469:
1200:
246:
1194:
128:
1206:
193:
50:. Historically, agostic complexes were the first examples of C–H sigma complexes to be observed spectroscopically and crystallographically, due to intramolecular interactions being particularly favorable and more often leading to robust complexes. Many catalytic transformations involving
306:. Anagostic interactions are more electrostatic in character. In terms of structures of anagostic interactions, the M┄H distances and M┄H−C angles fall into the ranges 2.3–2.9 Å and 110°–170°, respectively.
269:
The term agostic is reserved to describe two-electron, three-center bonding interactions between carbon, hydrogen, and a metal. Two-electron three-center bonding is clearly implicated in the complexation of
170:
data have shown that C−H and M┄H bond distances are 5-20% longer than expected for isolated metal hydride and hydrocarbons. The distance between the metal and the hydrogen is typically 1.8–2.3
556:
Von
Frantzius, Gerd; Streubel, Rainer; Brandhorst, Kai; Grunenberg, Jörg (2006). "How Strong is an Agostic Bond? Direct Assessment of Agostic Interactions Using the Generalized Compliance Matrix".
911:
237:
the highly electrophilic metal center has agostic interactions with the growing polymer chain. This increased rigidity influences the stereoselectivity of the polymerization process.
645:
Braga, D.; Grepioni, F.; Tedesco, E.; Biradha, K.; Desiraju, G. R. (1997). "Hydrogen
Bonding in Organometallic Crystals. 6. X−H┄M Hydrogen Bonds and M┄(H−X) Pseudo-Agostic Bonds".
294:
binds to metal centers often via agostic-like, three-centered Si┄H−M interactions. Because these interactions do not include carbon, however, they are not classified as agostic.
341:
1293:
108:
Short interactions between hydrocarbon substituents and coordinatively unsaturated metal complexes have been noted since the 1960s. For example, in tris(
701:
1411:
1338:
1019:
744:
629:
759:
28:
851:
482:
La Placa, Sam J.; Ibers, James A. (1965). "A Five-Coordinated d
Complex: Structure of Dichlorotris(triphenylphosphine)ruthenium(II)".
105:. Often such agostic interactions involve alkyl or aryl groups that are held close to the metal center through an additional σ-bond.
1437:
604:
250:
1273:
1258:
886:
881:
846:
225:
studies, the stabilization arising from an agostic interaction is estimated to be 10–15 kcal/mol. Recent calculations using
1082:
896:
891:
876:
1109:
779:
1070:
1060:
901:
121:
43:
1065:
694:
38:
on one of its ligands. The interaction is the result of two electrons involved in the C−H bond interaction with an empty
1012:
234:
952:
957:
511:"Evidence for Carbon–Hydrogen–Titanium Interactions: Synthesis and Crystal Structures of the Agostic alkyls [TiCl
233:. Agostic bonds sometimes play a role in catalysis by increasing 'rigidity' in transition states. For instance, in
1333:
1328:
1442:
1318:
1308:
1283:
1253:
769:
102:
35:
1099:
841:
805:
710:
687:
63:
20:
178:
signal that is shifted upfield from that of a normal aryl or alkane, often to the region normally assigned to
189:
is typically lowered to 70–100 Hz versus the 125 Hz expected for a normal sp carbon–hydrogen bond.
1360:
1263:
1235:
1005:
509:
Z. Dawoodi; M. L. H. Green; V. S. B. Mtetwa; K. Prout; A. J. Schultz; J. M. Williams; T. F. Koetzle (1986).
413:
378:
222:
91:
468:
986:
871:
229:
point to a weaker stabilisation (<10 kcal/mol). Thus, agostic interactions are stronger than most
132:
1404:
1365:
906:
59:
55:
1399:
150:
The nature of the interaction was foreshadowed in main group chemistry in the structural chemistry of
1323:
1214:
1077:
1036:
861:
724:
429:
226:
116:(II) center and a hydrogen atom on the ortho position of one of the nine phenyl rings. Complexes of
1225:
1055:
981:
800:
729:
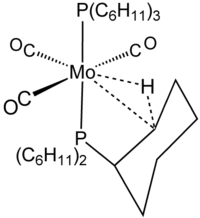
323:
167:
1389:
1144:
795:
749:
151:
109:
51:
1375:
1164:
1124:
1114:
976:
947:
937:
866:
625:
600:
457:
409:
374:
87:
1416:
1199:
1156:
1129:
942:
836:
739:
734:
654:
592:
565:
538:
491:
447:
437:
390:
355:
31:
1268:
1139:
815:
810:
319:
163:
1394:
433:
1303:
1104:
932:
831:
754:
583:
Nikonov, G. I. (2005). "Recent
Advances in Nonclassical Interligand SiH Interactions".
