24:
305:
169:
616:
486:
637:
It has been shown that when Aze is misincorporated into proteins in place of proline, Aze deters the growth of competing vegetation and poisons predators. Other studies have shown effects of Aze resulting in a wide range of toxic and teratogenic disorders, including in a range of malformations, in
585:
by α-bromination, followed by removal of hydrogen bromide from the intermediate γ-amino-α-bromobutyric acid and ring closure by treatment with a barium hydroxide solution. An optically active Aze was obtained by treatment of α,γ-diaminobutyric acid dihydrochloride with a mixture of nitrous and
657:. However, the lack of detailed toxicologic data and the need for more direct evidence about the damaging effects of the misincorporation of Aze on specific proteins are reasons why the toxicity of Aze to humans cannot be determined at this time. Molecular studies of human prolyl- and alanyl-
568:
group substituted on one of the ring carbon atoms. The main difference between Aze and proline is the ring of Aze has four members and the ring of proline has five. Aze has the ability to act as an analog of
665:. Even if Aze seems to fit into the active site of both tRNA synthetases (due to its double mimicry effect of alanine and proline), it is rejected by alanyl-tRNA synthetases post-transfer editing system.
932:
499:
594:
Azetidine-2-carboxylic acid has been known since 1955 to be present in rhizomes and fresh foliage of certain plants. It is known to occur in two species from the
354:
925:
586:
hydrochloric acids to yield γ-amino-α-chlorobutyric acid, followed by elimination of hydrogen chloride and cyclization by treatment with barium hydroxide.
857:
Song, Y; Zhou, H; Vo, MN; Shi, Y; Nawaz, MH; Vargas-Rodriguez, O; Diedrich, JK; Yates, JR; Kishi, S; Musier-Forsyth, K; Schimmel, P (22 December 2017).
1403:
1332:
918:
803:
319:
23:
1413:
205:
530:
1322:
941:
262:
1388:
819:
Rubenstein E.; H. Zhou; K.M. Krasinska; A. Chien; C.H. Becker. (2006). "Azetidine-2-carboxylic Acid in Garden Beets".
283:
1398:
1383:
506:
1234:
658:
176:
968:
86:
1040:
1004:
164:
1342:
1186:
1108:
126:
1408:
1337:
1327:
1250:
36:
859:"Double mimicry evades tRNA synthetase editing by toxic vegetable-sourced non-proteinogenic amino acid"
1347:
1136:
870:
705:
Rubenstein E.; T. McLaughlin; R.C. Winant; A. Sanchez; M. Eckart; K.M. Krasinska; A. Chien. (2008). "
1393:
1296:
1162:
1156:
986:
602:
300:
52:
619:
629:, and has also been detected in small quantities in table beets, garden beets, and sugar beets.
1362:
1270:
1030:
996:
896:
836:
799:
776:
724:
1128:
886:
878:
828:
766:
758:
716:
449:
377:
271:
146:
1050:
1010:
654:
565:
62:
874:
304:
168:
1208:
1114:
958:
891:
858:
771:
746:
477:
106:
615:
1377:
1357:
1174:
438:
428:
157:
832:
720:
1267:
1202:
1073:
596:
553:
1086:
1081:
250:
1316:
976:
910:
682:
661:
suggest that Aze is incorporated in proteins as proline with toxic consequences
608:
465:
882:
1352:
1283:
1180:
1100:
1035:
981:
650:
557:
533:
400:
195:
137:
581:
Optically inactive Aze was obtained in small yield from the neurotransmitter
1278:
1197:
1192:
950:
561:
900:
840:
780:
728:
747:"Azetidine-2-carboxylic Acid: a New Cyclic Imino Acid Occurring in Plants"
1214:
1168:
642:
626:
1302:
1060:
646:
570:
537:
418:
237:
177:
762:
1064:
1022:
638:
various animal species including ducks, hamsters, mice, and rabbits.
117:
476:
Except where otherwise noted, data are given for materials in their
225:
614:
97:
85:
75:
582:
216:
914:
328:
InChI=1S/C4H7NO2/c6-4(7)3-1-2-5-3/h3,5H,1-2H2,(H,6,7)/t3-/m0/s1
338:
InChI=1/C4H7NO2/c6-4(7)3-1-2-5-3/h3,5H,1-2H2,(H,6,7)/t3-/m0/s1
288:
573:
and can be incorporated into proteins in place of proline.
625:
Aze is also found in numerous plants from the bean family
494:
641:
Misincorporation of Aze into human proteins can alter
1266:
1259:
1243:
1227:
1149:
1127:
1099:
1072:
1059:
1020:
995:
967:
948:
204:
707:Azetidine-2-carboxylic Acid in the Food Chain".
