407:) from the other parent. Hb S beta thalassemia is the least common and is experienced in patients who have inherited beta thalassemia hemoglobin from one parent and HbS from the other. In addition, there is sickle cell trait (HbAS) which is defined by having HbA and HbS. This makes the individual heterozygous for sickle cell. Of the world population, it is estimated that there are about 300 million individuals with the sickle cell trait and about 100 million of those are in sub-Saharan Africa. There is also a higher prevalence of sickle cell trait in areas that malaria is commonly found, with the prevalence in some parts of Africa and Saudi Arabia being as high as 25% and 60%, respectively. Individuals who have HbAS have about 40%HbS, 56% HBA, and are usually asymptomatic unless there is a severe lack of oxygen to the body (hypoxia) which can lead to symptoms of sickle cell disease. However, HbAS does not cause vaso-occlusive crisis, which is known to be associated with sickle cell disease.
31:
348:
418:
to elongated crescents. The sickling reaction is reversible after re-oxygenating the hemoglobin, therefore, red blood cells can go through cycles of sickling and unsickling depending on the concentration of oxygen present in the bloodstream. Red blood cells that are sickle-shaped lack flexibility and
190:
which are located within the cytosol. Two globin chains that have heme groups combine to form hemoglobin. One of the chains is an alpha chain and the other is a non-alpha chain. Non-alpha chain nature in hemoglobin molecules varies due to different variables. Fetuses have a non-alpha chain called
254:
are considered to have a “silent” α-thalassemia whereas, if the mutation is on both then it is considered an α-thalassemia trait. α-thalassemia is mostly found in sub-tropical and tropical areas, where individuals who carry the gene is 80-90% of the population. Like other hemoglobin related
283:
in that lacks functional α-globin genes from both parents. Hb Bart’s is a tetramer of four gamma-globulin subunits and is ineffective at transporting oxygen to the tissues due to its very high oxygen affinity. This usually results in fatal hydrops fetalis and associated symptoms include
327:, accumulation of alpha-globin subunits and alpha tetramers begin to accumulate leading to damage of erythrocytes. People of Asian, Middle Eastern, and Mediterranean descent have a much higher incidences of β-thalassemia. It has been determined that there is a wide variation in
335:
of the disease due to more than 200 different thalassemia-associated mutations have being found in the beta-globin gene. Individuals with β-thalassemia major usually require medical attention within the first 2 years of life and require regular
118:), it will attach to the Iron II (Fe2+) of heme and it is this iron ion that can bind and unbind oxygen to transport oxygen throughout the body. All subunits must be present for hemoglobin to pick up and release oxygen under normal conditions.
191:
gamma and after birth it is then called beta. The beta chain will pair with the alpha chain. It is the combining of two alpha and non-alpha chains which create a hemoglobin molecule. Two alpha and two gamma chains form fetal hemoglobin or
271:
red blood cells. In contrast, mild α-thalassemia carriers could have symptoms of anemia due to other factors not related specifically to the disorder: poor diet, drop in hemoglobin levels due to blood loss, or other diseases.
94:, which transports oxygen from the lungs to the tissues. Hemoglobin A is the most common adult form of hemoglobin and exists as a tetramer containing two alpha subunits and two beta subunits (α2β2).
307:(β-thalassemia) is an inherited mutation of the β-globulin gene which causes the reduced synthesis of the β-globin chain of hemoglobin. The majority of the mutations are point mutations that affect
634:
Kato, Gregory J.; Piel, Frédéric B.; Reid, Clarice D.; Gaston, Marilyn H.; Ohene-Frempong, Kwaku; Krishnamurti, Lakshmanan; Smith, Wally R.; Panepinto, Julie A.; Weatherall, David J. (2018-03-15).
195:(HbF). After the first five to six months after birth, the combining of two alpha chains and two beta chains form adult hemoglobin (HbA). The genes that encode for the alpha chains are located on
275:
The most severe form of α -thalassemia is a condition that begins at infancy in which there is no expression of α-genes and results in a large production of hemoglobin Bart's
1286:
Tsaras, Geoffrey; Owusu-Ansah, Amma; Boateng, Freda Owusua; Amoateng-Adjepong, Yaw (June 2009). "Complications
Associated with Sickle Cell Trait: A Brief Narrative Review".