452:
417:
302:
Certain M┄H−C interactions are not classified as agostic but are described by the term
171:
98:
39:
596:
212:), highlighting an agostic interaction between the methyl group and the Ti(IV) center.
1431:
1352:
1312:
1245:
1174:
1047:
1028:
927:
394:
351:
230:
83:
47:
245:
1298:
101:, to describe this and many other interactions between a transition metal and a
1384:
1134:
856:
346:
117:
1193:
16:
Formation of a 3-center 2-electron bond between a transition metal and C–H bond
95:
71:
674:
359:
350:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
62:
featuring agostic interactions. Agostic interactions are observed throughout
1119:
1094:
968:
764:
442:
113:
679:
461:
542:
495:
290:, which is closely related to the agostic complex shown in the figure.
179:
174:, and the M┄H−C angle is in the range of 90°–140°. The presence of a H
127:
658:
569:
315:
291:
510:
192:
112:) ruthenium dichloride, a short interaction is observed between the
1205:
244:
191:
126:
67:
997:
209:
1001:
683:
175:
912:
Arene complexes of univalent gallium, indium, and thallium
418:"Agostic interactions in transition metal compounds"
1374:
1351:
1282:
1244:
1224:
1213:
1173:
1155:
1046:
1035:
966:
920:
824:
788:
717:
86:word for "to hold close to oneself", was coined by
381:(1983). "Carbon-hydrogen-transition metal bonds".
162:Agostic interactions are best demonstrated by
27:refers to the intramolecular interaction of a
1013:
695:
314:Agostic interactions serve a key function in
8:
1221:
1043:
1020:
1006:
998:
796:Oxidative addition / reductive elimination
702:
688:
680:
451:
441:
1412:Polyhedral skeletal electron pair theory
745:Polyhedral skeletal electron pair theory
587:. Advances in Organometallic Chemistry.
42:of the transition metal, resulting in a
369:
367:
334:
622:Metal Dihydrogen and σ-Bond Complexes
7:
852:Transition metal fullerene complexes
82:The term agostic, derived from the
887:Transition metal carbyne complexes
882:Transition metal carbene complexes
847:Transition metal indenyl complexes
347:Compendium of Chemical Terminology
146:, featuring an agostic interaction
14:
897:Transition metal alkyne complexes
892:Transition metal alkene complexes
221:On the basis of experimental and
122:three-center two-electron bonding
1204:
1198:
1192:
902:Transition-metal allyl complexes
467:
158:Characteristics of agostic bonds
877:Transition metal acyl complexes
182:ligands. The coupling constant
34:with an appropriately situated
44:three-center two-electron bond
1:
624:. New York: Kluwer Academic.
597:10.1016/s0065-3055(05)53006-5
535:J. Chem. Soc., Dalton Trans.
395:10.1016/0022-328X(83)85065-7
241:Related bonding interactions
58:are proposed to proceed via
46:. It is a special case of a
953:Shell higher olefin process
760:Dewar–Chatt–Duncanson model
120:are described as using the
94:, on the suggestion of the
1459:
1110:Metal–ligand multiple bond
842:Cyclopentadienyl complexes
806:β-hydride elimination
780:Metal–ligand multiple bond
29:coordinatively-unsaturated
1190:
907:Transition metal carbides
416:; Parkin, Gerard (2007).
1438:Organometallic chemistry
711:Organometallic chemistry
360:10.1351/goldbook.AT06984
74:, and polyenyl ligands.
