350:
327:
224:
249:
601:
356:
255:
29:
1231:
2517:
2502:
2547:
2532:
2562:
1160:
55:
2319:
Brandenberger R, Wei H, Zhang S, Lei S, Murage J, Fisk GJ, Li Y, Xu C, Fang R, Guegler K, Rao MS, Mandalam R, Lebkowski J, Stanton LW (2005). "Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation".
2012:
Boultwood J, Strickson AJ, Jabs EW, Cheng JF, Fidler C, Wainscoat JS (2000). "Physical mapping of the human ATX1 homologue (HAH1) to the critical region of the 5q- syndrome within 5q32, and immediately adjacent to the SPARC gene".
2386:
Banci L, Bertini I, Ciofi-Baffoni S, Chasapis CT, Hadjiliadis N, Rosato A (2005). "An NMR study of the interaction between the human copper(I) chaperone and the second and fifth metal-binding domains of the Menkes protein".
1174:
stems from initial characterization that showed that ATOX1 protected cells from reactive oxygen species. Since then, the primary role of ATOX1 has been established as a copper metallochaperone protein found in the
1310:
mechanism and imparts specificity for both the metal and transporter. Since the ligand exchange accelerates that transfer and the reaction has a shallow thermodynamic gradient, it is said to be under
1306:
mechanism, where Cu(I) transiently adopts a 3-coordinate geometry with cysteine ligands from ATOX1 and the associated transporter. The ligand exchange mechanism allows for faster exchange than a
2239:
Lutsenko S, Tsivkovskii R, Walker JM (2003). "Functional properties of the human copper-transporting ATPase ATP7B (the Wilson's disease protein) and regulation by metallochaperone Atox1".
1179:
of eukaryotes. A metallochaperone is an important protein that has metal trafficking and sequestration roles. As a metal sequestration protein, ATOX1 is capable of binding free metals
1975:
Wernimont AK, Huffman DL, Lamb AL, O'Halloran TV, Rosenzweig AC (2000). "Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins".
2206:"X-ray absorption spectroscopy of the copper chaperone HAH1 reveals a linear two-coordinate Cu(I) center capable of adduct formation with exogenous thiols and phosphines"
363:
262:
1215:, a ferroxidase required for iron metabolism, within the golgi apparatus. In addition to the metallochaperone function, recent reports have characterized ATOX1 as a
2357:
Anastassopoulou I, Banci L, Bertini I, Cantini F, Katsari E, Rosato A (2004). "Solution structure of the apo and copper(I)-loaded human metallochaperone HAH1".
1348:
treatment of Wilson's
Disease is under review. Since ATOX1 forms a stable complex tetrathiomolybdate, it is being studied as the potential therapeutic target.
1211:, Atx1 delivers Cu(I) to a homologous transporter, Ccc2. The delivery of copper to ATPase transporters is vital for the subsequent insertion of copper into
2602:
834:
185:
815:
2787:
2481:
1598:
1552:
1425:
3008:
1407:
2044:"Metallochaperone Atox1 transfers copper to the NH2-terminal domain of the Wilson's disease protein and regulates its catalytic activity"
349:
1034:
1825:
1520:
1041:
326:
2077:
Moore SD, Helmle KE, Prat LM, Cox DW (2003). "Tissue localization of the copper chaperone ATOX1 and its potential role in disease".
1287:
1889:"Characterization of the interaction between the Wilson and Menkes disease proteins and the cytoplasmic copper chaperone, HAH1p"
1484:
2942:
1394:
1373:
2595:
1390:
1831:
248:
223:
52:
2516:
2501:
2546:
2531:
1854:"HAH1 is a copper-binding protein with distinct amino acid residues mediating copper homeostasis and antioxidant defense"
1369:
165:
2561:
2161:"Kinetic analysis of the interaction of the copper chaperone Atox1 with the metal binding sites of the Menkes protein"
1338:
362:
261:
355:
254:
2588:
2474:
1315:
1254:
change is induced upon coordination to Cu(I). Cu(I) is coordinated in a distorted linear geometry to sulfurs of
2978:
1207:
1143:
879:
173:
1924:"Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis"
2983:
1186:
860:
2845:
2615:
1299:
2960:
2751:
2715:
2632:
1246:
located in between the first β-sheet and α-helix. The metal binding motif is largely solvent exposed in
2952:
2934:
2694:
2689:
2684:
2467:
2248:
1935:
1737:
1445:"Identification and functional expression of HAH1, a novel human gene involved in copper homeostasis"
1219:
237:
1837:
1267:
1263:
152:
2970:
2412:
2345:
2272:
2102:
2000:
1259:
1148:
1134:
197:
1334:
There is a link between ATOX1 levels and sensitivity of cells for Pt-based drugs like cisplatin.
