31:
187:
171: 9.5 to 10, which is a distinctive part of the spectrum. This signal shows the characteristic coupling to any protons on the α carbon with a small coupling constant typically less than 3.0 Hz. The C NMR spectra of aldehydes and ketones gives a suppressed (weak) but distinctive signal at
113:
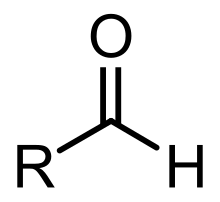
Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being
902:
reduction to produce alcohols, especially "oxo-alcohols". From the biological perspective, the key reactions involve addition of nucleophiles to the formyl carbon in the formation of imines (oxidative deamination) and hemiacetals (structures of aldose
1385:. The mechanism involves a pair of electrons from the carbonyl-group double bond transferring to the oxygen atom, leaving it single-bonded to carbon and giving the oxygen atom a negative charge. This intermediate ion rapidly reacts with
992:
is catalyzed by either acid or base. In neutral solution, the enol is the minority tautomer, reversing several times per second. But it becomes the dominant tautomer in strong acid or base solutions, and enolized aldehydes undergo
270:. Possibly because of the high reactivity of the formyl group, aldehydes are not common in several of the natural building blocks: amino acids, nucleic acids, lipids. Most sugars, however, are derivatives of aldehydes. These
455:
463:
1177:
add readily to the carbonyl group. In the product, the carbonyl carbon becomes sp-hybridized, being bonded to the nucleophile, and the oxygen center becomes protonated:
2888:
Kohlpaintner, C.; Schulte, M.; Falbe, J.; Lappe, P. and Weber, J. (2008) "Aldehydes, Aliphatic" in
Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim.
2549:
Nwaukwa, Stephen; Keehn, Philip (1982). "Oxidative cleavage of α-diols, α-diones, α-hydroxy-ketones and α-hydroxy- and α-keto acids with calcium hypochlorite ".
1985:, which are used in detergents. Some aldehydes are produced only on a small scale (less than 1000 tons per year) and are used as ingredients in flavours and
3036:
1645:
977:
Aldehydes (except those without an alpha carbon, or without protons on the alpha carbon, such as formaldehyde and benzaldehyde) can exist in either the
345:, ethylene to acetaldehyde in the presence of copper and palladium catalysts (acetaldehyde is also produced on a large scale by acetylene hydration). "
1551:) and this is heated with a base such as KOH, the terminal carbon is fully reduced to a methyl group. The Wolff–Kishner reaction may be performed as a
3955:
2470:
Reuss, G.; Disteldorf, W.; Gamer, A. O. and Hilt, A. (2005) "Formaldehyde" in
Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim.
3960:
2030:
aldehydes are named as derivatives of the longest carbon chain containing the aldehyde group. Thus, HCHO is named as a derivative of methane, and
2908:
2771:
2743:
2718:
2342:
1420:
994:
377:
1454:). Acid or base-catalyzed dehydration then leads to α,β-unsaturated carbonyl compounds. The combination of these two steps is known as the
138:
Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water,
278:, a sort of masked form of the parent aldehyde. For example, in aqueous solution only a tiny fraction of glucose exists as the aldehyde.
804:
1279:, a primary or secondary amine adds to the carbonyl group and a proton is transferred from the nitrogen to the oxygen atom to create a
634:
443:
2997:
2651:
2624:
2432:
2399:
2374:
1473:
reacts with an aldehyde as electrophile. The product of the Prins reaction varies with reaction conditions and substrates employed.
161:
1130:
arises because this reaction produces a precipitate of silver, whose presence can be used to test for the presence of an aldehyde.
