520:
497:
1022:
40:
925:, non-steroid anti-inflammatory drugs, etc.) to increase their nephrotoxic potential. As it must be given concurrently with probenecid it is advised that drugs that are known to interact with probenecid (e.g. drugs that probenecid interferes with the renal tubular secretion of, such as paracetamol, aciclovir, aminosalicylic acid, etc.) are also withheld.
821:
which decreases side effects to the kidney. Probenecid mitigates nephrotoxicity by inhibiting organic anion transport of the proximal tubule epithelial cells of the kidney. In addition, hydration must be administered to patients receiving cidofovir. 1 liter of normal saline is recommended in
937:. It also inhibits human polymerases, but this action is 8–600 times weaker than its actions on viral DNA polymerases. It also incorporates itself into viral DNA, hence inhibiting viral DNA synthesis during reproduction.
165:
1655:
830:
The major dose-limiting side effect of cidofovir is nephrotoxicity (i.e., kidney damage). Other common side effects (occurring in >1% of people treated with the drug) include:
1494:
Araya CE, Lew JF, Fennell RS, Neiberger RE, Dharnidharka VR (February 2006). "Intermediate-dose cidofovir without probenecid in the treatment of BK virus allograft nephropathy".
120:
755:, a cidofovir derivative with much higher activity against smallpox that can be taken orally has been developed. It has inhibitory effects on varicella-zoster virus replication
62:
1084:
592:
626:
1111:
Cundy, Kenneth C. "Clinical
Pharmacokinetics of the Antiviral Nucleotide Analogues Cidofovir and Adefovir." Clinical Pharmacokinetics 36.2 (1999): 127–143.
740:
743:
with successful case reports of its use. Despite this, the drug failed to demonstrate any efficacy in controlled studies. Cidofovir might have anti-
2101:
1666:
2635:
1947:
1894:"Effect of oral probenecid coadministration on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys"
2001:
1208:
889:(a uricosuric drug) and intravenous saline should always be administered with each cidofovir infusion to prevent this nephrotoxicity.
2161:
646:
1171:
767:
activity in a subgroup of transplant recipients. Cidofovir is being investigated as a complementary intralesional therapy against
2684:
783:
2521:
2484:
274:
150:
2094:
779:
654:
InChI=1S/C8H14N3O6P/c9-7-1-2-11(8(13)10-7)3-6(4-12)17-5-18(14,15)16/h1-2,6,12H,3-5H2,(H2,9,10,13)(H2,14,15,16)/t6-/m0/s1
376:
2652:
476:
897:
Hypersensitivity to cidofovir or probenecid (as probenecid needs to be given concurrently to avoid nephrotoxicity).
2709:
2704:
787:
2679:
2134:
1963:
1865:
759:
although no clinical trials have been done to date, likely due to the abundance of safer alternatives such as
2079:
2031:
2640:
2087:
1259:
Segarra-Newnham M, Vodolo KM (June 2001). "Use of cidofovir in progressive multifocal leukoencephalopathy".
106:
515:
2255:
1005:
261:
renal The above pharmacokinetic parameters are measured for cidofovir used in conjunction with probenecid.
233:
1056:"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)"
933:
Its active metabolite, cidofovir diphosphate, inhibits viral replication by selectively inhibiting viral
2587:
2536:
2392:
1130:
445:
2689:
2531:
2388:
1055:
969:
736:
243:
2517:
2480:
2232:
2056:
Brodfuehrer PR, Howell HG, Sapino Jr C, Vemishetti P (1994). "A practical synthesis of (S)-HPMPC".
948:
492:
291:
465:
2582:
2013:
1779:
1722:
1611:
1519:
1427:
1284:
80:
981:
817:
Cidofovir is only available as an intravenous formulation. Cidofovir is to be administered with
1691:
Fernández-Morano T, del Boz J, González-Carrascosa M, Tortajada B, de Troya M (December 2011).
1198:
17:
2005:
1943:
1915:
1830:
1771:
1714:
1603:
1568:
1511:
1476:
1419:
1384:
1335:
1276:
1241:
1204:
603:
189:
177:
52:
1224:
Chilukuri S, Rosen T (April 2003). "Management of acyclovir-resistant herpes simplex virus".
425:
2065:
1997:
1905:
1820:
1810:
1761:
1753:
1704:
1595:
1558:
1550:
1503:
1466:
1458:
1411:
1374:
1366:
1325:
1315:
1268:
1233:
1009:
980:
Cidofovir was discovered at the
Institute of Organic Chemistry and Biochemistry, Prague, by
882:
532:
300:
215:
385:
365:
2699:
2694:
2409:
2171:
1974:
1402:
Bradbury J (March 2002). "Orally available cidofovir derivative active against smallpox".