249:
61:
926:
8:
700:
698:
696:
470:100.1 °C (212.2 °F; 373.2 K)
1263:
1146:
1124:
1096:
1069:
933:
919:
911:
303:
167:
145:
15:
890:
770:
270:
674:
556:, 4 membered ring with nitrogen as its
359:
324:
299:
852:
850:
443:242 °C (468 °F; 515 K)
433:215 °C (419 °F; 488 K)
158:
331:Key: IADUEWIQBXOCDZ-VKHMYHEASA-N
125:
105:
7:
341:Key: IADUEWIQBXOCDZ-VKHMYHEABQ
240:
224:
14:
798:. Kluwer Academic. p. 222.
484:
22:
833:10.1016/j.phytochem.2006.01.028
721:10.1016/j.phytochem.2008.11.007
480:(at 25 °C , 100 kPa).
1:
1404:Non-proteinogenic amino acids
942:Non-proteinogenic amino acids
540:with the molecular formula C
17:Azetidine-2-carboxylic acid
1235:List of abiotic amino acids
1046:Azetidine-2-carboxylic acid
519:Azetidine-2-carboxylic acid
405:101.104 g/mol
40:Azetidine-2-carboxylic acid
1430:
883:10.1038/s41467-017-02201-z
796:Plant secondary metabolism
794:Seigler, David S. (1998).
606:(lily of the valley), and
1311:
474:
459:
370:
350:
315:
45:
35:
30:
21:
1005:gamma-Aminobutyric acid
1244:Engineered amino acids
1187:Dihydroxyphenylglycine
1109:2-Aminoisobutyric acid
622:
1414:Secondary amino acids
1251:Expanded genetic code
863:Nature Communications
618:
1137:Carboxyglutamic acid
1297:Aminolevulinic acid
1228:Abiotic amino acids
1163:Aminolevulinic acid
1157:4-Aminobenzoic acid
987:Diaminopimelic acid
969:bacterial cell wall
875:2017NatCo...8.2281S
751:Biochemical Journal
745:Fowden, L. (1956).
603:Convallaria majalis
450:Solubility in water
18:
1389:Cyclic amino acids
1363:Dehydroamino acids
1323:Encoded (proteins)
623:
620:Lily of the Valley
507:Infobox references
413:crystalline solid
16:
1399:Toxic amino acids
1384:Alpha-Amino acids
1371:
1370:
1333:Non-proteinogenic
1292:
1291:
1271:citric acid cycle
1223:
1222:
1145:
1144:
1123:
1122:
1095:
1094:
1031:ADDA (amino acid)
997:neurotransmitters
763:10.1042/bj0640323
612:(solomon's seal).
515:Chemical compound
513:
512:
284:CompTox Dashboard
87:Interactive image
1421:
1264:
1147:
1129:clotting factors
1125:
1097:
1070:
935:
928:
921:
912:
905:
904:
894:
854:
845:
844:
816:
810:
809:
791:
785:
784:
774:
742:
736:
733:
702:
691:
686:, 12th Edition,
679:
659:tRNA synthetases
497:
491:
488:
487:
378:Chemical formula
308:
307:
292:
290:
274:
253:
242:
228:
208:
179:
171:
160:
149:
129:
109:
89:
65:
26:
19:
1429:
1428:
1424:
1423:
1422:
1420:
1419:
1418:
1374:
1373:
1372:
1367:
1366:
1348:Secondary amino
1307:
1288:
1255:
1239:
1219:
1141:
1119:
1091:
1055:
1051:Quisqualic acid
1016:
1011:Quisqualic acid
991:
963:
944:
939:
909:
908:
856:
855:
848:
818:
817:
813:
806:
793:
792:
788:
744:
743:
739:
704:
703:
694:
680:
676:
671:
655:protein folding
635:
592:
579:
566:carboxylic acid
551:
547:
543:
516:
509:
504:
503:
502: ?)