1017:
996:
970:
949:
1330:
directly associated with ATOX1 malfunction, there is currently active research in a few areas:
3013:
2916:
2447:
2404:
2374:
2337:
2307:
2264:
2227:
2192:
2147:
2094:
2065:
2030:
1992:
1963:
1910:
1875:
1804:
1763:
1721:
1702:
1650:
1594:
1548:
1516:
1466:
1311:
1251:
1100:
600:
145:
45:
2832:
2623:
2437:
2396:
2366:
2329:
2297:
2256:
2217:
2182:
2172:
2137:
2127:
2086:
2055:
2022:
1984:
1953:
1943:
1900:
1865:
1794:
1753:
1745:
1692:
1684:
1640:
1632:
1456:
442:
373:
317:
272:
193:
2924:
2870:
2706:
1688:
1239:
1198:
417:
1726:"Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation"
1720:
Alvarez HM, Xue Y, Robinson CD, Canalizo-Hernández MA, Marvin RG, Kelly RA, Mondragón A,
2252:
2159:
Strausak D, Howie MK, Firth SD, Schlicksupp A, Pipkorn R, Multhaup G, Mercer JF (2003).
1939:
1741:
2652:
2442:
2425:
2260:
1758:
1725:
1697:
1672:
1645:
1620:
1243:
1190:
28:
2142:
2115:
1230:
749:
744:
739:
734:
729:
724:
719:
714:
709:
693:
677:
672:
667:
662:
657:
652:
647:
642:
3002:
2850:
2799:
2426:"Role of antioxidant-1 in extracellular superoxide dismutase function and expression"
2400:
2187:
1958:
1923:
1212:
1193:. As a metal trafficking protein, ATOX1 is responsible for shuttling copper from the
629:
2349:
2276:
2106:
2004:
177:
2725:
2416:
1171:
1164:
435:
214:
201:
2907:
2814:
2809:
2782:
2680:
2675:
2424:
Jeney V, Itoh S, Wendt M, Gradek Q, Ushio-Fukai M, Harrison DG, Fukai T (2005).
1430:
National Center for
Biotechnology Information, U.S. National Library of Medicine
1412:
National Center for
Biotechnology Information, U.S. National Library of Medicine
1159:
1118:
2580:
518:
2804:
2671:
2611:
2090:
334:
231:
181:
2286:"Binding of copper(I) by the Wilson disease protein and its copper chaperone"
1948:
1905:
1888:
2792:
1749:
1461:
1444:
1307:
1275:
1247:
1202:
1176:
1138:
779:
578:
456:
401:
388:
300:
287:
189:
2451:
2408:
2378:
2341:
2311:
2302:
2285:
2268:
2231:
2222:
2205:
2196:
2177:
2160:
2151:
2098:
2069:
2060:
2043:
2034:
1996:
1967:
1914:
1870:
1853:
1808:
1767:
1706:
1654:
2132:
2026:
1879:
1470:
1443:
Klomp LW, Lin SJ, Yuan DS, Klausner RD, Culotta VC, Gitlin JD (May 1997).
1081:
1076:
2773:
2760:
2730:
2720:
2666:
2641:
1065:
924:
905:
2893:
2888:
2855:
1327:
1255:
1194:
1181:
1122:
1103:
891:
846:
2370:
1799:
1782:
1636:
1286:
122:
118:
114:
110:
106:
102:
98:
94:
90:
86:
82:
78:
74:
1303:
1271:
1216:
1170:
ATOX1 is an abbreviation of the full name
Antioxidant Protein 1. The
1114:
1097:
1049:
801:
1278:
via this motif, but a physiological role, if any, is not yet known.
2333:
2840:
1988:
1295:
1285:
1229:
1158:
1130:
1126:
764:
760:
2883:
2878:
2777:
2661:
1591:
Binding, transport and storage of metal ions in biological cells
1242:-like βαββαβ fold and coordinates to Cu(I) via a MXCXXC binding
1110:
169:
2584:
2463:
1852:
Hung IH, Casareno RL, Labesse G, Mathews FS, Gitlin JD (1998).
1485:"Entrez Gene: ATOX1 ATX1 antioxidant protein 1 homolog (yeast)"
1197:
to ATPase transporters ATP7A and ATP7B that move copper to the
2459:
1887:
Larin D, Mekios C, Das K, Ross B, Yang AS, Gilliam TC (1999).
609:
1290:
Model of ligand exchange copper transfer from Atx1 to Ccc2
2116:"Genomic organization of ATOX1, a human copper chaperone"
1513:
Biological
Inorganic Chemistry, Structure and Reactivity
425:
1511:
Bertini I, Gray HB, Steifel EI, Valentine JS (2006).
590:
2969:
2951:
2933:
2915:
2906:
2869:
2831:
2750:
2743:
2705:
2651:
2640:
2631:
2622:
1010:
989:
963:
942:
1386:
1384:
1382:
1365:
1363:
1361:
2284:Wernimont AK, Yatsunyk LA, Rosenzweig AC (2004).
1922:Hamza I, Schaefer M, Klomp LW, Gitlin JD (1999).
372:
271:
1666:
1664:
1783:"Metallodrugs in medicinal inorganic chemistry"
1185:, in order to protect cells from generation of
16:Protein-coding gene in the species Homo sapiens
1506:
1504:
1502:
1500:
1498:
1496:
1494:
1391:GRCm38: Ensembl release 89: ENSMUSG00000018585
1274:. ATOX1 also binds Hg(II), Cd(II), Ag(I), and
1262:of 120°. The overall -1 charge of the primary
2596:
2475:
2042:Walker JM, Tsivkovskii R, Lutsenko S (2002).