64:organometallic chemistry
21:organometallic chemistry
872:Half sandwich compounds
531:)R] (R = Et or Me)"
443:10.1073/pnas.0610747104
235:Ziegler–Natta catalysis
987:Bioinorganic chemistry
266:
213:
147:
958:Ziegler–Natta process
862:Metal tetranorbornyls
620:Kubas, G. J. (2001).
422:Proc. Natl. Acad. Sci
316:alkene polymerization
248:
195:
130:
56:reductive elimination
1100:Coordinate (dipolar)
967:Related branches of
725:Crystal field theory
675:Agostic interactions
585:Adv. Organomet. Chem
543:10.1039/dt9860001629
414:Green, Malcolm L. H.
379:Green, Malcolm L. H.
265:and triphenylsilane.
227:compliance constants
1274:C–H···O interaction
1056:Electron deficiency
982:Inorganic chemistry
801:Migratory insertion
775:Agostic interaction
730:Ligand field theory
496:10.1021/ic50028a002
434:2007PNAS..104.6908B
352:agostic interaction
324:migratory insertion
168:Neutron diffraction
25:agostic interaction
1259:Resonance-assisted
867:Sandwich compounds
825:Types of compounds
750:Isolobal principle
410:Brookhart, Maurice
383:J. Organomet. Chem
375:Brookhart, Maurice
267:
214:
152:trimethylaluminium
148:
110:triphenylphosphine
52:oxidative addition
1425:
1424:
1376:Electron counting
1347:
1346:
1236:London dispersion
1188:
1187:
1165:Metal aromaticity
995:
994:
977:Organic chemistry
948:Olefin metathesis
938:Grignard reaction
837:Grignard reagents
659:10.1021/om9608364
631:978-0-306-46465-2
570:10.1021/om050489a
253:derived from (MeC
88:Maurice Brookhart
48:C–H sigma complex
1450:
1443:Chemical bonding
1417:Jemmis mno rules
1269:Dihydrogen bonds
1222:
1208:
1202:
1196:
1130:Hyperconjugation
1044:
1022:
1015:
1008:
999:
943:Monsanto process
740:d electron count
735:18-electron rule
704:
697:
690:
681:
663:
662:
653:(9): 1846–1856.
642:
636:
635:
617:
611:
610:
580:
574:
573:
553:
547:
546:
506:
500:
499:
479:
473:
472:
471:
465:
455:
445:
406:
400:
398:
371:
362:
339:
274:, e.g., in W(CO)
217:Strength of bond
32:transition metal
1458:
1457:
1453:
1452:
1451:
1449:
1448:
1447:
1428:
1427:
1426:
1421:
1370:
1343:
1286:
1278:
1240:
1227:
1217:
1209:
1203:
1197:
1184:
1169:
1151:
1039:
1031:
1026:
996:
991:
962:
916:
832:Gilman reagents
820:
816:Carbometalation
811:Transmetalation
784:
713:
708:
671:
666:
647:Organometallics
644:
643:
639:
632:
619:
618:
614:
607:
582:
581:
577:
558:Organometallics
555:
554:
550:
530:
526:
522:
518:
514:
508:
507:
503:
481:
480:
476:
466:
428:(17): 6908–14.