985:
934:
914:
728:
700:
223:
1848:
519:
496:
2646:
2428:
2198:
2113:
1970:
1825:
1798:
1766:
1741:
1563:
1538:
1471:
1446:
1379:
1354:
1330:
1303:
1085:"Drug and medical device highlights 2018: Helping you maintain and improve your health"
906:
768:
696:
2069:
1757:
1415:
1370:
1237:
2673:
2564:
2363:
2143:
1709:
1692:
1507:
1089:
1034:
752:
613:
508:
202:
2017:
1783:
1726:
1615:
1539:"Side-effects of cidofovir in the treatment of recurrent respiratory papillomatosis"
1523:
1462:
1431:
1288:
2603:
2374:
2251:
2189:
2125:
2121:
2117:
1447:"Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate"
953:
456:
133:
128:
1988:
Safrin S, Cherrington J, Jaffe HS (September 1997). "Clinical uses of cidofovir".
1131:"Vistide (cidofovir) dosing, indications, interactions, adverse effects, and more"
325:
1629:
1599:
72:
2612:
2504:
2470:
2456:
2451:
2246:
2207:
2203:
2194:
1304:"Human polyomavirus reactivation: disease pathogenesis and treatment approaches"
878:
841:
113:
2617:
2574:
2494:
2443:
2433:
2404:
2368:
2352:
2342:
2301:
2286:
2221:
1554:
959:
922:
918:
886:
818:
693:
568:
356:
2608:
2509:
2499:
2489:
2400:
2357:
2347:
2322:
2276:
2261:
2185:
2110:
910:
760:
748:
732:
704:
256:
66:
2009:
1910:
1893:
1834:
1775:
1718:
1607:
1572:
1515:
1480:
1423:
1388:
1339:
1280:
1245:
1021:
1919:
1320:
31:
2526:
2419:
2296:
2002:
10.1002/(SICI)1099-1654(199709)7:3<145::AID-RMV196>3.0.CO;2-0
1942:(2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust.
1013:
963:
764:
744:
336:
1586:
Soma MA, Albert DM (February 2008). "Cidofovir: to use or not to use?".
805:
It has been suggested as an antitumour agent, due to its suppression of
345:
2270:
2241:
2216:
2180:
874:
861:
727:
Its only indication that has received regulatory approval worldwide is
689:
311:
1272:
39:
2281:
1815:
993:
866:
436:
1665:. Gilead Sciences International Ltd. 7 November 2013. Archived from
739:
infections. Cidofovir has also been investigated as a treatment for
405:
2035:
1892:
Lacy SA, Hitchcock MJ, Lee WA, Tellier P, Cundy KC (August 1998).
794:
591:
582:
416:
731:
retinitis. Cidofovir has also shown efficacy in the treatment of
806:
747:
efficacy and might be used on a limited basis in the event of a
708:
396:
183:
2083:
1851:. U.S. Centers for Disease Control and Prevention. 26 May 2022.
196:
1697:
Journal of the
European Academy of Dermatology and Venereology
1063:
772:
1588:
Current
Opinion in Otolaryngology & Head and Neck Surgery
481:
159:
91:
1849:"Interim Clinical Guidance for the Treatment of Monkeypox"
877:
and elevated liver enzymes and rare side effects include:
172:
1200:
Principles and
Practice of Pediatric Infectious Disease
707:(an infection of the retina of the eye) in people with
671:
905:
It is known to interact with nephrotoxic agents (e.g.
1742:"In vitro activity of potential anti-poxvirus agents"
1977:. September 2010. Dosage and Administration: Dosage.
2596:
2573:
2552:
2545:
2469:
2442:
2418:
2387:
2335:
2315:
2231:
2170:
2160:
2151:
2142:
2133:
1355:"Cidofovir in the treatment of poxvirus infections"
612:
602:
580:
567:
531:
526:
507:
475:
455:
435:
415:
395:
375:
355:
335:
310:
290:
265:
255:
242:
232:
222:
214:
149:
144:
119:
105:
79:
61:
51:
46:
1799:"Cidofovir Activity against Poxvirus Infections"
1445:Magee WC, Hostetler KY, Evans DH (August 2005).
714:Cidofovir was approved for medical use in 1996.
324:
251:2.6 hours (active metabolites: 15–65 hours)
1693:"Topical cidofovir for viral warts in children"
1636:. American Society of Health-System Pharmacists
1004:Cidofovir can be synthesized from a pyrimidone
299:
2095:
1860:
1858:
699:medication primarily used as a treatment for
8:
464:
30:
1656:"Vistide : EPAR – Product Information"
1178:. Gilead Sciences Pty Ltd. 3 September 2013
2549:
2167:
2157:
2148:
2139:
2102:
2088:
2080:
1543:European Archives of Oto-Rhino-Laryngology
1203:. Elsevier Health Sciences. p. 1502.