1584:
643:copper ion transmembrane transporter activity
8:
1614:
1612:
1610:
1582:
1580:
1578:
1576:
1574:
1572:
1570:
1568:
1566:
1564:
1538:
1536:
1534:
1532:
1270:that contains a proximal positively charged
156:, ATX1, HAH1, antioxidant 1 copper chaperone
1370:GRCh38: Ensembl release 89: ENSG00000177556
2912:
2747:
2648:
2637:
2628:
2603:
2589:
2581:
2482:
2468:
2460:
2204:Ralle M, Lutsenko S, Blackburn NJ (2003).
1621:"Structural biology of copper trafficking"
775:
625:
413:
312:
209:
63:
2441:
2301:
2221:
2186:
2176:
2141:
2131:
2059:
1957:
1947:
1904:
1869:
1798:
1757:
1696:
1644:
1460:
725:protein maturation by copper ion transfer
1137:proteins are found in a wide variety of
745:negative regulation of apoptotic process
2788:Iron-responsive element-binding protein
2497:
1357:
1326:Although there are presently no known
1294:ATOX1 transfers Cu(I) to transporters
18:
2114:Liu PC, Koeller DM, Kaler SG (2004).
1689:10.1146/annurev-biochem-030409-143539
1671:Robinson NJ, Winge DR (7 June 2010).
377:
338:
333:
276:
235:
230:
7:
2525:: CRYSTAL STRUCTURE OF MERCURY-HAH1
2510:: CRYSTAL STRUCTURE OF CADMIUM-HAH1
1619:Boal AK, Rosenzweig AC (Oct 2009).
1226:Structure & metal coordination
1117:, ATOX1 plays a key role in copper
2555:: Solution structure of Cu(I) HAH1
2540:: CRYSTAL STRUCTURE OF COPPER-HAH1
2443:10.1161/01.RES.0000162001.57896.66
2261:10.1111/j.1749-6632.2003.tb07161.x
1007:
986:
960:
939:
915:
896:
870:
851:
825:
806:
730:copper ion transmembrane transport
595:
537:choroid plexus of fourth ventricle
513:
451:
430:
14:
2560:
2545:
2530:
2515:
2500:
2401:10.1111/j.1742-4658.2004.04526.x
1163:Schematic of copper homeostasis
668:copper-dependent protein binding
599:
361:
354:
348:
325:
260:
253:
247:
222:
27:
2943:Phosphoric acids and phosphates
2570:: Solution structure of apoHAH1
1121:as it delivers copper from the
735:cellular copper ion homeostasis
610:More reference expression data
579:More reference expression data
499:olfactory zone of nasal mucosa
1:
2676:Ferroportin (SLC11A3/SLC40A1)
1781:Mjos KD, Orvig C (Apr 2014).
1677:Annual Review of Biochemistry
1268:secondary coordination sphere
346:
245:
1928:Proc. Natl. Acad. Sci. U.S.A
1724:, O'Halloran TV (Jan 2010).
1593:. : Royal Soc Of Chemistry.
1515:. University Science Books.
740:response to oxidative stress
3009:Genes on human chromosome 5
1339:ammonium tetrathiomolybdate
1147:as ATX1, and all contain a
495:right lobe of thyroid gland
491:stromal cell of endometrium
3030:
1836:gene details page in the
1673:"Copper metallochaperones"
1266:is stabilized through the
1234:ATOX1 copper coordination
487:left lobe of thyroid gland
2769:
2495:
2091:10.1007/s00335-002-2172-9
1426:"Mouse PubMed Reference:"
1408:"Human PubMed Reference:"
1080:
1075:
1071:
1064:
1048:
1035:Chr 5: 151.74 – 151.77 Mb
1029:
1014:
993:
982:
967:
946:
935:
922:
918:
903:
899:
890:
877:
873:
858:
854:
845:
832:
828:
813:
809:
800:
785:
778:
774:
758:
663:copper chaperone activity
648:metallochaperone activity
628:
624:
607:
598:
589:
576:
525:
516:
463:
454:
424:
416:
412:
395:
382:
345:
324:
315:
311:
294:
281:
244:
221:
212:
208:
163:
160:
150:
143:
138:
71:
66:
49:
44:
39:
35:
26:
21:
2979:Calcium-sensing receptor
2726:Calreticulin/mobilferrin
1949:10.1073/pnas.96.23.13363
1906:10.1074/jbc.274.40.28497
1589:Maret W, Wedd A (2014).
1545:Metallomics and the cell
1302:. Transfer occurs via a
1208:Saccharomyces cerevisiae
1144:Saccharomyces cerevisiae
1042:Chr 11: 55.34 – 55.35 Mb
379:11 B1.3|11 33.07 cM
2984:Calcium-binding protein
1750:10.1126/science.1179907
1547:. Dordrecht: Springer.
1462:10.1074/jbc.272.14.9221
1187:reactive oxygen species
1106:that is encoded by the
2303:10.1074/jbc.M311213200
2223:10.1074/jbc.M303474200
2178:10.1074/jbc.M212437200
2061:10.1074/jbc.M203845200
1871:10.1074/jbc.273.3.1749
1291:
1235:
1189:and mismetallation of
1167:
1151:metal binding domain.