106:
Hemoglobin A (HbA) is the most common adult form of hemoglobin and exists as a tetramer containing two alpha subunits and two beta subunits (α2β2). Each subunit contains a
2136:
114:) molecules can bind to. In addition to oxygen, subunit assembly and quaternary structure are known to play important roles in Hb affinity. When hemoglobin binds to O2 (
263:
and are diagnosed if it is found after routine hematological analyses or before birth screenings. Single α-globin gene carriers usually have no profound fatigue or
98:(HbA2) is a less common adult form of hemoglobin and is composed of two alpha and two delta-globin subunits. This hemoglobin makes up 1-3% of hemoglobin in adults.
255:
disorders (sickle cell and β-thalassemia), it is hypothesized that α-thalassemia is selected for within populations due to carriers being better protected against
702:
Barrick, Doug; Lukin, Jonathan A; Simplaceanu, Virgil; Ho, Chien (2004), "Nuclear
Magnetic Resonance Spectroscopy in the Study of Hemoglobin Cooperativity",
250:(α-thalassemia) is defined by a lack of α-globin chain production in hemoglobin, and those who carry a mutation impacting the α-globin chain on only one
211:
Due to the numerous steps and processes during hemoglobin synthesis, there are many places in which errors can occur. Heme synthesis involves multiple
1455:
419:
stick to the walls of blood vessels decreasing or stopping the flow of oxygen to nearby tissues. This decrease in oxygen to the tissues cause
127:
1396:
719:
596:
130:
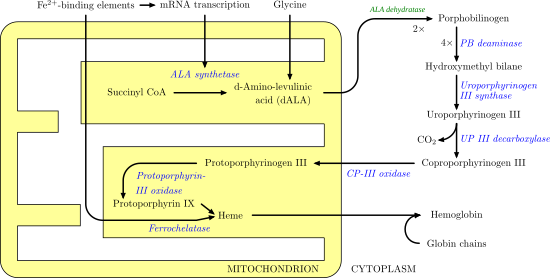
Biosynthesis of heme which involves many enzymatic steps which begin in the mitochondrion and ends in the cytoplasm of the cell.
439:
to help the patients body produce healthy red blood cells, and medications to help alleviate the symptoms listed previously.
635:
423:
which presents itself in muscle pain and injury to tissues. Some symptoms of sickle cell anemia include fever, fatigue from
403:. HB SS which is the most common and severe form of sickle cell. Hb SC is due to inheriting Hb S from one parent and Hb C (
319:) are considered to have β-thalassemia minor (carrier or trait β-thalassemia), while those who have two gene mutations (
362:
on the top of the diagram. The inset image shows a cross-section of a normal red blood cell with normal hemoglobin.
1448:
620:
1191:
1523:
1427:
170:. This molecule moves back into the mitochondrion where it reacts with protoporphyrin-III oxidase to produce
1601:
1582:
1545:
891:
436:
167:
1441:
1321:
Li, Eileena J.; Carroll, Vanessa G. (September 2014). "Sickle Cell Trait and Renal
Papillary Necrosis".
420:
367:
308:
256:
163:
591:. Walker, Patricia Frye., Barnett, Elizabeth D. (Elizabeth Day). St. Louis, Mo.: Elsevier Mosby. 2007.
2156:
1726:
1712:
1698:
1684:
219:
or deletions in genes coding for the globin chain can occur. This results in globin gene disorders (
1992:
1962:
1886:
1528:
400:
236:
224:
1976:
1658:
1354:
684:
614:
410:
Patients that are homozygous for HbS have multi-stranded fibers that induce a change in shape of
323:
or compound heterozygosity) are diagnosed with β-thalassemia or intermedia. Due to the lack of
1957:
1852:
1372:
1346:
1338:
1303:
1263:
1233:
1215:
1172:
1164:
1115:
1073:
1034:
1016:
972:
964:
932:
924:
872:
810:
802:
725:
715:
676:
668:
602:
592:
562:
432:
337:
268:
247:
171:
47:
1862:
1821:
1751:
1433:
1330:
1295:
1223:
1207:
1154:
1081:
1065:
1024:
1008:
914:
906:
862:
852:
707:
660:
650:
377:
371:
304:
276:
220:
192:
2116:
1971:
1423:
2018:
2013:
1228:
1195:
1086:
1053:
1029:
996:
415:
411:
370:). The inset image shows a cross-section of a sickle cell with sickle hemoglobin. From:
366::Demonstrates abnormal, sickled red blood cells blocking blood flow in a blood vessel (
355:
232:
175:
91:
83:
711:
524:
387:
Sickle hemoglobin (HbS) is the most common variant of hemoglobin and arises due to an
215:
and when these enzymes are deficient or do not function properly consequences such as
2150:
2123:
2082:
1997:
1804:
1586:
1569:
1508:
464:
392:
293:
200:
196:
147:
115:
95:
1358:
2111:
2052:
1928:
1914:
1900:
688:
479:
474:
469:
359:
341:
312:
260:
155:
1299:
1159:
1142:
769:
2059:
1472:
1012:
955:
Muncie, Herbert L.; Campbell, James (2009-08-15). "Alpha and beta thalassemia".
