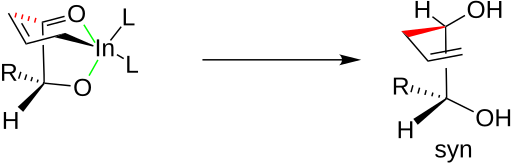
chair conformation. Under chelation control, the allyl group attacks the carbonyl carbon from the less hindered side opposite to that of the R group. Once the C-C bond is fully formed, the indium is released, producing the syn diol. A similar chelated structure is relevant to the allylation of β-oxy aldehydes results in anti diols.
256:
The addition of allylindium reagents to electrophilic hydrazones, illustrated below, has been reported to synthesize only one enantiomer of the chiral product with up to 97% selectivity using binol as a chiral additive. Similarly, a chiral amino alcohol allows for extremely high enantioselectivity in
245:
Numerous investigations have found an explanation for this effect. The oxygens of the carbonyl and the hydroxyl group chelate the indium of the organoindium intermediate as illustrated below on the left by the two green bonds. The incipient C-C bond, illustrated in red, creates a six-member ring in a
234:
The addition of allylindium reagents to aldehydes substituted at α or β carbons can be very diastereoselective in aqueous systems. For example, if chelation control is present in an α-oxy aldehyde, the product is expected to be the syn diastereomer. A sample reaction of chelation versus non-chelation
223:
of the substituents on both the intermediate and carbonyl. An α-attack from the nucleophile (at the position bearing the halogen) is distinguishable from a γ-attack (at the double bond) by inspecting the products. The scheme below gives an example of two different products formed from the same
184:), and others. Solvent often affects the solubility, rate of the reaction, yield, stability, regioselectivity, and stereoselectivity. Indium mediates the allylation of a wide variety of electrophiles. The examples in the following scheme illustrate the breadth of applications of IMA.
110:
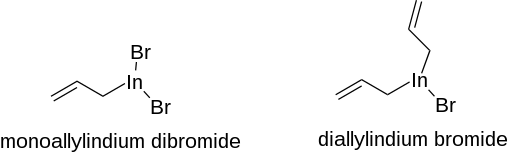
OrganoIn(III) compounds are also prepared by treating In metal with alkyl halides. This reaction gives mixed organoindium halides. Illustrative is the reaction of allyl bromide with a THF suspension of indium. Both monoallylindium dibromide and diallylindium bromide are produced.
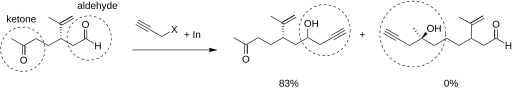
209:, however, give high yields. Research has shown that in reactions of an indium intermediate with an electrophilic compound of both aldehyde and ketone, the reaction proceeded with the aldehyde. The electrophilic compound is shown below.
22:
is the chemistry of compounds containing In-C bonds. The main application of organoindium chemistry is in the preparation of semiconducting components for microelectronic applications. The area is also of some interest in
163:
where the indium, allyl halide, and electrophile are all mixed in a one-pot process. Indium alkylates more readily than other metals, such as Mg, Pb, Bi, or Zn and does not require a promoter or organic
257:
the allylation of ketones. The indium-mediated allylation in water is especially useful in carbohydrate synthesis (such as sialic acids), without using protecting groups.
633:
224:
nucleophile under α-regioselectivity (α) and γ-regioselectivity (γ). This regioselectivity does not appear to depend on conjugation or the degree of substitution.
309:
Shen, Zhi-Liang; Wang, Shun-Yi; Chok, Yew-Keong; Xu, Yun-He; Loh, Teck-Peng (2013). "Organoindium
Reagents: The Preparation and Application in Organic Synthesis".
387:
Uhl, Werner; Graupner, Rene; Layh, Marcus; Schütz, Uwe (1995). "In4{C(SiMe3)3}4 mit In4-tetraeder und In4Se4{C(SiMe3)3}4 mit In4Se4-heterocubanstruktur".