2925:§R-5.6.1, Aldehydes, thioaldehydes, and their analogues, A Guide to IUPAC Nomenclature of Organic Compounds: recommendations 1993
1276:
3029:
1924:
483:
1248:
can be stable. Acetals are stable, but revert to the aldehyde in the presence of acid. Aldehydes can react with water to form
2987:
3859:
1666:
2117:. If the presence of another functional group demands the use of a suffix, the aldehyde group is named with the prefix
1630:
1534:
1192:
In many cases, a water molecule is removed after the addition takes place; in this case, the reaction is classed as an
451:
1283:. In the case of a primary amine, a water molecule can be eliminated from the carbinolamine intermediate to yield an
1126:
complex. This reagent converts aldehydes to carboxylic acids without attacking carbon–carbon double bonds. The name
3988:
3432:
3022:
1338:
1260:
727:
989:
3993:
1567:
832:
754:
401:
2667:
1615:
3469:
1392:
656:
581:
3942:
2415:
G. Berthier, J. Serre (1966). "General and
Theoretical Aspects of the Carbonyl Group". In Saul Patai (ed.).
1412:
1055:). The preferred oxidant in industry is oxygen or air. In the laboratory, popular oxidizing agents include
3842:
1762:
1733:
is an organic chemical compound with two aldehyde groups. The nomenclature of dialdehydes have the ending
1056:
1028:
604:
494:
2332:
1512:
This reaction is used as a test for aldehydes and is useful for separation or purification of aldehydes.
1345:, which are usually orange crystalline solids. This reaction forms the basis of a test for aldehydes and
3949:
3837:
2018:
The common names for aldehydes do not strictly follow official guidelines, such as those recommended by
1408:
1396:
1205:
913:
849:
821:
808:
798:
239:
115:
2159:) would yield a carboxylic acid with a trivial name, the aldehyde may be named by replacing the suffix
3918:
3363:
2840:
Chen, Che-Hong; Ferreira, Julio Cesar
Batista; Gross, Eric R.; Rosen, Daria Mochly (1 January 2014).
2641:
1197:
1134:
650:
642:
447:
381:
2937:§R-5.7.1, Carboxylic acids, A Guide to IUPAC Nomenclature of Organic Compounds: recommendations 1993
1981:. Many other aldehydes find commercial applications, often as precursors to alcohols, the so-called
3224:
2905:
2202:
1229:
1159:
1099:
887:
Aldehydes participate in many reactions. From the industrial perspective, important reactions are:
768:
682:
546:
350:
2789:"Separation of Aldehydes and Reactive Ketones from Mixtures Using a Bisulfite Extraction Protocol"
3983:
2027:
1455:
1163:
1036:
1006:
701:
690:
593:
558:
502:
442:
A variety of reagent systems achieve aldehydes under chromium-free conditions. One such are the
1680:
reactions. The aldehyde serves as the dienophile component, giving a pyran or related compound.
1341:
can also be the nucleophile and after the elimination of water, resulting in the formation of a
186:
2787:
Furigay, Maxwell H.; Boucher, Maria M.; Mizgier, Nikola A.; Brindle, Cheyenne S. (2018-04-02).
1958:. Acetaldehyde once was a dominating product, but production levels have declined to less than
3908:
3878:
3636:
3258:
2993:
2949:
2871:
2822:
2804:
2767:
2739:
2714:
2647:
2620:
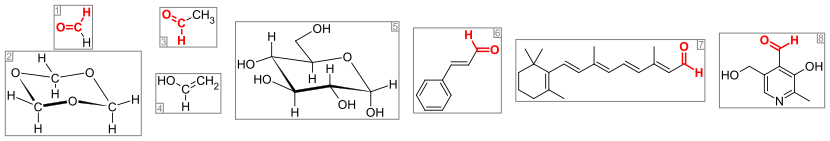
2494:
2428:
2395:
2370:
2338:
2201:(dehydrogenated alcohol). In the past, aldehydes were sometimes named after the corresponding
2185:
1773:
1552:
1288:
1201:
1193:
1072:
1064:
857:
746:
338:
39:
2419:. PATAI'S Chemistry of Functional Groups. Vol. 1. John Wiley & Sons. pp. 1–77.