741:progressive multifocal leukoencephalopathy
518:
495:
364:
1909:
1824:
1814:
1765:
1708:
1562:
1470:
1378:
1329:
1319:
822:conjunction with each dose of cidofovir.
384:
944:activity against the following viruses:
1630:"Cidofovir Monograph for Professionals"
1537:Broekema FI, Dikkers FG (August 2008).
1308:Clinical & Developmental Immunology
1047:
873:Whereas uncommon side effects include:
651:
631:
491:
344:
279:
1933:
1931:
1929:
1197:Long SS, Prober CG, Fischer M (2012).
509:
29:
1451:Antimicrobial Agents and Chemotherapy
1166:
1164:
1162:
1160:
1158:
1156:
1154:
1152:
1125:
1123:
1121:
1119:
1117:
444:
424:
71:
7:
1797:Andrei G, Snoeck R (December 2010).
988:and is marketed with the brand name
793:It has been used topically to treat
132:
751:incident involving smallpox cases.
404:
315:
634:O=C1/N=C(\C=C/N1C(OCP(=O)(O)O)CO)N
561:
25:
1710:10.1111/j.1468-3083.2010.03961.x
1508:10.1111/j.1399-3046.2005.00391.x
1020:
549:
543:
38:
1463:10.1128/AAC.49.8.3153-3162.2005
659:Key:VWFCHDSQECPREK-LURJTMIESA-N
18:Acyclic nucleoside phosphonates
1302:De Gascun CF, Carr MJ (2013).
1172:"Product Information VISTIDE®"
858:Decreased intraocular pressure
786:approval on 30 April 1998 and
555:
537:
1:
2070:10.1016/S0040-4039(00)76875-4
1940:Australian Medicines Handbook
1758:10.1016/s0166-3542(02)00198-5
1416:10.1016/S0140-6736(02)08115-1
1371:10.1016/S0166-3542(02)00008-6
1261:The Annals of Pharmacotherapy
1238:10.1016/S0733-8635(02)00093-1
1600:10.1097/MOO.0b013e3282f43408
992:by Gilead in the US, and by
282:({oxy}methyl)phosphonic acid
1990:Reviews in Medical Virology
790:approval on 23 April 1997.
2726:
782:approval on 26 June 1996,
527:Chemical and physical data
2630:
1663:European Medicines Agency
1555:10.1007/s00405-008-0658-0
1496:Pediatric Transplantation
1353:De Clercq E (July 2002).
667:
642:
622:
618:260 °C (500 °F)
270:
37:
2032:"Press Releases: Gilead"
1740:Kern ER (January 2003).
1037:, a prodrug of cidofovir
2685:Anti-herpes virus drugs
2553:Nucleic acid inhibitors
763:. Cidofovir shows anti-
1911:10.1006/toxs.1998.2481
1898:Toxicological Sciences
1176:TGA eBusiness Services
2588:Peginterferon alfa-2a
2537:Tenofovir alafenamide
1964:"Vistide (cidofovir)"
1938:Rossi S, ed. (2013).
2532:Tenofovir disoproxil
2518:Nucleotide analogues
2481:Nucleoside analogues
1226:Dermatologic Clinics
970:Human papillomavirus
2233:Pyrimidine analogue
2058:Tetrahedron Letters
1672:on 22 February 2014
1321:10.1155/2013/373579
984:, and developed by
949:Human herpesviruses
929:Mechanism of action
192:(Prescription only)
168:(Prescription only)
34:
2657:Never to phase III
2583:Interferon alfa 2b
1746:Antiviral Research
1359:Antiviral Research
1135:Medscape Reference
778:It first received
2667:
2666:
2626:
2625:
2465:
2464:
2383:
2382:
2331:
2330:
2311:
2310:
1949:978-0-9805790-9-3
1809:(12): 2803–2830.
1703:(12): 1487–1489.