154:
344:
Beachley O. T.; Pazik J. C.; Glassman T. E.; Churchill M. R.; Fettinger J.C.; Blom R. (1988). "Synthesis, characterization and structural studies of In(C
599:
Chan, T.-H.; Li, C.-J. A Concise
Chemical Synthesis of (+) 3-Deoxy-D-glycero-D-galacto-nonulsonic acid (KDN) J. Chem. Soc., Chem. Commun. 1992, 747-748.
431:
481:
Li, C.-J.; Chan, T. H. Organic
Syntheses Using Indium-Mediated and Catalyzed Reactions In Aqueous Media, Tetrahedron 1999, 55, 11149-11176
626:
172:). Although indium mediated allylations can be carried out in aqueous media, a variety of other solvents may be used including THF (
85:
619:
213:
168:. IMAs have advantages over other carbon bond forming reactions because of their ability to be carried out in water (see
27:. Most organoindium compounds feature the In(III) oxidation state, akin to its lighter congeners Ga(III) and B(III).
1188:
1183:
1178:
1173:
1168:
1163:
1158:
1153:
1148:
1143:
1138:
1133:
1123:
1066:
960:
881:
876:
733:
352:
Me) by x-ray diffraction and electron diffraction techniques and a reinvestigation of the crystalline state of In(C
250:
1331:
1224:
926:
896:
886:
866:
854:
822:
787:
755:
723:
718:
678:
46:
693:
657:
611:
261:
1269:
1264:
1259:
1254:
1249:
1244:
1239:
1234:
1229:
1214:
1204:
1055:
1030:
1025:
1010:
995:
975:
970:
921:
849:
832:
782:
777:
772:
767:
743:
703:
290:
239:
268:
107:
To obtain the trialkyl derivatives, alkylation of indium trihalides with organolithium reagents is typical.
464:
Li, C.-J.; Chan, T.-H. Organic
Reactions in Aqueous Media with Indium, Tetrahedron Lett. 1991, 32, 7017-7020
1219:
1209:
1020:
1005:
990:
980:
965:
906:
891:
871:
861:
842:
837:
827:
817:
760:
728:
285:
698:
688:
228:
1128:
1043:
985:
948:
943:
931:
901:
807:
802:
797:
738:
713:
93:
936:
748:
683:
673:
116:
1015:
1000:
812:
792:
916:
189:
587:
280:
427:
326:
181:
177:
24:
498:
Frimpong, K; Wzorek, J; Lawlor, C; Spencer, K; Mitzel. T; J. Org. Chem. 2009, 74, 5861–5870.
600:
560:
499:
482:
465:
419:
396:
369:
318:
160:
551:"Allylindation in Aqueous Media: Methyl 3-(Hydroxymethyl)-4-Methyl-2-Methylenepentanoate".
173:
169:
77:
34:
523:
Haddad, T.D; Hirayama, L.C; Buckley, J.J; Singaram, B. J. Org. Chem. 2012, 77, 889–898.
220:
486:
1325:
1288:
469:
400:
219:
The regioselectivity of allylation mediated by indium in water is dependent on the
148:
144:
52:
30:
423:
147:, give an allyl-In(III) intermediate, second, this allyl indide reacts with an
101:
564:
514:
Law, M.C; Cheung, T.W; Wong, K.Y; Chan, T.H. J. Org. Chem. 2007, 72, 923–929.
446:
Yasuda, M; Haga, M; Nagaoka, Y; Baba, A. Eur. J. Org. Chem. 2010, 5359–5363.
45:
Monovalent In is relatively more common than Ga(I) or B(I). One example is
330:
123:
A variety of organoindium(III) species such as InRX and solvates of RXIn, R
604:
206:
373:
165:
503:
322:
643:
414:
Kopasz, J. P.; Hallock, R. B.; Beachley, O. T. (1986). "TrisIndium".
140:
81:
541:
Paquette, L.A; Mitzel, T.M. J. Am. Chem. Soc. 1996, 118, 1931–1937.
89:
60:
29:
615:
80:
is a colorless, volatile solid. It is the preferred source of
577:
Cook, G.R; Kargbo, R; Maity, B. Org. Lett. 2005, 7, 2767–2770.
532:
Isaac, M.B; Chan, T.H. Tetrahedron Lett. 1995, 36, 8957–8960.