493:
489:
485:
481:
452:
394:
390:
386:
380:
366:
363:
358:
357:
346:
343:
342:
339:
333:
332:
329:
323:
322:
311:
293:
286:
277:
257:
243:
231:
211:
198:
189:
152:
132:
112:
92:
79:
68:
55:
41:
12:
11:
5:
1427:
1425:
1417:
1416:
1411:
1406:
1401:
1396:
1391:
1386:
1376:
1375:
1369:
1368:
1365:
1360:
1355:
1350:
1345:
1340:
1335:
1330:
1325:
1313:
1312:
1309:
1308:
1306:
1305:
1300:
1293:
1290:
1289:
1287:
1286:
1281:
1275:
1273:
1261:
1257:
1256:
1254:
1253:
1247:
1245:
1241:
1240:
1238:
1237:
1231:
1229:
1225:
1224:
1221:
1220:
1218:
1217:
1212:
1209:Hydroxyproline
1206:
1200:
1195:
1190:
1184:
1178:
1172:
1166:
1160:
1153:
1151:
1143:
1142:
1140:
1139:
1133:
1131:
1121:
1120:
1118:
1117:
1115:Enduracididine
1112:
1105:
1103:
1093:
1092:
1090:
1089:
1084:
1078:
1076:
1067:
1057:
1056:
1054:
1053:
1048:
1043:
1038:
1033:
1027:
1025:
1018:
1017:
1015:
1014:
1008:
1001:
999:
993:
992:
990:
989:
984:
979:
973:
971:
965:
964:
962:
961:
959:Hydroxyproline
955:
953:
946:
945:
940:
938:
937:
930:
923:
915:
907:
906:
846:
827:(9): 898–903.
821:Phytochemistry
811:
804:
786:
757:(2): 323–331.
737:
709:Phytochemistry
692:
673:
672:
670:
667:
634:
631:
591:
588:
578:
575:
549:
545:
541:
514:
511:
510:
505:
483:
482:
478:standard state
475:
472:
471:
468:
462:
461:
457:
456:
453:
448:
445:
444:
441:
435:
434:
431:
425:
424:
421:
415:
414:
411:
407:
406:
403:
397:
396:
392:
388:
384:
381:
376:
373:
372:
368:
367:
365:
364:
361:
353:
352:
351:
348:
347:
345:
344:
340:
337:
336:
334:
330:
327:
326:
318:
317:
316:
313:
312:
310:
309:
296:
294:
282:
279:
278:
276:
275:
267:
265:
259:
258:
256:
255:
246:
244:
236:
233:
232:
230:
229:
221:
219:
213:
212:
210:
209:
201:
199:
194:
191:
190:
188:
187:
183:
181:
173:
172:
162:
154:
153:
151:
150:
142:
140:
134:
133:
131:
130:
122:
120:
114:
113:
111:
110:
102:
100:
94:
93:
91:
90:
82:
80:
73:
70:
69:
67:
66:
58:
56:
51:
48:
47:
43:
42:
39:
33:
32:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1426:
1415:
1412:
1410:
1407:
1405:
1402:
1400:
1397:
1395:
1392:
1390:
1387:
1385:
1382:
1381:
1379:
1364:
1361:
1359:
1358:D-amino acids
1356:
1354:
1351:
1349:
1346:
1344:
1341:
1339:
1336:
1334:
1331:
1329:
1326:
1324:
1320:
1318:
1314:
1310:
1304:
1301:
1298:
1295:
1294:
1285:
1282:
1280:
1277:
1276:
1274:
1272:
1269:
1265:
1262:
1260:Intermediates
1258:
1252:
1249:
1248:
1246:
1242:
1236:
1233:
1232:
1230:
1226:
1216:
1213:
1210:
1207:
1204:
1201:
1199:
1196:
1194:
1191:
1188:
1185:
1182:
1179:
1176:
1175:Penicillamine
1173:
1171:(insectiside)
1170:
1167:
1164:
1161:
1158:
1155:
1154:
1152:
1148:
1138:
1135:
1134:
1132:
1130:
1126:
1116:
1113:
1110:
1107:
1106:
1104:
1102:
1098:
1088:
1085:
1083:
1080:
1079:
1077:
1075:
1074:Beta-peptides
1071:
1068:
1066:
1062:
1058:
1052:
1049:
1047:
1044:
1042:
1039:
1037:
1034:
1032:
1029:
1028:
1026:
1024:
1019:
1012:
1009:
1006:
1003:
1002:
1000:
998:
994:
988:
985:
983:
980:
978:
975:
974:
972:
970:
966:
960:
957:
956:
954:
952:
947:
943:
936:
931:
929:
924:
922:
917:
916:
913:
902:
898:
893:
888:
884:
880:
876:
872:
868:
864:
860:
853:
851:
847:
842:
838:
834:
830:
826:
822:
815:
812:
807:
805:0-412-01981-7
801:
797:
790:
787:
782:
778:
773:
768:
764:
760:
756:
752:
748:
741:
738:
735:
732:
730:
726:
722:
718:
714:
708:
701:
699:
697:
693:
689:
685:
684:
678:
675:
668:
666:
664:
660:
656:
652:
648:
644:
639:
632:
630:
628:
621:
617:
613:
611:
610:
605:
604:
599:
598:
589:
587:
584:
576:
574:
572:
567:
563:
559:
555:
539:
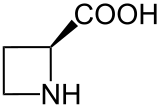
536:homologue of
535:
532:
529:) is a plant
528:
524:
521:(abbreviated
520:
508:
501:
496:
479:
473:
469:
467:
464:
463:
458:
455:5.0 g/100 ml
454:
451:
447:
446:
442:
440:
439:Boiling point
437:
436:
432:
430:
429:Melting point
427:
426:
422:
420:
417:
416:
412:
409:
408:
404:
402:
399:
398:
382:
379:
375:
374:
369:
360:
356:
349:
335:
325:
321:
314:
306:
302:
301:DTXSID0044020
298:
297:
295:
285:
281:
280:
273:
269:
268:
266:
264:
261:
260:
252:
248:
247:
245:
239:
235:
234:
227:
223:
222:
220:
218:
215:
214:
207:
203:
202:
200:
197:
193:
192:
185:
184:
182:
180:
175:
174:
170:
166:
163:
161:
159:ECHA InfoCard
156:
155:
148:
144:
143:
141:
139:
136:
135:
128:
127:ChEMBL1165239
124:
123:
121:
119:
116:
115:
108:
104:
103:
101:
99:
96:
95:
88:
84:
83:
81:
77:
72:
71:
64:
60:
59:
57:
54:
50:
49:
44:
38:
34:
29:
25:
20:
1409:Plant toxins
1315:
1268:Mitochondria
1203:Homocysteine
1183:(Alzheimers)
1165:(metabolism)
1045:
866:
862:
824:
820:
814:
795:
789:
754:
750:
740:
734:
712:
710:
706:
687:
681:
677:
662:
640:
636:
624:
607:
601:
597:Asparagaceae
595:
593:
580:
554:heterocyclic
526:
522:
518:
517:
46:Identifiers
1353:Imino acids
1317:Amino acids
1177:(chelation)
977:Lanthionine
869:(1): 2281.
683:Merck Index
609:Polygonatum
552:. Aze is a
531:non-protein
466:Flash point
423:1.275 g/cm
410:Appearance
371:Properties
362:O=C(O)1NCC1
165:100.016.693
1394:Azetidines
1378:Categories
1343:Glucogenic
1284:Citrulline
1211:(collagen)
1181:Norleucine
1101:Antibiotic
1041:thialysine
1036:Canavanine
982:Norleucine
715:(1): 1–5.
669:References
651:hemoglobin
590:Occurrence
558:heteroatom
534:amino acid
401:Molar mass
272:5GZ3E0L9ZU
196:IUPHAR/BPS
138:ChemSpider
107:CHEBI:6198
74:3D model (
53:CAS Number
37:IUPAC name
1338:Ketogenic
1328:Essential
1279:Ornithine
1198:Norvaline
1193:Sarcosine
1087:β-Leucine
1082:β-Alanine
951:cell wall
577:Synthesis
564:), and a
562:azetidine
186:218-362-5
178:EC Number
63:2133-34-8
1215:Hypusine
1169:Canaline
901:29273753
841:16516254
781:13363844
729:19101705
643:collagen
633:Toxicity
627:Fabaceae
460:Hazards
254: L-
1303:Cystine
1299:(5-ALA)
1205:(heart)
1061:Medical
1013:(toxic)
892:5741666
871:Bibcode
772:1199734
663:in vivo
647:keratin
571:proline
538:proline
500:what is
498: (
419:Density
395:
238:PubChem
1189:(DHPG)
1159:(PABA)
1065:Health
1023:toxins
1021:human
1007:(GABA)
949:Plant
899:
889:
839:
802:
779:
769:
727:
653:, and
495:verify
492:
355:SMILES
226:C08267
118:ChEMBL
31:Names
1319:types
1150:Other
1111:(AIB)
320:InChI
251:16486
147:15628
98:ChEBI
76:JSmol
897:PMID
837:PMID
800:ISBN
777:PMID
725:PMID
688:6089
583:GABA
560:(an
263:UNII
217:KEGG
206:4686
887:PMC
879:doi
829:doi
767:PMC
759:doi
717:doi
527:Azc
525:or
523:Aze
289:EPA
241:CID
1380::
1321::
895:.
885:.
877:.
865:.
861:.
849:^
835:.
825:67
823:.
775:.
765:.
755:64
753:.
749:.
723:.
713:70
711:.
695:^
649:,
645:,
600:-
548:NO
391:NO
1063:/
934:e
927:t
920:v
903:.
881::
873::
867:8
843:.
831::
808:.
783:.
761::
731:.
719::
690:.
550:2
546:7
544:H
542:4
490:N
393:2
389:7
387:H
385:4
383:C
291:)
287:(
78:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.