1273:10.1345/aph.10338
1093:. 14 October 2020
893:Contraindications
679:
678:
604:Specific rotation
593:Interactive image
477:CompTox Dashboard
200:
187:
175:
163:
95:
16:(Redirected from
2717:
2710:Primary alcohols
2705:Phosphonic acids
2597:Multiple/unknown
2550:
2546:Multiple/general
2316:Not TK activated
2168:
2158:
2149:
2140:
2104:
2097:
2090:
2081:
2074:
2073:
2053:
2047:
2046:
2044:
2043:
2034:. Archived from
2028:
2022:
2021:
1985:
1979:
1978:
1968:
1960:
1954:
1953:
1935:
1924:
1923:
1913:
1889:
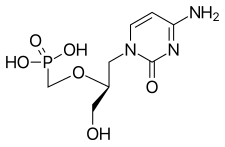
1883:
1882:
1880:
1879:
1870:
1862:
1853:
1852:
1845:
1839:
1838:
1828:
1818:
1816:10.3390/v2122803
1794:
1788:
1787:
1769:
1737:
1731:
1730:
1712:
1688:
1682:
1681:
1679:
1677:
1671:
1660:
1652:
1646:
1645:
1643:
1641:
1626:
1620:
1619:
1583:
1577:
1576:
1566:
1534:
1528:
1527:
1491:
1485:
1484:
1474:
1457:(8): 3153–3162.
1442:
1436:
1435:
1399:
1393:
1392:
1382:
1350:
1344:
1343:
1333:
1323:
1299:
1293:
1292:
1256:
1250:
1249:
1221:
1215:
1214:
1194:
1188:
1187:
1185:
1183:
1168:
1147:
1146:
1144:
1142:
1127:
1112:
1109:
1103:
1102:
1100:
1098:
1081:
1075:
1074:
1072:
1070:
1060:nctr-crs.fda.gov
1052:
1024:
917:, IV pentamide,
883:Fanconi syndrome
675:
674:
595:
575:
563:
557:
551:
545:
539:
522:
511:
500:
499:
485:
483:
468:
448:
428:
408:
388:
368:
348:
328:
318:
317:
303:
247:
205:
198:
195:
185:
182:
174:
171:
161:
158:
136:
93:
90:
75:
42:
35:
33:
21:
2725:
2724:
2720:
2719:
2718:
2716:
2715:
2714:
2680:Gilead Sciences
2670:
2669:
2668:
2663:
2662:
2647:Clinical trials
2622:
2592:
2569:
2541:
2461:
2438:
2414:
2410:Podophyllotoxin
2379:
2327:
2307:
2227:
2172:Purine analogue
2153:
2129:
2108:
2078:
2077:
2055:
2054:
2050:
2041:
2039:
2030:
2029:
2025:
1987:
1986:
1982:
1975:Gilead Sciences
1966:
1962:
1961:
1957:
1950:
1937:
1936:
1927:
1891:
1890:
1886:
1877:
1875:
1868:
1864:
1863:
1856:
1847:
1846:
1842:
1796:
1795:
1791:
1739:
1738:
1734:
1690:
1689:
1685:
1675:
1673:
1669:
1658:
1654:
1653:
1649:
1639:
1637:
1628:
1627:
1623:
1585:
1584:
1580:
1536:
1535:
1531:
1493:
1492:
1488:
1444:
1443:
1439:
1401:
1400:
1396:
1352:
1351:
1347:
1301:
1300:
1296:
1258:
1257:
1253:
1223:
1222:
1218:
1211:
1196:
1195:
1191:
1181:
1179:
1170:
1169:
1150:
1140:
1138:
1129:
1128:
1115:
1110:
1106:
1096:
1094:
1083:
1082:
1078:
1068:
1066:
1054:
1053:
1049:
1044:
1031:
1002:
986:Gilead Sciences
978:
962:(including the
935:DNA polymerases
931:
915:aminoglycosides
903:
895:
871:
828:
815:
803:
729:cytomegalovirus
725:
720:
701:cytomegalovirus
670:
668:
663:
660:
655:
650:
649:
638:
635:
630:
629:
598:
573:
560:
554:
548:
542:
503:
479:
471:
451:
431:
411:
391:
371:
351:
331:
314:
306:
286:
283:
278:
277:
245:
234:Protein binding
224:Bioavailability
216:Pharmacokinetic
210:
203:
140:
108:
101:
82:
28:
23:
22:
15:
12:
11:
5:
2723:
2721:
2713:
2712:
2707:
2702:
2697:
2692:
2687:
2682:
2672:
2671:
2665:
2664:
2661:
2660:
2659:
2658:
2655:
2644:
2638:
2632:
2631:
2628:
2627:
2624:
2623:
2621:
2620:
2615:
2606:
2600:
2598:
2594:
2593:
2591:
2590:
2585:
2579:
2577:
2571:
2570:
2568:
2567:
2562:
2556:
2554:
2547:
2543:
2542:
2540:
2539:
2534:
2529:
2513:
2512:
2507:
2502:
2497:
2492:
2476:
2474:
2467:
2466:
2463:
2462:
2460:
2459:
2454:
2448:
2446:
2440:
2439:
2437:
2436:
2424:
2422:
2416:
2415:
2413:
2412:
2407:
2397:
2395:
2385:
2384:
2381:
2380:
2378:
2377:
2372:
2360:
2355:
2350:
2345:
2339:
2337:
2333:
2332:
2329:
2328:
2326:
2325:
2319:
2317:
2313:
2312:
2309:
2308:
2306:
2305:
2292:
2291:
2290:
2289:
2284:
2279:
2266:
2265:
2259:
2249:
2237:
2235:
2229:
2228:
2226:
2225:
2212:
2211:
2201:
2199:Valganciclovir
2192:
2176:
2174:
2165:
2155:
2146:
2137:
2131:
2130:
2109:
2107:
2106:
2099:
2092:
2084:
2076:
2075:
2048:
2023:
1996:(3): 145–156.