742:
324:
315:
of the hemoglobin β gene and gene product. Individuals with one gene mutation (
228:
52:
17:
1255:
1107:
1069:
857:
840:
554:
30:
2106:
2064:
1967:
1490:
447:
388:
251:
87:
79:
1342:
1334:
1219:
1168:
1077:
1054:"Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management"
1020:
968:
928:
806:
672:
606:
166:(ALA). ALA then moves to the cytosol and after a series of reactions creates
2047:
347:
328:
289:
126:
1350:
1307:
1267:
1176:
1119:
1038:
976:
936:
919:
890:
Piel, Frédéric B.; Weatherall, David J. (2014-11-13). Longo, Dan L. (ed.).
876:
729:
680:
664:
566:
340:
to survive. Patients who present the disorder later usually do not require
1237:
1211:
910:
814:
1373:"Sickle Cell Disease | National Heart, Lung, and Blood Institute (NHLBI)"
655:
332:
320:
316:
216:
187:
1464:
279:. The most common cause of Hb Bart’s is the inheritance of a deletion
159:
151:
867:
1481:
428:
424:
396:
280:
264:
212:
143:
292:
of the skeleton, and cardiovascular deformities that could lead to
1533:
1190:
Ashiotis, Th.; Zachariadis, Z.; Sofroniadou, K.; Loukopoulos, D.;
706:, Methods in Enzymology, vol. 379, Elsevier, pp. 28–54,
456:
391:
substitution in the beta-globin subunit at the sixth residue from
346:
285:
199:, while the genes that encode for non-alpha chains are located on
125:
1642:
1630:
1625:
1557:
1468:
494:
489:
435:
which aid with increasing the number of normal red blood cells,
139:
107:
43:
1437:
1872:
1613:
504:
499:
404:
174:. Iron is then enzymatically inserted into protoporphyrin via
154:
of the cell. First, in the mitochondrion, the condensation of
1397:"Sickle cell anemia - Diagnosis and treatment - Mayo Clinic"
793:
Weatherall, D. J. (1980–1981). "The thalassemia syndromes".
42:
subunits are shown in red and blue, and the iron-containing
267:
because they have a compensating increase in the number of
990:
988:
986:
223:) which can be either abnormal globin chain variants (
839:
Farashi, Samaneh; Harteveld, Cornelis L. (May 2018).
2099:
2073:
2038:
2031:
2006:
1985:
1950:
1843:
1783:
1744:
1677:
1666:
1657:
1580:
1505:
1498:
1489:
1480:
235:. These hemoglobinopathies are often inherited as
227:) or reduced chain synthesis in erythroid cells (
1141:Cao, Antonio; Galanello, Renzo (February 2010).
431:, and organ failure. Current treatments include
344:and are diagnosed with thalassemia intermedia.
997:"Classification of the Disorders of Hemoglobin"
704:Energetics of Biological Macromolecules, Part D
284:intra-uterine anemia, slowing of brain growth,
82:tetramer, accounting for over 97% of the total
1449:
8:
1001:Cold Spring Harbor Perspectives in Medicine
2035:
1674:
1663:
1502:
1495:
1486:
1456:
1442:
1434:
2137:disorders of globin and globulin proteins
1426:at the U.S. National Library of Medicine
1254:Ashorobi, Damilola; Bhatt, Ruchi (2019),
1227:
1158:
1085:
1028:
995:Forget, B. G.; Bunn, H. F. (2013-02-01).
918:
866:
856:
654:
34:The structure of adult human hemoglobin.