408:
407:
403:
373:
372:
365:
340:
336:
332:
320:stereochemistry
312:
300:
298:Anagostic bonds
289:
285:
281:
277:
273:
264:
260:
256:
243:
219:
207:
203:
199:
196:Structure of (C
188:
164:crystallography
160:
145:
141:
136:
80:
17:
12:
11:
5:
1456:
1454:
1446:
1445:
1440:
1430:
1429:
1423:
1422:
1420:
1419:
1414:
1409:
1408:
1407:
1402:
1397:
1392:
1381:
1379:
1372:
1371:
1369:
1368:
1363:
1357:
1355:
1349:
1348:
1345:
1344:
1342:
1341:
1336:
1331:
1326:
1321:
1316:
1306:
1301:
1296:
1290:
1288:
1280:
1279:
1277:
1276:
1271:
1266:
1261:
1256:
1250:
1248:
1242:
1241:
1239:
1238:
1232:
1230:
1219:
1215:Intermolecular
1211:
1210:
1191:
1189:
1186:
1185:
1183:
1182:
1179:
1177:
1171:
1170:
1168:
1167:
1161:
1159:
1153:
1152:
1150:
1149:
1148:
1147:
1142:
1132:
1127:
1122:
1117:
1112:
1107:
1102:
1097:
1092:
1087:
1086:
1085:
1075:
1074:
1073:
1068:
1063:
1052:
1050:
1041:
1037:Intramolecular
1033:
1032:
1029:Chemical bonds
1027:
1025:
1024:
1017:
1010:
1002:
993:
992:
990:
989:
984:
979:
973:
971:
964:
963:
961:
960:
955:
950:
945:
940:
935:
933:Cativa process
930:
924:
922:
918:
917:
915:
914:
909:
904:
899:
894:
889:
884:
879:
874:
869:
864:
859:
854:
849:
844:
839:
834:
828:
826:
822:
821:
819:
818:
813:
808:
803:
798:
792:
790:
786:
785:
783:
782:
777:
772:
767:
762:
757:
752:
747:
742:
737:
732:
727:
721:
719:
715:
714:
709:
707:
706:
699:
692:
684:
678:
677:
670:
669:External links
667:
665:
664:
637:
630:
612:
605:
575:
564:(1): 118–121.
548:
528:
524:
520:
516:
512:
501:
490:(6): 778–783.
474:
401:
363:
333:
331:
328:
311:
308:
299:
296:
287:
283:
279:
275:
271:
262:
258:
254:
242:
239:
231:hydrogen bonds
218:
215:
205:
201:
197:
186:
159:
156:
143:
139:
134:
99:Jasper Griffin
79:
76:
15:
13:
10:
9:
6:
4:
3:
2:
1455:
1444:
1441:
1439:
1436:
1435:
1433:
1418:
1415:
1413:
1410:
1406:
1403:
1401:
1398:
1396:
1393:
1391:
1390:Hückel's rule
1388:
1387:
1386:
1383:
1382:
1380:
1377:
1373:
1367:
1364:
1362:
1359:
1358:
1356:
1354:
1353:Bond cleavage
1350:
1340:
1337:
1335:
1332:
1330:
1327:
1325:
1322:
1320:
1319:Intercalation
1317:
1314:
1310:
1309:Metallophilic
1307:
1305:
1302:
1300:
1297:
1295:
1292:
1291:
1289:
1285:
1281:
1275:
1272:
1270:
1267:
1265:
1262:
1260:
1257:
1255:
1252:
1251:
1249:
1247:
1243:
1237:
1234:
1233:
1231:
1229:
1226:Van der Waals
1223:
1220:
1216:
1212:
1207:
1201:
1195:
1181:
1180:
1178:
1176:
1172:
1166:
1163:
1162:
1160:
1158:
1154:
1146:
1143:
1141:
1138:
1137:
1136:
1133:
1131:
1128:
1126:
1123:
1121:
1118:
1116:
1113:
1111:
1108:
1106:
1103:
1101:
1098:
1096:
1093:
1091:
1088:
1084:
1081:
1080:
1079:
1076:
1072:
1069:
1067:
1064:
1062:
1059:
1058:
1057:
1054:
1053:
1051:
1049:
1045:
1042:
1038:
1034:
1030:
1023:
1018:
1016:
1011:
1009:
1004:
1003:
1000:
988:
985:
983:
980:
978:
975:
974:
972:
970:
965:
959:
956:
954:
951:
949:
946:
944:
941:
939:
936:
934:
931:
929:
928:Carbonylation
926:
925:
923:
919:
913:
910:
908:
905:
903:
900:
898:
895:
893:
890:
888:
885:
883:
880:
878:
875:
873:
870:
868:
865:
863:
860:
858:
855:
853:
850:
848:
845:
843:
840:
838:
835:
833:
830:
829:
827:
823:
817:
814:
812:
809:
807:
804:
802:
799:
797:
794:
793:
791:
787:
781:
778:
776:
773:
771:
768:
766:
763:
761:
758:
756:
755:π backbonding
753:
751:
748:
746:
743:
741:
738:
736:
733:
731:
728:
726:
723:
722:
720:
716:
712:
705:
700:
698:
693:
691:
686:
685:
682:
676:
673:
672:
668:
660:
656:
652:
648:
641:
638:
633:
627:
623:
616:
613:
608:
606:9780120311538
602:
598:
594:
590:
586:
579:
576:
571:
567:
563:
559:
552:
549:
544:
540:
536:
532:
505:
502:
497:
493:
489:
485:
478:
475:
470:
463:
459:
454:
449:
444:
439:
435:
431:
427:
423:
419:
415:
411:
405:
402:
396:
392:
388:
384:
380:
376:
370:
368:
364:
361:
357:
353:
349:
348:
343:
338:
335:
329:
327:
325:
322:, as well as
321:
317:
309:
307:
305:
297:
295:
293:
252:
251:sigma complex
247:
240:
238:
236:
232:
228:
224:
223:computational
216:
211:
194:
190:
185:
181:
177:
173:
169:
165:
157:
155:
153:
137:
129:
125:
123:
119:
115:
111:
106:
104:
100:
97:
93:
92:Malcolm Green
89:
85:
84:Ancient Greek
77:
75:
73:
69:
65:
61:
60:intermediates
57:
53:
49:
45:
41:
37:
33:
30:
26:
22:
1395:Baird's rule
1115:Charge-shift
1089:
1078:Hypervalence
921:Applications
857:Metallocenes
774:
650:
646:
640:
621:
615:
588:
584:
578:
561:
557:
551:
534:
504:
487:
483:
477:
425:
421:
404:
386:
382:
345:
337:
313:
303:
301:
268:
220:
183:
161:
149:
107:
81:
24:
18:
1385:Aromaticity
1361:Heterolysis
1339:Salt bridge
1284:Noncovalent
1254:Low-barrier
1135:Aromaticity
1125:Conjugation
1105:Pi backbond
770:spin states
591:: 217–309.
537:(8): 1629.
484:Inorg. Chem
389:: 395–408.
118:borohydride
1432:Categories
1313:aurophilic
1294:Mechanical
718:Principles
330:References
96:classicist
72:alkylidene
1405:spherical
1366:Homolysis
1329:Cation–pi
1304:Chalcogen
1264:Symmetric
1120:Hapticity
969:chemistry
789:Reactions
765:Hapticity
304:anagostic
124:model.
114:ruthenium
40:d-orbital
1334:Anion–pi
1324:Stacking
1246:Hydrogen
1157:Metallic
1048:Covalent
1040:(strong)
462:17442749
310:Function
103:C−H bond
36:C−H bond
1299:Halogen
1145:bicyclo
1090:Agostic
453:1855361
430:Bibcode
261:)Mn(CO)
180:hydride
78:History
1400:Möbius
1228:forces
1218:(weak)
628:
603:
460:
450:
292:Silane
1378:rules
1287:other
1175:Ionic
1083:3c–4e
1071:8c–2e
1066:4c–2e
1061:3c–2e
342:IUPAC
204:)TiCl
68:alkyl
1140:homo
1095:Bent
626:ISBN
601:ISBN
458:PMID
318:and
278:(PCy
210:dmpe
142:(CO)
90:and
54:and
655:doi
593:doi
566:doi
539:doi
527:PMe
519:PCH
515:(Me
492:doi
448:PMC
438:doi
426:104
391:doi
387:250
356:doi
354:".
176:NMR
133:PCy
131:Mo(
66:in
19:In
1434::
651:16
649:.
599:.
589:53
562:25
560:.
533:.
523:CH
486:.
456:.
446:.
436:.
424:.
420:.
412:;
385:.
377:;
366:^
344:,
326:.
249:A
187:CH
166:.
154:.
70:,
23:,
1315:)
1311:(
1021:e
1014:t
1007:v
703:e
696:t
689:v
661:.
657::
634:.
609:.
595::
572:.
568::
545:.
541::
529:2
525:2
521:2
517:2
513:3
498:.
494::
488:4
464:.
440::
432::
399:.
397:.
393::
358::
288:2
286:H
284:2
282:)
280:3
276:3
272:2
270:H
263:3
259:4
257:H
255:5
208:(
206:3
202:5
200:H
198:2
184:J
172:Å
144:3
140:2
138:)
135:3
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.