2961:Magnesium transporter
2752:Iron-binding proteins
2716:Duodenal cytochrome B
2188:10536/DRO/DU:30002199
2133:10.1186/1471-2156-4-4
2027:10.1007/s004390051020
1322:Clinical significance
1289:
1233:
1162:
340:Chromosome 11 (mouse)
2953:Magnesium metabolism
2935:Phosphate metabolism
2685:Transferrin receptor
2241:Ann. N. Y. Acad. Sci
1830:genome location and
1314:control rather than
1220:transcription factor
715:copper ion transport
549:olfactory epithelium
503:left coronary artery
479:right adrenal cortex
238:Chromosome 5 (human)
67:List of PDB id codes
40:Available structures
2253:2003NYASA.986..204L
1940:1999PNAS...9613363H
1838:UCSC Genome Browser
1742:2010Sci...327..331A
1264:coordination sphere
1199:trans-Golgi network
710:metal ion transport
483:left adrenal cortex
475:right adrenal gland
471:right lobe of liver
2971:Calcium metabolism
1292:
1236:
1203:secretory vesicles
1168:
880:ENSMUSG00000018585
703:Biological process
687:Cellular component
653:copper ion binding
636:Molecular function
561:left lobe of liver
2996:
2995:
2992:
2991:
2917:Sodium metabolism
2902:
2901:
2833:Copper metabolism
2827:
2826:
2823:
2822:
2739:
2738:
2578:
2577:
2371:10.1021/bi0487591
1977:Nat. Struct. Biol
1899:(40): 28497–504.
1800:10.1021/cr400460s
1736:(5963): 331–334.
1637:10.1021/cr900104z
1631:(10): 4760–4779.
1600:978-1-84973-599-5
1554:978-94-007-5561-1
1337:The mechanism of
1091:
1090:
1087:
1086:
1060:
1059:
1025:
1024:
1004:
1003:
978:
977:
957:
956:
931:
930:
912:
911:
886:
885:
867:
866:
841:
840:
822:
821:
770:
769:
750:copper ion export
673:metal ion binding
620:
619:
616:
615:
585:
584:
572:
571:
510:
509:
408:
407:
307:
306:
202:ATOX1 - orthologs
134:
133:
130:
129:
50:Ortholog search:
3021:
2913:
2748:
2649:
2638:
2629:
2624:Transition metal
2616:Metal metabolism
2605:
2598:
2591:
2582:
2564:
2549:
2534:
2519:
2504:
2484:
2477:
2470:
2461:
2455:
2445:
2420:
2382:
2365:(41): 13046–53.
2353:
2315:
2305:
2296:(13): 12269–76.
2280:
2235:
2225:
2216:(25): 23163–70.
2200:
2190:
2180:
2155:
2145:
2135:
2110:
2073:
2063:
2038:
2008:
1971:
1961:
1951:
1918:
1908:
1883:
1873:
1813:
1812:
1802:
1793:(8): 4540–4563.
1787:Chemical Reviews
1778:
1772:
1771:
1761:
1717:
1711:
1710:
1700:
1668:
1659:
1658:
1648:
1625:Chemical Reviews
1616:
1605:
1604:
1586:
1559:
1558:
1543:Banci L (2013).
1540:
1527:
1526:
1508:
1489:
1488:
1481:
1475:
1474:
1464:
1440:
1434:
1433:
1422:
1416:
1415:
1404:
1398:
1388:
1377:
1367:
1125:to transporters
1101:metallochaperone
1073:
1072:
1044:
1037:
1020:
1008:
999:
987:
983:RefSeq (protein)
973:
961:
952:
940:
916:
897:
871:
852:
826:
807:
776:
626:
612:
603:
596:
581:
521:
519:Top expressed in
514:
459:
457:Top expressed in
452:
431:
414:
404:
391:
380:
365:
358:
352:
341:
329:
313:
303:
290:
279:
264:
257:
251:
240:
226:
210:
204:
155:
148:
125:
64:
58:
37:
36:
31:
19:
3029:
3028:
3024:
3023:
3022:
3020:
3019:
3018:
2999:
2998:
2997:
2988:
2965:
2947:
2929:
2898:
2871:Zinc metabolism
2865:
2819:
2765:
2735:
2707:Iron(III) oxide
2701:
2643:
2633:Iron metabolism
2618:
2609:
2579:
2574:
2571:
2565:
2556:
2550:
2541:
2535:
2526:
2520:
2511:
2505:
2491:
2488:
2458:
2423:
2385:
2356:
2322:Nat. Biotechnol
2318:
2283:
2238:
2203:
2171:(23): 20821–7.
2158:
2113:
2076:
2054:(31): 27953–9.
2041:
2011:
1974:
1934:(23): 13363–8.