1980:
1971:package insert
1955:
1948:
1925:
1884:
1873:www.gilead.com
1854:
1840:
1789:
1752:(1–2): 35–40.
1732:
1683:
1647:
1621:
1578:
1549:(8): 871–879.
1529:
1486:
1437:
1410:(9311): 1041.
1394:
1345:
1294:
1267:(6): 741–744.
1251:
1232:(2): 311–320.
1216:
1210:978-1437727029
1209:
1189:
1148:
1113:
1104:
1076:
1046:
1045:
1043:
1040:
1039:
1038:
1030:
1027:
1026:
1025:
1012:derivative of
1001:
998:
977:
974:
973:
972:
967:
956:
951:
930:
927:
907:amphotericin B
902:
899:
894:
891:
870:
869:
864:
859:
856:
853:
850:
847:
844:
839:
836:
832:
827:
824:
814:
813:Administration
811:
802:
799:
769:papillomatosis
724:
721:
719:
716:
677:
676:
665:
664:
662:
661:
658:
656:
653:
645:
644:
643:
640:
639:
637:
636:
633:
625:
624:
623:
620:
619:
616:
610:
609:
606:
600:
599:
597:
596:
588:
586:
578:
577:
571:
565:
564:
558:
552:
546:
540:
535:
529:
528:
524:
523:
513:
505:
504:
502:
501:
488:
486:
473:
472:
470:
469:
461:
459:
453:
452:
450:
449:
441:
439:
433:
432:
430:
429:
421:
419:
413:
412:
410:
409:
401:
399:
393:
392:
390:
389:
381:
379:
373:
372:
370:
369:
361:
359:
353:
352:
350:
349:
341:
339:
333:
332:
330:
329:
321:
319:
308:
307:
305:
304:
296:
294:
288:
287:
285:
284:
281:
273:
272:
271:
268:
267:
263:
262:
259:
253:
252:
249:
240:
239:
236:
230:
229:
226:
220:
219:
212:
211:
209:
208:
193:
180:
169:
155:
153:
147:
146:
142:
141:
139:
138:
125:
123:
117:
116:
111:
109:administration
103:
102:
100:
99:
97:
87:
85:
77:
76:
69:
59:
58:
55:
49:
48:
44:
43:
27:Antiviral drug
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2722:
2711:
2708:
2706:
2703:
2701:
2698:
2696:
2693:
2691:
2688:
2686:
2683:
2681:
2678:
2677:
2675:
2656:
2654:
2651:
2650:
2648:
2645:
2642:
2639:
2637:
2634:
2633:
2629:
2619:
2616:
2614:
2610:
2607:
2605:
2602:
2601:
2599:
2595:
2589:
2586:
2584:
2581:
2580:
2578:
2576:
2572:
2566:
2565:Brincidofovir
2563:
2561:
2558:
2557:
2555:
2551:
2548:
2544:
2538:
2535:
2533:
2530:
2528:
2525:
2523:
2519:
2515:
2514:
2511:
2508:
2506:
2503:
2501:
2498:
2496:
2493:
2491:
2488:
2486:
2482:
2478:
2477:
2475:
2472:
2468:
2458:
2455:
2453:
2450:
2449:
2447:
2445:
2441:
2435:
2432:
2430:
2426:
2425:
2423:
2421:
2417:
2411:
2408:
2406:
2402:
2399:
2398:
2396:
2394:
2390:
2386:
2376:
2373:
2370:
2366:
2365:
2364:early protein
2361:
2359:
2356:
2354:
2351:
2349:
2346:
2344:
2341:
2340:
2338:
2334:
2324:
2321:
2320:
2318:
2314:
2303:
2299:
2298:
2294:
2293:
2288:
2285:
2283:
2280:
2278:
2275:
2274:
2273:
2272:
2268:
2267:
2263:
2260:
2257:
2253:
2250:
2248:
2244:
2243:
2239:
2238:
2236:
2234:
2230:
2223:
2219:
2218:
2214:
2213:
2209:
2205:
2202:
2200:
2196:
2193:
2191:
2187:
2183:
2182:
2178:
2177:
2175:
2173:
2169:
2166:
2163:
2159:
2156:
2152:DNA-synthesis
2150:
2147:
2145:
2141:
2138:
2136:
2132:
2127:
2123:
2119:
2115:
2112:
2105:
2100:
2098:
2093:
2091:
2086:
2085:
2082:
2071:
2067:
2063:
2059:
2052:
2049:
2038:on 2013-02-08
2037:
2033:
2027:
2024:
2019:
2015:
2011:
2007:
2003:
1999:
1995:
1991:
1984:
1981:
1976:
1972:
1965:
1959:
1956:
1951:
1945:
1941:
1934:
1932:
1930:
1926:
1921:
1917:
1912:
1907:
1904:(2): 97–106.