934:
near 17. Note, however, this is much more acidic than an alkane or ether hydrogen, which has
3613:
3107:
3045:
2889:
2861:
2853:
2812:
2796:
2612:
2589:
2558:
2529:
2471:
2452:
2420:
2387:
2362:
1947:
1875:
1817:
1746:
1447:
1416:
1400:
1362:
1225:
1103:
872:
842:
776:
742:
358:
287:
86:
82:
49:
30:
2936:
2924:
3832:
3591:
3586:
3569:
3552:
3353:
3102:
2912:
2693:
2129:
1870:
1805:
1704:
1582:
1264:
1143:
1012:
719:
709:
623:
585:
471:
467:
389:
373:
354:
346:
150:
2022:, but these rules are useful. IUPAC prescribes the following nomenclature for aldehydes:
1303:) can also add to the carbonyl group. After the elimination of water, this results in an
3903:
3898:
3774:
3769:
3764:
3557:
3524:
3308:
3290:
3280:
2866:
2841:
2817:
2788:
2762:
Shriner, R. L.; Hermann, C. K. F.; Morrill, T. C.; Curtin, D. Y.; Fuson, R. C. (1997).
2244:
2152:
1994:
1951:
1885:
1880:
1853:
1828:
1685:
1600:
1462:
1427:
1382:
1107:
1048:
917:
675:
669:
550:
510:
459:
397:
342:
259:
228:
206:
119:
2562:
2136:
may be used to indicate which carbon atom is part of the aldehyde group; for example,
1110:
solution to give a precipitate of silver(I) oxide, and then adding just enough dilute
105:. Aldehydes are a common motif in many chemicals important in technology and biology.
3977:
3923:
3871:
3802:
3688:
3678:
3673:
3663:
3658:
3608:
3603:
3519:
3514:
3504:
3358:
3313:
3275:
3263:
3234:
3112:
2939:, IUPAC, Commission on Nomenclature of Organic Chemistry, Blackwell Scientific, 1993.
2927:, IUPAC, Commission on Nomenclature of Organic Chemistry, Blackwell Scientific, 1993.
2086:
1974:
1932:
1848:
1811:
1777:
1750:
1677:
1676:
Aldehydes can, typically in the presence of suitable catalysts, serve as partners in
1292:
1280:
1217:
1032:
1024:
824:
598:
520:
506:
291:
255:
214:
2738:. Vol. A: Structure and Mechanisms (5th ed.). Springer. pp. 601–608.
3854:
3741:
3736:
3713:
3464:
3303:
3229:
3166:
3161:
3139:
3095:
3080:
3070:
2490:"Oxidation with the Chromium Trioxide-Pyridine Complex Prepared in situ: 1-Decanal"
2280:
2254:
2210:
1990:
1928:
1838:
1822:
1799:
1793:
1155:
1068:
892:
664:
490:
393:
210:
202:
143:
139:
2893:
2456:
944:
near 50 approximately, and is even more acidic than a ketone α-hydrogen which has
286:
There are several methods for preparing aldehydes, but the dominant technology is
1244:
and water. Simple hemiacetals are usually unstable, although cyclic ones such as
380:. Oxidation can be achieved by heating the alcohol with an acidified solution of
3913:
3866:
3827:
3708:
3596:
3581:
3576:
3564:
3129:
3124:
3090:
3085:
3075:
3053:
2616:
2489:
2328:
2258:
2232:
1982:
1970:
1955:
1378:
1366:
1174:
1080:
1060:
867:
790:
647:
2857:
1389:, such as from the HCN molecule, to form the alcohol group of the cyanohydrin.
3822:
3813:
3693:
3648:
3544:
3509:
3499:
3439:
3375:
3298:
3246:
2915:, web page, University of Wisconsin Colleges, accessed on line August 4, 2007.
2424:
2386:. PATAI'S Chemistry of Functional Groups. Vol. 2. John Wiley & Sons.