1921:
1886:
1851:
1847:
1845:Further reading
1821:
1816:
1780:
1779:
1775:
1719:
1718:
1714:
1670:
1669:
1662:
1618:
1617:
1608:
1601:
1588:
1587:
1562:
1555:
1542:
1541:
1530:
1523:
1510:
1509:
1492:
1483:
1482:
1478:
1442:
1441:
1437:
1424:
1423:
1419:
1406:
1405:
1401:
1389:
1380:
1368:
1359:
1355:
1347:
1343:
1324:
1304:ligand exchange
1284:
1228:
1191:metalloproteins
1157:
1082:View/Edit Mouse
1077:View/Edit Human
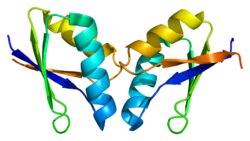
1040:
1033:
1030:Location (UCSC)
1016:
995:
969:
948:
861:ENSG00000177556
754:
698:
682:
658:protein binding
608:
577:
568:
563:
559:
557:skin of abdomen
555:
551:
547:
543:
539:
535:
531:
517:
506:
501:
497:
493:
489:
485:
481:
477:
473:
469:
455:
399:
386:
378:
368:
367:
366:
359:
339:
316:Gene location (
298:
285:
277:
267:
266:
265:
258:
236:
213:Gene location (
164:
151:
144:
73:
51:
17:
12:
11:
5:
3027:
3025:
3017:
3016:
3011:
3001:
3000:
2994:
2993:
2990:
2989:
2987:
2986:
2981:
2975:
2973:
2967:
2966:
2964:
2963:
2957:
2955:
2949:
2948:
2946:
2945:
2939:
2937:
2931:
2930:
2928:
2927:
2921:
2919:
2910:
2904:
2903:
2900:
2899:
2897:
2896:
2891:
2886:
2881:
2875:
2873:
2867:
2866:
2864:
2863:
2858:
2853:
2848:
2843:
2837:
2835:
2829:
2828:
2825:
2824:
2821:
2820:
2818:
2817:
2812:
2807:
2802:
2797:
2796:
2795:
2785:
2780:
2770:
2767:
2766:
2764:
2763:
2757:
2755:
2745:
2741:
2740:
2737:
2736:
2734:
2733:
2728:
2723:
2718:
2712:
2710:
2703:
2702:
2700:
2699:
2698:
2697:
2692:
2678:
2669:
2664:
2662:DMT1 (SLC11A2)
2658:
2656:
2653:Iron(II) oxide
2646:
2635:
2626:
2620:
2619:
2610:
2608:
2607:
2600:
2593:
2585:
2576:
2575:
2573:
2572:
2566:
2559:
2557:
2551:
2544:
2542:
2536:
2529:
2527:
2521:
2514:
2512:
2506:
2499:
2496:
2493:
2492:
2489:
2487:
2486:
2479:
2472:
2464:
2457:
2456:
2421:
2383:
2354:
2334:10.1038/nbt971
2316:
2281:
2236:
2201:
2156:
2111:
2074:
2039:
2009:
1972:
1919:
1884:
1864:(3): 1749–54.
1848:
1846:
1843:
1842:
1841:
1820:
1819:External links
1817:
1815:
1814:
1773:
1722:Penner-Hahn JE
1712:
1683:(1): 537–562.
1660:
1606:
1599:
1560:
1553:
1528:
1522:978-1891389436
1521:
1490:
1476:
1455:(14): 9221–6.
1435:
1417:
1399:
1378:
1356:
1354:
1351:
1350:
1349:
1345:
1341:
1335:
1323:
1320:
1283:
1282:Metal transfer
1280:
1252:conformational
1227:
1224:
1156:
1153:
1113:in humans. In
1089:
1088:
1085:
1084:
1079:
1069:
1068:
1062:
1061:
1058:
1057:
1055:
1053:
1046:
1045:
1038:
1031:
1027:
1026:
1023:
1022:
1012:
1011:
1005:
1002:
1001:
991:
990:
984:
980:
979:
976:
975:
965:
964:
958:
955:
954:
944:
943:
937:
933:
932:
929:
928:
920:
919:
913:
910:
909:
901:
900:
894:
888:
887:
884:
883:
875:
874:
868:
865:
864:
856:
855:
849:
843:
842:
839:
838:
830:
829:
823:
820:
819:
811:
810:
804:
798:
797:
792:
787:
783:
782:
772:
771:
768:
767:
756:
755:
753:
752:
747:
742:
737:
732:
727:
722:
717:
712:
706:
704:
700:
699:
697:
696:
690:
688:
684:
683:
681:
680:
678:ATPase binding
675:
670:
665:
660:
655:
650:
645:
639:
637:
633:
632:
622:
621:
618:
617:
614:
613:
605:
604:
593:
587:
586:
583:
582:
574:
573:
570:
569:
567:
566:
562:
558:
554:
550:
546:
542:
538:
534:
530:
526:
523:
522:
511:
508:
507:
505:
504:
500:
496:
492:
488:
484:
480:
476:
472:
468:
464:
461:
460:
448:
447:
439:
428:
422:
421:
418:RNA expression
410:
409:
406:
405:
397:
393:
392:
384:
381:
376:
370:
369:
360:
353:
347:
343:
342:
337:
331:
330:
322:
321:
309:
308:
305:
304:
296:
292:
291:
283:
280:
275:
269:
268:
259:
252:
246:
242:
241:
234:
228:
227:
219:
218:
206:
205:
162:
158:
157:
149:
141:
140:
136:
135:
132:
131:
128:
127:
69:
68:
60:
59:
48:
42:
41:
33:
32:
24:
23:
15:
13:
10:
9:
6:
4:
3:
2:
3026:
3015:
3012:
3010:
3007:
3006:
3004:
2985:
2982:
2980:
2977:
2976:
2974:
2972:
2968:
2962:
2959:
2958:
2956:
2954:
2950:
2944:
2941:
2940:
2938:
2936:
2932:
2926:
2923:
2922:
2920:
2918:
2914:
2911:
2909:
2905:
2895:
2892:
2890:
2887:
2885:
2882:
2880:
2877:
2876:
2874:
2872:
2868:
2862:
2859:
2857:
2854:
2852:
2851:Ceruloplasmin
2849:
2847:
2844:
2842:
2839:
2838:
2836:
2834:
2830:
2816:
2813:
2811:
2808:
2806:
2803:
2801:
2800:Ceruloplasmin
2798:
2794:
2791:
2790:
2789:
2786:
2784:
2781:
2779:
2775:
2772:
2771:
2768:
2762:
2759:
2758:
2756:
2753:
2749:
2746:
2742:
2732:
2729:
2727:
2724:
2722:
2719:
2717:
2714:
2713:
2711:
2708:
2704:
2696:
2693:
2691:
2688:
2687:
2686:
2682:
2679:
2677:
2673:
2670:
2668:
2665:
2663:
2660:
2659:
2657:
2654:
2650:
2647:
2645:
2642:Absorption in
2639:
2636:
2634:
2630:
2627:
2625:
2621:
2617:
2613:
2606:
2601:
2599:
2594:
2592:
2587:
2586:
2583:
2569:
2563:
2558:
2554:
2548:
2543:
2539:
2533:
2528:
2524:
2518:
2513:
2509:
2503:
2498:
2494:
2485:
2480:
2478:
2473:
2471:
2466:
2465:
2462:
2453:
2449:
2444:
2439:
2435:
2431:
2427:
2422:
2418:
2414:
2410:
2406:
2402:
2398:
2395:(3): 865–71.