1903:
1899:
1895:
1888:
1885:
1874:
1867:
1861:
1859:
1855:
1850:
1844:
1841:
1836:
1832:
1827:
1822:
1817:
1812:
1808:
1804:
1800:
1793:
1790:
1785:
1781:
1777:
1773:
1768:
1763:
1759:
1755:
1751:
1747:
1743:
1736:
1733:
1728:
1724:
1720:
1716:
1711:
1706:
1702:
1698:
1694:
1687:
1684:
1668:
1664:
1657:
1651:
1648:
1635:
1631:
1625:
1622:
1617:
1613:
1609:
1605:
1601:
1597:
1593:
1589:
1582:
1579:
1574:
1570:
1565:
1560:
1556:
1552:
1548:
1544:
1540:
1533:
1530:
1525:
1521:
1517:
1513:
1509:
1505:
1501:
1497:
1490:
1487:
1482:
1478:
1473:
1468:
1464:
1460:
1456:
1452:
1448:
1441:
1438:
1433:
1429:
1425:
1421:
1417:
1413:
1409:
1405:
1398:
1395:
1390:
1386:
1381:
1376:
1372:
1368:
1364:
1360:
1356:
1349:
1346:
1341:
1337:
1332:
1327:
1322:
1317:
1313:
1309:
1305:
1298:
1295:
1290:
1286:
1282:
1278:
1274:
1270:
1266:
1262:
1255:
1252:
1247:
1243:
1239:
1235:
1231:
1227:
1220:
1217:
1212:
1206:
1202:
1201:
1193:
1190:
1177:
1173:
1167:
1165:
1163:
1161:
1159:
1157:
1155:
1153:
1149:
1136:
1132:
1126:
1124:
1122:
1120:
1118:
1114:
1108:
1105:
1092:
1091:
1090:Health Canada
1086:
1080:
1077:
1065:
1061:
1057:
1051:
1048:
1041:
1036:
1035:Brincidofovir
1033:
1032:
1028:
1023:
1019:
1018:
1017:
1015:
1011:
1007:
999:
997:
995:
991:
987:
983:
975:
971:
968:
965:
961:
957:
955:
952:
950:
947:
946:
945:
943:
940:It possesses
938:
936:
928:
926:
924:
920:
916:
912:
908:
900:
898:
892:
890:
888:
884:
880:
876:
868:
865:
863:
860:
857:
854:
851:
848:
845:
843:
840:
837:
834:
833:
831:
825:
823:
820:
812:
810:
808:
800:
798:
796:
791:
789:
785:
781:
776:
774:
770:
766:
762:
758:
754:
753:Brincidofovir
750:
746:
742:
738:
734:
730:
722:
717:
715:
712:
710:
706:
702:
698:
695:
691:
687:
684:, brand name
683:
673:
666:
657:
652:
648:
641:
632:
628:
621:
617:
615:
614:Melting point
611:
607:
605:
601:
594:
590:
589:
587:
584:
579:
572:
570:
566:
536:
534:
530:
525:
521:
517:
514:
512:
510:ECHA InfoCard
506:
498:
494:
493:DTXSID3043734
490:
489:
487:
478:
474:
467:
463:
462:
460:
458:
454:
447:
443:
442:
440:
438:
434:
427:
423:
422:
420:
418:
414:
407:
403:
402:
400:
398:
394:
387:
383:
382:
380:
378:
374:
367:
363:
362:
360:
358:
354:
347:
343:
342:
340:
338:
334:
327:
323:
322:
320:
313:
309:
302:
298:
297:
295:
293:
289:
280:
276:
269:
264:
260:
258:
254:
250:
248:
241:
237:
235:
231:
227:
225:
221:
217:
213:
206:
194:
191:
181:
179:
170:
167:
157:
156:
154:
152:
148:
143:
135:
130:
127:
126:
124:
122:
118:
115:
112:
110:
104:
98:
89:
88:
86:
84:
78:
74:
70:
68:
64:
60:
56:
54:
50:
47:Clinical data
45:
41:
36:
19:
2604:Filociclovir
2559:
2516:
2479:
2427:
2375:Tromantadine
2362:
2295:
2269:
2252:Trifluridine
2240:
2215:
2190:Valaciclovir
2179:
2064:(20): 3243.