1058:Journal of the Royal Society of Medicine
29:
516:
162:by ALA synthase takes place to produce
90:is an oxygen-binding protein, found in
612:
259:. Most carriers of α-thalassemia are
1249:
1247:
1136:
1134:
1106:Needs, Todd; Lynch, David T. (2019),
1101:
1099:
1097:
950:
948:
946:
795:Texas Reports on Biology and Medicine
485:Hemoglobin protein subunits (genes):
7:
834:
832:
830:
828:
826:
824:
764:
762:
583:
581:
555:"Biochemistry, Hemoglobin Synthesis"
553:Farid, Yostina; Lecat, Paul (2019),
548:
546:
544:
542:
540:
186:Globin synthesis takes place in the
845:Blood Cells, Molecules and Diseases
1052:Somervaille, Tim (November 2001).
841:"Molecular basis of α-thalassemia"
427:, swelling of the hands and feet,
25:
231:) during the cellular process of
146:steps that take place within the
27:Normal human hemoglobin in adults
1288:The American Journal of Medicine
378:health/health-topics/topics/sca/
899:New England Journal of Medicine
399:. There are different forms of
311:, transcriptional control, and
142:synthesis involves a series of
65:adult hemoglobin, hemoglobin A1
643:Nature Reviews Disease Primers
358:are shown flowing freely in a
1:
712:10.1016/s0076-6879(04)79002-3
110:group that diatomic oxygen (O
1300:10.1016/j.amjmed.2008.12.020
1160:10.1097/GIM.0b013e3181cd68ed
1013:10.1101/cshperspect.a011684
78:, is the most common human
2173:
1070:10.1177/014107680109401119
858:10.1016/j.bcmd.2017.09.004
2132:
1262:, StatPearls Publishing,
1114:, StatPearls Publishing,
957:American Family Physician
561:, StatPearls Publishing,
372:http://www.nhlbi.nih.gov/
1428:Medical Subject Headings
1335:10.1177/0009922814533418
1196:"Thalassaemia in Cyprus"
1200:British Medical Journal
437:bone marrow transplants
1192:Stamatoyannopoulos, G.
774:sickle.bwh.harvard.edu
770:"Hemoglobin Synthesis"
747:sickle.bwh.harvard.edu
619:: CS1 maint: others (
374:
131:
102:Structure and function
57:
46:groups in green. From
1212:10.1136/bmj.2.5857.38
911:10.1056/NEJMra1404415
743:"Hemoglobin Overview"
636:"Sickle cell disease"
452:Hemoglobin variants:
421:vaso-occlusive crisis
368:vaso-occlusive crisis
350:
207:Clinical significance
168:coproporphyringen III
164:5-aminolevulinic acid
129:
63:(HbA), also known as
33:
1147:Genetics in Medicine
892:"The α-Thalassemias"
656:10.1038/nrdp.2018.10
525:"Hemoglobinopathies"
1993:Glycated hemoglobin
1963:Carbaminohemoglobin
1323:Clinical Pediatrics
1256:"Sickle Cell Trait"
401:sickle cell disease
383:Sickle cell disease
237:autosomal recessive
1401:www.mayoclinic.org
1143:"Beta-thalassemia"
1108:"Beta Thalassemia"
589:Immigrant medicine
433:blood transfusions
375:
338:blood transfusions
257:malaria falciparum
225:sickle cell anemia
221:hemoglobinopathies
132:
58:
2144:
2143:
2095:
2094:
2091:
2090:
2027:
2026:
1958:Carboxyhemoglobin
1946:
1945:
1839:
1838:
1653:
1652:
1377:www.nhlbi.nih.gov
1329:(10): 1013–1015.
905:(20): 1908–1916.
248:Alpha-thalassemia
243:Alpha-thalassemia
178:to produce heme.