2394:
2390:
2384:
2380:
2376:
2372:
2368:
2364:
2360:
2355:
2351:
2347:
2343:
2339:
2335:
2331:
2328:(6): 707–16.
2327:
2323:
2317:
2313:
2309:
2304:
2299:
2295:
2291:
2290:J. Biol. Chem
2287:
2282:
2278:
2274:
2270:
2266:
2262:
2258:
2254:
2250:
2247:(1): 204–11.
2246:
2242:
2237:
2233:
2229:
2224:
2219:
2215:
2211:
2210:J. Biol. Chem
2207:
2202:
2198:
2194:
2189:
2184:
2179:
2174:
2170:
2166:
2165:J. Biol. Chem
2162:
2157:
2153:
2149:
2144:
2139:
2134:
2129:
2125:
2121:
2117:
2112:
2108:
2104:
2100:
2096:
2092:
2088:
2085:(10): 563–8.
2084:
2080:
2075:
2071:
2067:
2062:
2057:
2053:
2049:
2048:J. Biol. Chem
2045:
2040:
2036:
2032:
2028:
2024:
2020:
2016:
2010:
2006:
2002:
1998:
1994:
1990:
1989:10.1038/78999
1986:
1983:(9): 766–71.
1982:
1978:
1973:
1969:
1965:
1960:
1955:
1950:
1945:
1941:
1937:
1933:
1929:
1925:
1920:
1916:
1912:
1907:
1902:
1898:
1894:
1893:J. Biol. Chem
1890:
1885:
1881:
1877:
1872:
1867:
1863:
1859:
1858:J. Biol. Chem
1855:
1850:
1849:
1844:
1839:
1835:
1834:
1829:
1828:
1823:
1822:
1818:
1810:
1806:
1801:
1796:
1792:
1788:
1784:
1777:
1774:
1769:
1765:
1760:
1755:
1751:
1747:
1743:
1739:
1735:
1731:
1727:
1723:
1716:
1713:
1708:
1704:
1699:
1694:
1690:
1686:
1682:
1678:
1674:
1667:
1665:
1661:
1656:
1652:
1647:
1642:
1638:
1634:
1630:
1626:
1622:
1615:
1613:
1611:
1607:
1602:
1596:
1592:
1585:
1583:
1581:
1579:
1577:
1575:
1573:
1571:
1569:
1567:
1565:
1561:
1556:
1550:
1546:
1539:
1537:
1535:
1533:
1529:
1524:
1518:
1514:
1507:
1505:
1503:
1501:
1499:
1497:
1495:
1491:
1486:
1480:
1477:
1472:
1468:
1463:
1458:
1454:
1450:
1446:
1439:
1436:
1431:
1427:
1421:
1418:
1413:
1409:
1403:
1400:
1396:
1392:
1387:
1385:
1383:
1379:
1375:
1371:
1366:
1364:
1362:
1358:
1352:
1340:
1336:
1333:
1332:
1331:
1329:
1321:
1319:
1317:
1316:thermodynamic
1313:
1309:
1305:
1301:
1297:
1288:
1281:
1279:
1277:
1273:
1269:
1265:
1261:
1257:
1253:
1250:-ATOX1 and a
1249:
1245:
1241:
1232:
1225:
1223:
1221:
1218:
1214:
1213:ceruloplasmin
1210:
1209:
1204:
1200:
1196:
1192:
1188:
1184:
1183:
1178:
1173:
1166:
1161:
1154:
1152:
1150:
1146:
1145:
1140:
1136:
1132:
1128:
1124:
1120:
1116:
1112:
1109:
1105:
1102:
1099:
1095:
1083:
1078:
1074:
1070:
1067:
1063:
1056:
1054:
1051:
1047:
1043:
1039:
1036:
1032:
1028:
1021:
1019:
1013:
1009:
1006:
1000:
998:
992:
988:
985:
981:
974:
972:
966:
962:
959:
953:
951:
945:
941:
938:
936:RefSeq (mRNA)
934:
927:
926:
921:
917:
914:
908:
907:
902:
898:
895:
893:
889:
882:
881:
876:
872:
869:
863:
862:
857:
853:
850:
848:
844:
837:
836:
831:
827:
824:
818:
817:
812:
808:
805:
803:
799:
796:
793:
791:
788:
784:
781:
777:
773:
766:
762:
757:
751:
748:
746:
743:
741:
738:
736:
733:
731:
728:
726:
723:
721:
720:ion transport
718:
716:
713:
711:
708:
707:
705:
702:
701:
695:
692:
691:
689:
686:
685:
679:
676:
674:
671:
669:
666:
664:
661:
659:
656:
654:
651:
649:
646:
644:
641:
640:
638:
635:
634:
631:
630:Gene ontology
627:
623:
611:
606:
602:
597:
594:
592:
588:
580:
575:
564:
560:
556:
553:hair follicle
552:
548:
544:
540:
536:
532:
528:
527:
524:
520:
515:
512:
502:
498:
494:
490:
486:
482:
478:
474:
470:
466:
465:
462:
458:
453:
450:
449:
446:
444:
440:
438:
437:
433:
432:
429:
427:
423:
419:
415:
411:
403:
398:
394:
390:
385:
375:
371:
364:
357:
351:
344:
336:
332:
328:
323:
319:
314:
310:
302:
297:
293:
289:
284:
274:
270:
263:
256:
250:
243:
239:
233:
229:
225:
220:
216:
211:
207:
203:
199:
195:
191:
187:
183:
179:
175:
171:
167:
159:
154:
147:
142:
137:
126:
124:
120:
116:
112:
108:
104:
100:
96:
92:
88:
84:
80:
76:
70:
65:
62:
61:
57:
54:
47:
43:
38:
34:
30:
25:
20:
2860:
2567:
2552:
2537:
2522:
2507:
2436:(7): 723–9.
2433:
2429:
2392:
2388:
2362:
2359:Biochemistry
2358:
2325:
2321:
2293:
2289:
2244:
2240:
2213:
2209:
2168:
2164:
2123:
2119:
2082:
2079:Mamm. Genome
2078:
2051:
2047:
2021:(1): 127–9.
2018:
2014:
1980:
1976:
1931:
1927:
1896:
1892:
1861:
1857:
1832:
1826:
1790:
1786:
1776:
1733:
1729:
1715:
1680:
1676:
1628:
1624:
1590:
1544:
1512:
1479:
1452:
1448:
1438:
1429:
1420:
1411:
1402:
1325:
1293:
1238:ATOX1 has a
1237:
1206:
1180:
1172:nomenclature
1169:
1165:cell biology
1142:
1141:, including
1107:
1093:
1092:
1015:
994:
968:
947:
923:
904:
878:
859:
833:
814:
794:
789:
533:right kidney
441:
434:
299:151,772,532
286:151,742,316
161:External IDs
72:
2925:Na/K-ATPase
2908:Electrolyte
2815:Lactoferrin
2810:Hemosiderin
2783:Hemojuvelin
2681:Transferrin
2490:PDB gallery
1449:J Biol Chem
1119:homeostasis
400:55,352,065
387:55,337,467
139:Identifiers
3003:Categories
2672:Hephaestin
2612:Metabolism
2015:Hum. Genet
1397:, May 2017
1376:, May 2017
1353:References
1260:bond angle
1258:to form a
1240:ferrodoxin
1139:eukaryotes
1135:Homologous
467:C1 segment
445:(ortholog)
182:HomoloGene
2793:Aconitase
2430:Circ. Res
2120:BMC Genet
1318:control.
1308:diffusion
1276:cisplatin
1217:cyclin D1
1177:cytoplasm
1149:conserved
1018:NP_033850
997:NP_004036
971:NM_009720
950:NM_004045
780:Orthologs
565:esophagus
190:GeneCards
3014:Proteins
2774:Hepcidin
2761:Ferritin
2731:Ferritin
2721:Integrin
2667:Ferritin
2644:duodenum
2452:15761197
2409:15670166
2379:15476398
2350:27764390
2342:15146197
2312:14709553
2277:39325916
2269:12763797
2232:12686548
2197:12679332
2152:12594858
2107:19978302
2099:12420134
2070:12029094
2035:10982193
2005:30817425
1997:10966647
1968:10557326
1915:10497213
1809:24456146
1768:19965379
1707:20205585
1655:19824702
1393:–
1372:–
1328:diseases
1155:Function
1066:Wikidata
759:Sources:
529:yolk sac
2894:SLC39A4
2889:SLC30A1
2856:SLC31A1
2417:1130281
2249:Bibcode
1936:Bibcode
1880:9430722
1759:3658115
1738:Bibcode
1730:Science
1698:3986808
1646:2768115
1471:9083055
1395:Ensembl
1374:Ensembl
1312:kinetic
1256:cystine
1195:cytosol
1182:in vivo
1123:cytosol
1115:mammals
1104:protein
892:UniProt
847:Ensembl
786:Species
765:QuickGO
694:cytosol
420:pattern
178:1333855
146:Aliases
2450:
2415:
2407:
2389:FEBS J
2377:
2348:
2340:
2310:
2275:
2267:
2230:
2195:
2150:
2143:150598
2140:
2105:
2097:
2068:
2033:
2003:
1995:
1966:
1956:
1913:
1878:
1824:Human
1807:
1766:
1756:
1705:
1695:
1653:
1643:
1597:
1551:
1519:
1469:
1272:lysine
1098:copper
1052:search
1050:PubMed
925:O08997
906:O00244
802:Entrez
591:BioGPS
545:embryo
278:5q33.1
170:602270
2861:ATOX1
2846:ATP7B
2841:ATP7A
2744:Other
2413:S2CID
2346:S2CID
2273:S2CID
2126:: 4.