2061:
2057:
2051:
2040:. Retrieved
2036:the original
2026:
1993:
1989:
1983:
1958:
1939:
1901:
1897:
1887:
1876:. Retrieved
1872:
1843:
1806:
1802:
1792:
1749:
1745:
1735:
1700:
1696:
1686:
1674:. Retrieved
1667:the original
1662:
1650:
1638:. Retrieved
1633:
1624:
1594:(1): 86–90.
1591:
1587:
1581:
1546:
1542:
1532:
1502:(1): 32–37.
1499:
1495:
1489:
1454:
1450:
1440:
1407:
1403:
1397:
1362:
1358:
1348:
1311:
1307:
1297:
1264:
1260:
1254:
1229:
1225:
1219:
1199:
1192:
1180:. Retrieved
1175:
1139:. Retrieved
1134:
1107:
1095:. Retrieved
1088:
1079:
1067:. Retrieved
1059:
1050:
1003:
989:
982:Antonín Holý
979:
954:Adenoviruses
941:
939:
932:
904:
901:Interactions
896:
872:
829:
826:Side effects
816:
804:
792:
777:
756:
726:
713:
685:
681:
680:
669:
457:NIAID ChemDB
244:Elimination
151:Legal status
145:Legal status
2690:Pyrimidones
2643:from market
2613:Taribavirin
2505:Telbivudine
2471:Hepatitis B
2457:Tecovirimat
2452:Methisazone
2247:Idoxuridine
2208:Famciclovir
2204:Penciclovir
2195:Ganciclovir
2144:Herpesvirus
2135:Baltimore I
2116:(primarily
1365:(1): 1–13.
996:elsewhere.
879:tachycardia
842:Neutropenia
735:-resistant
718:Medical use
576: g·mol
516:100.166.433
301:113852-37-2
266:Identifiers
114:Intravenous
53:Trade names
2674:Categories
2618:Moroxydine
2575:Interferon
2495:Lamivudine
2444:Poxviridae
2434:Rifampicin
2405:Resiquimod
2369:Fomivirsen
2353:Letermovir
2343:Amenamevir
2302:Cytarabine
2287:Sorivudine
2256:+tipiracil
2222:Vidarabine
2114:antivirals
2042:2007-12-05
1878:2019-06-05
1676:5 February
1640:5 February
1314:: 373579.
1182:5 February
1141:4 February
1042:References
1006:derivative
960:poxviruses
923:tacrolimus
919:vancomycin
887:Probenecid
819:probenecid
771:caused by
694:injectable
581:3D model (
569:Molar mass
426:CHEBI:3696
386:768M1V522C
357:ChemSpider
292:CAS Number
275:IUPAC name
2653:Phase III
2641:Withdrawn
2609:Ribavirin
2560:Cidofovir
2510:Clevudine
2500:Lobucavir
2490:Entecavir
2401:Imiquimod
2358:Maribavir
2348:Docosanol
2323:Foscarnet
2277:Brivudine
2262:Edoxudine
2186:Aciclovir
2164:activated
2154:inhibitor
2111:DNA virus
1866:"Details"
1634:Drugs.com
1010:protected
1000:Synthesis
911:foscarnet
846:Hair loss
761:aciclovir
749:bioterror
733:aciclovir
723:DNA virus
705:retinitis
697:antiviral
682:Cidofovir
446:ChEMBL152
257:Excretion
246:half-life
107:Routes of
81:Pregnancy
73:Monograph
67:Drugs.com
32:Cidofovir
2527:Adefovir
2429:assembly
2420:Vaccinia
2297:cytosine
2018:32366514
2010:10398479
1835:21994641
1784:23136929
1776:12615301
1727:32295082
1719:21261749
1616:22895067
1608:18197029
1573:18458927
1524:24131709
1516:16499584
1481:16048917
1432:22903225
1424:11937193
1389:12076747
1340:23737811
1289:32026770
1281:11408993
1246:12757254
1097:17 April
1029:See also
1014:glycidol
964:smallpox
942:in vitro
852:Headache
849:Weakness
838:Vomiting
765:BK virus
757:in vitro
745:smallpox
672:(verify)
337:DrugBank
228:complete
121:ATC code
83:category
2271:thymine