172:protoporphyrin IX
16:(Redirected from
2164:
2036:
1675:
1664:
1503:
1496:
1487:
1458:
1451:
1444:
1435:
1411:
1410:
1408:
1407:
1393:
1387:
1386:
1384:
1383:
1369:
1363:
1362:
1318:
1312:
1311:
1283:
1277:
1276:
1275:
1274:
1251:
1242:
1241:
1231:
1187:
1181:
1180:
1162:
1138:
1129:
1128:
1127:
1126:
1103:
1092:
1091:
1089:
1049:
1043:
1042:
1032:
992:
981:
980:
952:
941:
940:
922:
896:
887:
881:
880:
870:
860:
836:
819:
818:
790:
784:
783:
781:
780:
766:
757:
756:
754:
753:
739:
733:
732:
699:
693:
692:
658:
640:
631:
625:
624:
618:
610:
585:
576:
575:
574:
573:
550:
535:
534:
532:
531:
521:
305:Beta-thalassemia
300:Beta-thalassemia
182:Globin synthesis
21:
18:Adult hemoglobin
2172:
2171:
2167:
2166:
2165:
2163:
2162:
2161:
2147:
2146:
2145:
2140:
2128:
2117:Cytochrome P450
2087:
2069:
2023:
2002:
1981:
1972:Deoxyhemoglobin
1942:
1938:
1934:
1924:
1920:
1910:
1906:
1896:
1892:
1882:
1878:
1868:
1858:
1835:
1831:
1827:
1817:
1813:
1808:
1800:
1796:
1779:
1775:
1771:
1761:
1757:
1740:
1736:
1732:
1727:HbE Portland II
1722:
1718:
1708:
1704:
1694:
1690:
1669:
1649:
1576:
1507:Alpha locus on
1476:
1462:
1420:
1415:
1414:
1405:
1403:
1395:
1394:
1390:
1381:
1379:
1371:
1370:
1366:
1320:
1319:
1315:
1285:
1284:
1280:
1272:
1270:
1253:
1252:
1245:
1206:(5857): 38–42.
1189:
1188:
1184:
1140:
1139:
1132:
1124:
1122:
1105:
1104:
1095:
1064:(11): 602–603.
1051:
1050:
1046:
994:
993:
984:
954:
953:
944:
894:
889:
888:
884:
838:
837:
822:
792:
791:
787:
778:
776:
768:
767:
760:
751:
749:
741:
740:
736:
722:
701:
700:
696:
638:
633:
632:
628:
611:
599:
587:
586:
579:
571:
569:
552:
551:
538:
529:
527:
523:
522:
518:
513:
460:
445:
416:biconcave disks
412:red blood cells
385:
356:red blood cells
302:
294:cardiac failure
245:
209:
184:
137:
124:
113:
104:
76:
72:
28:
23:
22:
15:
12:
11:
5:
2170:
2168:
2160:
2159:
2149:
2148:
2142:
2141:
2133:
2130:
2129:
2127:
2126:
2121:
2120:
2119:
2114:
2103:
2101:
2097:
2096:
2093:
2092:
2089:
2088:
2086:
2085:
2079:
2077:
2071:
2070:
2068:
2067:
2062:
2057:
2056:
2055:
2044:
2042:
2033:
2029:
2028:
2025:
2024:
2022:
2021:
2019:Erythrocruorin
2016:
2010:
2008:
2004:
2003:
2001:
2000:
1995:
1989:
1987:
1983:
1982:
1980:
1979:
1977:Sulfhemoglobin
1974:
1965:
1960:
1954:
1952:
1948:
1947:
1944:
1943:
1941:
1940:
1936:
1932:
1926:
1922:
1918:
1912:
1908:
1904:
1898:
1894:
1890:
1884:
1880:
1876:
1870:
1866:
1860:
1856:
1849:
1847:
1841:
1840:
1837:
1836:
1834:
1833:
1829:
1825:
1819:
1815:
1811:
1806:
1802:
1798:
1794:
1787:
1785:
1781:
1780:
1778:
1777:
1773:
1769:
1763:
1759:
1755:
1748:
1746:
1742:
1741:
1739:
1738:
1734:
1730:
1724:
1720:
1716:
1713:HbE Portland I
1710:
1706:
1702:
1696:
1692:
1688:
1681:
1679:
1672:
1661:
1655:
1654:
1651:
1650:
1648:
1647:
1646:
1645:
1635:
1634:
1633:
1628:
1618:
1617:
1616:
1606:
1605:
1604:
1593:
1591:
1578:
1577:
1575:
1574:
1573:
1572:
1562:
1561:
1560:
1550:
1549:
1548:
1538:
1537:
1536:
1531:
1526:
1515:
1513:
1500:
1493:
1484:
1478:
1477:
1463:
1461:
1460:
1453:
1446:
1438:
1432:
1431:
1419:
1418:External links
1416:
1413:
1412:
1388:
1364:
1313:
1294:(6): 507–512.
1278:
1243:
1194:(1973-04-07).
1182:
1130:
1093:
1044:
1007:(2): a011684.
982:
963:(4): 339–344.