2103:S2CID
2001:S2CID
1959:23953
1833:ATOX1
1827:ATOX1
1300:ATP7B
1296:ATP7A
1244:motif
1205:. In
1131:ATP7B
1127:ATP7A
1108:ATOX1
1096:is a
1094:ATOX1
835:11927
795:Mouse
790:Human
761:Amigo
443:Mouse
436:Human
383:Start
318:Mouse
282:Start
215:Human
194:ATOX1
153:ATOX1
22:ATOX1
2884:TMC8
2879:TMC6
2778:HAMP
2695:TFR2
2690:TFR1
2568:1tl5
2553:1tl4
2538:1fee
2523:1fe4
2508:1fe0
2448:PMID
2405:PMID
2375:PMID
2338:PMID
2308:PMID
2265:PMID
2228:PMID
2193:PMID
2148:PMID
2095:PMID
2066:PMID
2031:PMID
1993:PMID
1964:PMID
1911:PMID
1876:PMID
1805:PMID
1764:PMID
1703:PMID
1651:PMID
1595:ISBN
1549:ISBN
1517:ISBN
1467:PMID
1298:and
1129:and
1111:gene
426:Bgee
374:Band
335:Chr.
273:Band
232:Chr.
186:2984
166:OMIM
123:4YEA
119:4YDX
115:4QOT
111:3IWX
107:3IWL
103:3CJK
99:2LQ9
95:2K1R
91:1TL5
87:1TL4
83:1FEE
79:1FE4
75:1FE0
56:RCSB
53:PDBe
2805:HFE
2683:to
2438:doi
2397:doi
2393:272
2367:doi
2330:doi
2298:doi
2294:279
2257:doi
2245:986
2218:doi
2214:278
2183:hdl
2173:doi
2169:278
2138:PMC
2128:doi
2087:doi
2056:doi
2052:277
2023:doi
2019:106
1985:doi
1954:PMC
1944:doi
1901:doi
1897:274
1866:doi
1862:273
1795:doi
1791:114
1754:PMC
1746:doi
1734:327
1693:PMC
1685:doi
1641:PMC
1633:doi
1629:109
1457:doi
1453:272
1344:MoS
1248:Apo
1201:or
816:475
541:lip
396:End
295:End
198:OMA
174:MGI
46:PDB
3005::
2614::
2446:.
2434:96
2432:.
2428:.
2411:.
2403:.
2391:.
2373:.
2363:43
2361:.
2344:.
2336:.
2326:22
2324:.
2306:.
2292:.
2288:.
2271:.
2263:.
2255:.
2243:.
2226:.
2212:.
2208:.
2191:.
2181:.
2167:.
2163:.
2146:.
2136:.
2122:.
2118:.
2101:.
2093:.
2083:13
2081:.
2064:.
2050:.
2046:.
2029:.
2017:.
1999:.
1991:.
1979:.
1962:.
1952:.
1942:.
1932:96
1930:.
1926:.
1909:.
1895:.
1891:.
1874:.
1860:.
1856:.
1803:.
1789:.
1785:.
1762:.
1752:.
1744:.
1732:.
1728:.
1701:.
1691:.
1681:79
1679:.
1675:.
1663:^
1649:.
1639:.
1627:.
1623:.
1609:^
1563:^
1531:^
1493:^
1465:.
1451:.
1447:.
1428:.
1410:.
1381:^
1360:^
1222:.
1133:.
763:/
402:bp
389:bp
301:bp
288:bp
196:;
192::
188:;
184::
180:;
176::
172:;
168::
121:,
117:,
113:,
109:,
105:,
101:,
97:,
93:,
89:,
85:,
81:,
77:,
2776:/
2754::
2709::
2674:/
2655::
2604:e
2597:t
2590:v
2483:e
2476:t
2469:v
2454:.
2440::
2419:.
2399::
2381:.
2369::
2352:.
2332::
2314:.
2300::
2279:.
2259::
2251::
2234:.
2220::
2199:.
2185::
2175::
2154:.
2130::
2124:4
2109:.
2089::
2072:.
2058::
2037:.
2025::
2007:.
1987::
1981:7
1970:.
1946::
1938::
1917:.
1903::
1882:.
1868::
1840:.
1811:.
1797::
1770:.
1748::
1740::
1709:.
1687::
1657:.
1635::
1603:.
1557:.
1525:.
1487:.
1473:.
1459::
1432:.
1414:.
1346:4
1342:2
320:)
217:)
200::
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.