131:
In are thought to rapidly interconvert at room temperature.
455:
Koszinowski, K. J. Am. Chem. Soc. 2010, 132, 6032–6040.
201:
Organoindium intermediates do not react with –OH or –CO
159:
The reaction is conducted under the conditions of a
1280:
56:Structure of CpIn, which is a polymer (red = In)
16:Chemistry of compounds with a carbon-indium bond
627:
8:
634:
620:
612:
646:with other elements in the periodic table
96:, such as InP, InAs, AlInGaNP, etc. InMe
586:Haddad, T.D; Hirayama, L.C; Taynton, P;
180:), room temperature ionic liquids, NMF (
68:, an In(I) tetrahedrane (dark gray = In)
59:
51:
301:
663:
590:. Tetrahedron Lett. 2008, 49, 508–511.
7:
1305:Academic research, no widespread use
389:Journal of Organometallic Chemistry
139:IMAs proceed in two steps: first,
14:
360:) by x-ray diffraction studies".
135:Indium-mediated allylations (IMA)
86:metalorganic vapour phase epitaxy
1197:
1116:
1048:
953:
653:
418:. Vol. 24. pp. 89–91.
267:
260:
249:
238:
227:
212:
188:
153:
115:
235:control is illustrated below.
1:
487:10.1016/S0040-4020(99)00641-9
470:10.1016/0040-4039(91)85028-4
401:10.1016/0022-328X(95)05399-A
1348:
424:10.1002/9780470132555.ch27
1194:
1113:
665:
661:
651:
205:H groups. Reactions with
47:cyclopentadienylindium(I)
565:10.15227/orgsyn.077.0107
291:Organothallium Chemistry
286:Organogallium Chemistry
94:compound semiconductors
92:) of indium-containing
1300:Many uses in chemistry
1295:Core organic chemistry
69:
57:
37:
20:Organoindium chemistry
63:
55:
33:
605:10.1039/C39920000747
416:Inorganic Syntheses
374:10.1021/om00095a007
281:Krische allylation
70:
58:
38:
1319:
1318:
1275:
1274:
553:Organic Syntheses
504:10.1021/jo900763u
433:978-0-471-83441-0
323:10.1021/cr300051y
182:n-methylformamide
178:dimethylformamide
73:Organoindium(III)
25:organic synthesis
1339:
1332:Indium compounds
1311:
1306:
1301:
1296:
1201:
1200:
1120:
1119:
1052:
1051:
957:
956:
654:
636:
629:
622:
613:
607:
597:
591:
584:
578:
575:
569:
568:
548:
542:
539:
533:
530:
524:
521:
515:
512:
506:
496:
490:
479:
473:
462:
456:
453:
447:
444:
438:
437:
411:
405:
404:
384:
378:
377:
368:(5): 1051–1059.
341:
335:
334:
311:Chemical Reviews
306:
271:
264:
253:
242:
231:
216:
192:
161:Barbier reaction
157:
143:reacts with the
119:
1347:
1346:
1342:
1341:
1340:
1338:
1337:
1336:
1322:
1321:
1320:
1315:
1314:
1309:
1304:
1299:
1294:
1276:
1198:
1117:
1049:
954:
647:
640:
610:
598:
594:
585:
581:
576:
572:
550:
549:
545:
540:
536:
531:
527:
522:
518:
513:
509:
497:
493:
480:
476:
463:
459:
454:
450:
445:
441:
434:
413:
412:
408:
386:
385:
381:
362:Organometallics
359:
355:
351:
347:
343:
342:
338:
308:
307:
303:
299:
277:
204:
199:
174:tetrahydrofuran
170:Green chemistry
158:
152:
137:
130:
126:
99:
78:Trimethylindium
75:
67:
43:
41:Organoindium(I)