942:
882:
820:
785:
758:
734:
720:
694:
626:
597:
577:
536:
515:
514:
512:
509:
508:
507:
502:
497:
495:Alpha globin 2
492:
490:Alpha globin 1
483:
482:
477:
472:
467:
462:
458:
444:
441:
384:
381:
317:heterozygocity
301:
298:
244:
241:
208:
205:
183:
180:
176:ferrochelatase
136:
135:Heme synthesis
133:
123:
120:
111:
103:
100:
84:red blood cell
74:
70:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2169:
2158:
2155:
2154:
2152:
2139:
2138:
2131:
2125:
2124:Methemalbumin
2122:
2118:
2115:
2113:
2110:
2109:
2108:
2105:
2104:
2102:
2098:
2084:
2083:Leghemoglobin
2081:
2080:
2078:
2076:
2072:
2066:
2063:
2061:
2058:
2054:
2051:
2050:
2049:
2046:
2045:
2043:
2041:
2037:
2034:
2030:
2020:
2017:
2015:
2014:Chlorocruorin
2012:
2011:
2009:
2005:
1999:
1998:Methemoglobin
1996:
1994:
1991:
1990:
1988:
1984:
1978:
1975:
1973:
1969:
1968:Oxyhemoglobin
1966:
1964:
1961:
1959:
1956:
1955:
1953:
1949:
1930:
1927:
1916:
1913:
1902:
1899:
1888:
1885:
1874:
1871:
1864:
1861:
1854:
1851:
1850:
1848:
1846:
1842:
1823:
1820:
1809:
1803:
1792:
1789:
1788:
1786:
1782:
1767:
1764:
1753:
1750:
1749:
1747:
1743:
1728:
1725:
1714:
1711:
1700:
1697:
1686:
1683:
1682:
1680:
1676:
1673:
1671:
1665:
1662:
1660:
1656:
1644:
1641:
1640:
1639:
1636:
1632:
1629:
1627:
1624:
1623:
1622:
1619:
1615:
1612:
1611:
1610:
1607:
1603:
1600:
1599:
1598:
1595:
1594:
1592:
1590:
1588:
1584:
1579:
1571:
1568:
1567:
1566:
1563:
1559:
1556:
1555:
1554:
1551:
1547:
1544:
1543:
1542:
1539:
1535:
1532:
1530:
1527:
1525:
1522:
1521:
1520:
1517:
1516:
1514:
1512:
1510:
1504:
1501:
1497:
1494:
1492:
1488:
1485:
1483:
1479:
1474:
1470:
1467:that contain
1466:
1459:
1454:
1452:
1447:
1445:
1440:
1439:
1436:
1429:
1425:
1422:
1421:
1417:
1402:
1398:
1392:
1389:
1378:
1374:
1368:
1365:
1360:
1356:
1352:
1348:
1344:
1340:
1336:
1332:
1328:
1324:
1317:
1314:
1309:
1305:
1301:
1297:
1293:
1289:
1282:
1279:
1269:
1265:
1261:
1257:
1250:
1248:
1244:
1239:
1235:
1230:
1225:
1221:
1217:
1213:
1209:
1205:
1201:
1197:
1193:
1186:
1183:
1178:
1174:
1170:
1166:
1161:
1156:
1152:
1148:
1144:
1137:
1135:
1131:
1121:
1117:
1113:
1109:
1102:
1100:
1098:
1094:
1088:
1083:
1079:
1075:
1071:
1067:
1063:
1059:
1055:
1048:
1045:
1040:
1036:
1031:
1026:
1022:
1018:
1014:
1010:
1006:
1002:
998:
991:
989:
987:
983:
978:
974:
970:
966:
962:
958:
951:
949:
947:
943:
938:
934:
930:
926:
921:
920:10044/1/40453
916:
912:
908:
904:
900:
893:
886:
883:
878:
874:
869:
864:
859:
854:
850:
846:
842:
835:
833:
831:
829:
827:
825:
821:
816:
812:
808:
804:
800:
796:
789:
786:
775:
771:
765:
763:
759:
748:
744:
738:
735:
731:
727:
723:
721:9780121827830
717:
713:
709:
705:
698:
695:
690:
686:
682:
678:
674:
670:
666:
665:10044/1/57817
662:
657:
652:
648:
644:
637:
630:
627:
622:
616:
608:
604:
600:
598:9780323034548
594:
590:
584:
582:
578:
568:
564:
560:
556:
549:
547:
545:
543:
541:
537:
526:
520:
517:
510:
506:
503:
501:
498:
496:
493:
491:
488:
487:
486:
481:
478:
476:
473:
471:
468:
466:
465:Hemoglobin A2
463:
461:
455:
454:
453:
450:
449:
442:
440:
438:
434:
430:
426:
422:
417:
413:
408:
406:
402:
398:
394:
393:glutamic acid
390:
382:
380:
379:
373:
369:
365:
361:
357:
353:
349:
345:
343:
339:
334:
330:
326:
322:
318:
314:
310:
306:
299:
297:
295:
291:
287:
282:
278:
273:
270:
266:
262:
258:
253:
249:
242:
240:
238:
234:
233:hematopoiesis
230:
226:
222:
218:
214:
206:
204:
202:
201:chromosome 11
198:
197:chromosome 16
194:
189:
181:
179:
177:
173:
169:
165:
161:
157:
153:
149:
148:mitochondrion
145:
141:
134:
128:
121:
119:
117:
116:oxyhemoglobin
109:
101:
99:
97:
96:Hemoglobin A2
93:
89:
85:
81:
77:
66:
62:
56:
54:
49:
45:
41:
37:
32:
19:
2134:
2112:Cytochrome b
2074:
2053:Metmyoglobin
2039:
1844:
1790:
1765:
1670:development:
1667:
1637:
1620:
1608:
1596:
1581:
1564:
1552:
1540:
1518:
1506:
1473:hemoproteins
1424:Hemoglobin+A
1404:. Retrieved
1400:
1391:
1380:. Retrieved
1376:
1367:
1326:
1322:
1316:
1291:
1287:
1281:
1271:, retrieved
1259:
1203:
1199:
1185:
1153:(2): 61–76.
1150:
1146:
1123:, retrieved
1111:
1061:
1057:
1047:
1004:
1000:
960:
956:
902:
898:
885:
848:
844:
798:
794:
788:
777:. Retrieved
773:
750:. Retrieved
746:
737:
703:
697:
646:
642:
629:
588:
570:, retrieved
558:
528:. Retrieved
519:
505:Delta globin
484:
480:Hemoglobin O
475:Hemoglobin F
470:Hemoglobin C
451:
446:
409:
405:hemoglobin C
386:
376:
363:
360:blood vessel
351:
342:transfusions
321:homozygosity
303:
274:
261:asymptomatic
246:
210:
193:hemoglobin F
185:
156:succinyl CoA
138:
105:
92:erythrocytes
86:hemoglobin.
68:
64:
61:Hemoglobin A
60:
59:
51:
39:
35:
2157:Hemoglobins
2060:Neuroglobin
1986:Other human
1699:HbE Gower 2
1685:HbE Gower 1
801:: 323–333.
500:Beta globin
325:beta-globin
309:translation
290:deformities
277:(Hb Bart's)
229:thalassemia
55:Hemoglobin.
53:Proteopedia
2107:Cytochrome
2065:Cytoglobin
1845:pathology:
1668:stages of
1583:Beta locus
1491:Hemoglobin
1406:2019-04-11
1382:2019-04-11
1273:2019-04-10
1260:StatPearls
1125:2019-04-10
1112:StatPearls
868:1887/79403
779:2019-04-11
752:2019-04-10
572:2019-04-10
559:StatPearls
530:2009-02-06
511:References
448:Hemoglobin
389:amino acid
329:phenotypes
269:microcytic
252:chromosome
88:Hemoglobin
80:hemoglobin
2135:see also
2048:Myoglobin
1951:Compounds
1822:HbF/Fetal
1752:HbF/Fetal
1678:Embryonic
1659:Tetramers
1343:0009-9228
1220:0007-1447
1169:1098-3600
1078:0141-0768
1021:2157-1422
969:1532-0650
929:0028-4793
851:: 43–53.
807:0040-4675
673:2056-676X
649:: 18010.
615:cite book
607:489070888
333:genotypes
217:mutations
188:ribosomes
144:enzymatic
122:Synthesis
2151:Category
2007:Nonhuman
1499:Subunits
1465:Proteins
1359:13268104
1351:24807983
1308:19393983
1268:30725815
1177:20098328
1120:30285376
1039:23378597
977:19678601
937:25390741
877:29032940
730:15051350
681:29542687
567:30725597
443:See also
354::Normal
313:splicing
239:traits.