2242:uridine
2217:adenine
2181:guanine
2120:, also
1920:9742650
1826:3185586
1803:Viruses
1767:9628899
1564:2441494
1472:1196213
1380:9533828
1331:3659475
1137:. WebMD
990:Vistide
976:History
875:anaemia
862:Uveitis
690:topical
688:, is a
686:Vistide
574:279.189
533:Formula
346:DB00369
312:PubChem
207:Rx-only
204:WARNING
176::
137:)
131: (
129:J05AB12
96: D
57:Vistide
2700:Ethers
2695:Amines
2636:WHO-EM
2522:NtRTIs
2485:NARTIs
2282:FV-100
2016:
2008:
1946:
1918:
1833:
1823:
1782:
1774:
1764:
1725:
1717:
1614:
1606:
1571:
1561:
1522:
1514:
1479:
1469:
1430:
1422:
1404:Lancet
1387:
1377:
1338:
1328:
1287:
1279:
1244:
1207:
1069:22 Oct
1008:and a
994:Pfizer
966:virus)
958:Human
867:Iritis
855:Chills
835:Nausea
703:(CMV)
627:SMILES
466:001049
437:ChEMBL
406:C06909
238:<6%
201:
188:
178:℞-only
164:
2473:(VII)
2336:Other
2126:D06BB
2122:S01AD
2014:S2CID
1967:(PDF)
1869:(PDF)
1780:S2CID
1723:S2CID
1670:(PDF)
1659:(PDF)
1612:S2CID
1520:S2CID
1428:S2CID
1285:S2CID
913:, IV
801:Other
795:warts
647:InChI
608:-97.3
583:JSmol
417:ChEBI
366:54636
326:60613
2124:and
2006:PMID
1944:ISBN
1916:PMID
1831:PMID
1772:PMID
1715:PMID
1678:2014
1642:2014
1604:PMID
1569:PMID
1512:PMID
1477:PMID
1420:PMID
1385:PMID
1336:PMID
1312:2013
1277:PMID
1242:PMID
1205:ISBN
1184:2014
1143:2014
1099:2024
1071:2023
881:and
807:FGF2
709:AIDS
397:KEGG
377:UNII
218:data
63:AHFS
2389:HPV
2118:J05
2066:doi
1998:doi
1973:).
1906:doi
1821:PMC
1811:doi
1762:PMC
1754:doi
1705:doi
1596:doi
1559:PMC
1551:doi
1547:265
1504:doi
1467:PMC
1459:doi
1412:doi
1408:359
1375:PMC
1367:doi
1326:PMC
1316:doi
1269:doi
1234:doi
1064:FDA
788:EMA
784:TGA
780:FDA
773:HPV
737:HSV
692:or
482:EPA
316:CID
190:POM
134:WHO
2676::
2649::
2393:MC
2162:TK
2062:35
2060:.
2012:.
2004:.
1992:.
1928:^
1914:.
1902:44
1900:.
1896:.
1871:.
1857:^
1829:.
1819:.
1805:.
1801:.
1778:.
1770:.
1760:.
1750:57
1748:.
1744:.
1721:.
1713:.
1701:25
1699:.
1695:.
1661:.
1632:.
1610:.
1602:.
1592:16
1590:.
1567:.
1557:.
1545:.
1541:.
1518:.
1510:.
1500:10
1498:.
1475:.
1465:.
1455:49
1453:.
1449:.
1426:.
1418:.
1406:.
1383:.
1373:.
1363:55
1361:.
1357:.
1334:.
1324:.
1310:.
1306:.
1283:.
1275:.
1265:35
1263:.
1240:.
1230:21
1228:.
1174:.
1151:^
1133:.
1116:^
1087:.
1062:.
1058:.
1016:.
921:,
909:,
885:.
809:.
797:.
775:.
711:.
547:14
197:US
184:UK
173:CA
166:S4
160:AU
92:AU
2611:/
2524::
2520:/
2487::
2483:/
2431::
2403:/
2391:/
2371:)
2367:(
2304:)
2300:(
2264:)
2258:)
2254:(
2245:(
2224:)
2220:(
2210:)
2206:/
2197:/
2188:/
2184:(
2128:)
2103:e
2096:t
2089:v
2072:.
2068::
2045:.
2020:.
2000::
1994:7
1969:(
1952:.
1922:.
1908::
1881:.
1837:.
1813::
1807:2
1786:.
1756::
1729:.
1707::
1680:.
1644:.
1618:.
1598::
1575:.
1553::
1526:.
1506::
1483:.
1461::
1434:.
1414::
1391:.
1369::
1342:.
1318::
1291:.
1271::
1248:.
1236::
1213:.
1186:.
1145:.
1101:.
1073:.
585:)
562:P
559:6
556:O
553:3
550:N
544:H
541:8
538:C
484:)
480:(
199::
186::
162::
94::
65:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.