35:Trimethylindium
17:
12:
11:
5:
1345:
1343:
1335:
1334:
1324:
1323:
1317:
1316:
1313:
1312:
1307:
1302:
1297:
1292:
1289:Chemical bonds
1285:
1284:
1282:
1278:
1277:
1273:
1272:
1267:
1262:
1257:
1252:
1247:
1242:
1237:
1232:
1227:
1222:
1217:
1212:
1207:
1202:
1195:
1192:
1191:
1186:
1181:
1176:
1171:
1166:
1161:
1156:
1151:
1146:
1141:
1136:
1131:
1126:
1121:
1114:
1111:
1110:
1106:
1105:
1102:
1099:
1096:
1093:
1090:
1087:
1084:
1081:
1078:
1075:
1072:
1069:
1064:
1061:
1058:
1053:
1046:
1041:
1037:
1036:
1033:
1028:
1023:
1018:
1013:
1008:
1003:
998:
993:
988:
983:
978:
973:
968:
963:
958:
951:
946:
940:
939:
934:
929:
924:
919:
914:
909:
904:
899:
894:
889:
884:
879:
874:
869:
864:
859:
857:
852:
846:
845:
840:
835:
830:
825:
820:
815:
810:
805:
800:
795:
790:
785:
780:
775:
770:
765:
763:
758:
752:
751:
746:
741:
736:
731:
726:
721:
716:
710:
709:
706:
701:
696:
691:
686:
681:
676:
670:
669:
666:
664:
662:
660:
652:
649:
648:
641:
639:
638:
631:
624:
616:
609:
608:
592:
579:
570:
543:
534:
525:
516:
507:
491:
474:
457:
448:
439:
432:
406:
379:
357:
353:
349:
345:
336:
317:(1): 271–401.
300:
298:
295:
294:
293:
288:
283:
276:
273:
221:steric effects
202:
198:
195:
194:
193:
136:
133:
128:
124:
121:
120:
97:
74:
71:
65:
42:
39:
15:
13:
10:
9:
6:
4:
3:
2:
1344:
1333:
1330:
1329:
1327:
1308:
1303:
1298:
1293:
1290:
1287:
1286:
1283:
1279:
1271:
1268:
1266:
1263:
1261:
1258:
1256:
1253:
1251:
1248:
1246:
1243:
1241:
1238:
1236:
1233:
1231:
1228:
1226:
1223:
1221:
1218:
1216:
1213:
1211:
1208:
1206:
1203:
1196:
1193:
1190:
1187:
1185:
1182:
1180:
1177:
1175:
1172:
1170:
1167:
1165:
1162:
1160:
1157:
1155:
1152:
1150:
1147:
1145:
1142:
1140:
1137:
1135:
1132:
1130:
1127:
1125:
1122:
1115:
1112:
1108:
1107:
1103:
1100:
1097:
1094:
1091:
1088:
1085:
1082:
1079:
1076:
1073:
1070:
1068:
1065:
1062:
1059:
1057:
1054:
1047:
1045:
1042:
1039:
1038:
1034:
1032:
1029:
1027:
1024:
1022:
1019:
1017:
1014:
1012:
1009:
1007:
1004:
1002:
999:
997:
994:
992:
989:
987:
984:
982:
979:
977:
974:
972:
969:
967:
964:
962:
959:
952:
950:
947:
945:
942:
941:
938:
935:
933:
930:
928:
925:
923:
920:
918:
915:
913:
910:
908:
905:
903:
900:
898:
895:
893:
890:
888:
885:
883:
880:
878:
875:
873:
870:
868:
865:
863:
860:
858:
856:
853:
851:
848:
847:
844:
841:
839:
836:
834:
831:
829:
826:
824:
821:
819:
816:
814:
811:
809:
806:
804:
801:
799:
796:
794:
791:
789:
786:
784:
781:
779:
776:
774:
771:
769:
766:
764:
762:
759:
757:
754:
753:
750:
747:
745:
742:
740:
737:
735:
732:
730:
727:
725:
722:
720:
717:
715:
712:
711:
707:
705:
702:
700:
697:
695:
692:
690:
687:
685:
682:
680:
677:
675:
672:
671:
667:
659:
656:
655:
650:
645:
642:Compounds of
637:
632:
630:
625:
623:
618:
617:
614:
606:
602:
596:
593:
589:
583:
580:
574:
571:
566:
562:
559:: 107. 2000.