1482:Globins
1238:4695698
1229:1588975
1087:1282256
1030:3552344
815:7034274
689:3870507
213:enzymes
160:glycine
152:cytosol
50:: 1GZX
2075:plant:
2040:human:
1534:pseudo
1430:(MeSH)
1357:
1349:
1341:
1306:
1266:
1236:
1226:
1218:
1175:
1167:
1118:
1084:
1076:
1037:
1027:
1019:
975:
967:
935:
927:
875:
813:
805:
728:
718:
687:
679:
671:
605:
595:
565:
429:stroke
425:anemia
397:valine
286:oedema
281:allele
265:anemia
2100:Other
2032:Other
1863:Barts
1784:Adult
1745:Fetal
1355:S2CID
895:(PDF)
685:S2CID
639:(PDF)
414:from
1643:HBE1
1631:HBG2
1626:HBG1
1558:HBQ1
1529:HBA2
1524:HBA1
1469:heme
1347:PMID
1339:ISSN
1304:PMID
1264:PMID
1234:PMID
1216:ISSN
1173:PMID
1165:ISSN
1116:PMID
1074:ISSN
1035:PMID
1017:ISSN
973:PMID
965:ISSN
933:PMID
925:ISSN
873:PMID
811:PMID
803:ISSN
726:PMID
716:ISBN
677:PMID
669:ISSN
621:link
603:OCLC
593:ISBN
563:PMID
457:Hb A
331:and
158:and
150:and
140:Heme
108:heme
44:heme
38:and
1929:HbO
1915:HbE
1901:HbC
1887:HbS
1873:HbD
1853:HbH
1805:HbA
1791:HbA
1766:HbA
1614:HBD
1602:HBB
1585:on
1570:HBM
1546:HBZ
1331:doi
1296:doi
1292:122
1224:PMC
1208:doi
1155:doi
1082:PMC
1066:doi
1025:PMC
1009:doi
915:hdl
907:doi
903:371
863:hdl
853:doi
708:doi
661:hdl
651:doi
395:to
67:or
48:PDB
2153::
1931:(α
1917:(α
1903:(α
1889:(α
1875:(α
1865:(γ
1855:(β
1824:(α
1810:(α
1793:(α
1768:(α
1754:(α
1729:(ζ
1715:(ζ
1701:(α
1687:(ζ
1587:11
1509:16
1399:.
1375:.
1353:.
1345:.
1337:.
1327:53
1325:.
1302:.
1290:.
1258:,
1246:^
1232:.
1222:.
1214:.
1202:.
1198:.
1171:.
1163:.
1151:12
1149:.
1145:.
1133:^
1110:,
1096:^
1080:.
1072:.
1062:94
1060:.
1056:.
1033:.
1023:.
1015:.
1003:.
999:.
985:^
971:.
961:80
959:.
945:^
931:.
923:.
913:.
901:.
897:.
871:.
861:.
849:70
847:.
843:.
823:^
809:.
799:40
797:.
772:.
761:^
745:.
724:,
714:,
683:.
675:.
667:.
659:.
645:.
641:.
617:}}
613:{{
601:.
580:^
557:,
539:^
459:1C
296:.
288:,
203:.
1970:/
1939:)
1937:2
1935:β
1933:2
1925:)
1923:2
1921:β
1919:2
1911:)
1909:2
1907:β
1905:2
1897:)
1895:2
1893:β
1891:2
1883:)
1881:2
1879:β
1877:2
1869:)
1867:4
1859:)
1857:4
1832:)
1830:2
1828:γ
1826:2
1818:)
1816:2
1814:δ
1812:2
1807:2
1801:)
1799:2
1797:β
1795:2
1776:)
1774:2
1772:β
1770:2
1762:)
1760:2
1758:γ
1756:2
1737:)
1735:2
1733:β
1731:2
1723:)
1721:2
1719:γ
1717:2
1709:)
1707:2
1705:ε
1703:2
1695:)
1693:2
1691:ε
1689:2
1638:ε
1621:γ
1609:δ
1597:β
1589::
1565:μ
1553:θ
1541:ζ
1519:α
1511::
1475:)
1471:(
1457:e
1450:t
1443:v
1409:.
1385:.
1361:.
1333::
1310:.
1298::
1240:.
1210::
1204:2
1179:.
1157::
1090:.
1068::
1041:.
1011::
1005:3
979:.
939:.
917::
909::
879:.
865::
855::
817:.
782:.
755:.
710::
691:.
663::
653::
647:4
623:)
609:.
533:.
364:B
352:A
112:2
75:2
73:β
71:2
69:α
40:β
36:α
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.