558:
554:
547:
544:
538:
535:
529:
526:
520:
517:
511:
508:
505:
501:
495:
492:
488:
484:
478:
475:
471:
467:
461:
458:
452:
449:
443:
440:
435:
429:
425:
421:
417:
410:
407:
402:
398:
394:
390:
383:
380:
375:
371:
367:
363:
340:
337:
332:
328:
324:
320:
316:
312:
305:
302:
296:
292:
289:
287:
284:
282:
279:
278:
274:
272:
270:
265:
263:
258:
254:
252:
247:
243:
241:
236:
232:
230:
225:
222:
217:
215:
210:
208:
196:
191:
187:
186:
185:
183:
179:
175:
171:
167:
162:
156:
150:
146:
142:
134:
132:
118:
114:
113:
112:
108:
105:
103:
95:
91:
87:
83:
79:
72:
64:Structure of
62:
54:
50:
48:
40:
36:
32:
28:
26:
21:
1310:Bond unknown
911:
595:
582:
573:
556:
552:
546:
537:
528:
519:
510:
494:
477:
460:
451:
442:
415:
409:
392:
388:
382:
365:
361:
339:
314:
310:
304:
266:
259:
255:
248:
244:
237:
233:
226:
218:
211:
200:
149:electrophile
145:allyl halide
138:
122:
109:
106:
76:
44:
19:
18:
588:Singaram, B
197:Selectivity
297:References
102:pyrophoric
1291:to carbon
395:: C1–C5.
207:carbonyls
127:In, and X
1326:Category
331:23110495
275:See also
176:), DMF (
1109:
166:solvent
1281:Legend
644:carbon
430:
329:
141:indium
82:indium
90:MOVPE
428:ISBN
327:PMID
84:for
1255:CEs
1250:CCf
1245:CBk
1240:CCm
1235:CAm
1230:CPu
1225:CNp
1215:CPa
1210:CTh
1189:CYb
1184:CTm
1179:CEr
1174:CHo
1169:CDy
1164:CTb
1159:CGd
1154:CEu
1149:CSm
1144:CPm
1139:CNd
1134:CPr
1129:CCe
1124:CLa
1104:Og
1101:Ts
1098:Lv
1095:Mc
1092:Fl
1089:Nh
1086:Cn
1083:Rg
1080:Ds
1077:Mt
1074:Hs
1071:Bh
1067:CSg
1063:Db
1060:Rf
1044:CRa
1040:Fr
1035:Rn
1031:CAt
1026:CPo
1021:CBi
1016:CPb
1011:CTl
1006:CHg
1001:CAu
996:CPt
991:CIr
986:COs
981:CRe
971:CTa
966:CHf
961:CLu
949:CBa
944:CCs
937:CXe
927:CTe
922:CSb
917:CSn
912:CIn
907:CCd
902:CAg
897:CPd
892:CRh
887:CRu
882:CTc
877:CMo
872:CNb
867:CZr
855:CSr
850:CRb
843:CKr
838:CBr
833:CSe
828:CAs
823:CGe
818:CGa
813:CZn
808:CCu
803:CNi
798:CCo
793:CFe
788:CMn
783:CCr
773:CTi
768:CSc
761:CCa
749:CAr
744:CCl
729:CSi
724:CAl
719:CMg
714:CNa
708:Ne
679:CBe
674:CLi
668:He
601:doi
561:doi
500:doi
483:doi
466:doi
420:doi
397:doi
393:493
370:doi
319:doi
315:113
100:is
49:.
1328::
1270:No
1265:Md
1260:Fm
1220:CU
1205:Ac
1056:Lr
976:CW
932:CI
862:CY
778:CV
756:CK
739:CS
734:CP
704:CF
699:CO
694:CN
689:CC
684:CB
658:CH
557:77
555:.
426:.
391:.
364:.
325:.
313:.
151::
104:.
635:e
628:t
621:v
603::
567:.
563::
502::
489:.
485::
472:.
468::
436:.
422::
403:.
399::
376:.
372::
366:7
358:5
356:H
354:5
350:4
348:H
346:5
333:.
321::
203:2
129:2
125:2
98:3
88:(
66:4
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.