2361:. PATAI'S Chemistry of Functional Groups. Vol. 1. John Wiley & Sons.
2275:
1724:
1233:
786:
762:
695:
678:
535:
385:
275:
94:
2808:
2593:
2475:
3789:
3703:
3668:
3653:
3641:
3484:
3459:
3268:
1656:
1482:
1404:
1342:
1323:
954:
near 20. This acidification of the α-hydrogen in aldehyde is attributed to:
846:
837:
243:
127:
2875:
2826:
1411:
reactions, yielding a substituted alcohol group. Related reactions include
341:. Industry oxidizes methanol to formaldehyde on a large scale, and, in the
2391:
2366:
1695:
In hydroacylation an aldehyde is added over an unsaturated bond to form a
3797:
3751:
3718:
3414:
3194:
3149:
3134:
2646:. Chichester, West Sussex: John Wiley & Sons, Ltd. pp. 199–202.
2064:
1978:
1920:
1912:
1843:
1833:
1769:
1431:
1084:
985:
812:
714:
475:
362:
267:
263:
17:
2305:
1240:
conditions, the hemiacetal and the alcohol can further react to form an
349:" and cheap oxygen is the oxidant of choice. For sensitive substrates,
3759:
3683:
3534:
3529:
3494:
3479:
3474:
3444:
3427:
3251:
3178:
3144:
2227:
1986:
1896:
Of all aldehydes, formaldehyde is produced on the largest scale, about
1865:
1650:
1358:
1346:
1308:
1249:
1245:
1111:
1076:
962:
896:
781:
759:
738:
732:
577:
498:
295:
232:
218:
2960:: 289–327. From page 290: "Je le décrirai dans ce mémoire sous le nom
3847:
3779:
3623:
3332:
3325:
3219:
3200:
3189:
3173:
3119:
2534:
2517:
2285:
2055:
2047:
2002:
1998:
1916:
1781:
1765:
1742:
1696:
1690:
1635:
1620:
1605:
1587:
1539:
1470:
1466:
1435:
1241:
1114:
solution to redissolve the precipitate in aqueous ammonia to produce
978:
862:
815:
628:
567:
540:
366:
271:
3014:
2577:
2842:"Targeting Aldehyde Dehydrogenase 2: New Therapeutic Opportunities"
1907:. It is mainly used in the production of resins when combined with
1267:. The mechanism of formation is identical to hemiacetal formation.
3728:
3698:
3631:
3489:
3454:
3449:
3422:
3370:
3337:
3241:
3065:
2800:
2578:"Einwirkung von Natriumhypochlorit auf Amide ungesättigter Säuren"
2218:
2019:
1671:
1659:
1593:
1451:
1443:
1439:
1304:
1284:
1088:
573:
563:
479:
236:
185:
29:
1232:
adds to the carbonyl group and a proton is transferred to form a
969:
The formyl proton itself does not readily undergo deprotonation.
3156:
2447:
Bertleff, W.; Roeper, M. and Sava, X. (2003) "Carbonylation" in
2334:
Advanced
Organic Chemistry: Reactions, Mechanisms, and Structure
2270:
1908:
1572:
1237:
1221:
1208:. There are many variations of nucleophilic addition reactions.
982:
921:
3018:
945:
935:
925:
2253:"ant". This word can be recognized in the simplest aldehyde,
372:
Laboratories may instead apply a wide variety of specialized
1741:. Short aliphatic dialdehydes are sometimes named after the
1481:
Aldehydes characteristically form "addition compounds" with
149:
Aldehydes can be identified by spectroscopic methods. Using
2643:
Practical
Methods for Biocatalysis and Biotransformations 2
70:
2734:
Carey, Francis A.; Sundberg, Richard J. (2007).
2518:"Oxidation of some α-hydroxy-acids with lead tetraacetate"
1023:). Typically this conversion is accomplished by catalytic
146:
completely so. The volatile aldehydes have pungent odors.
958:
the electron-withdrawing quality of the formyl center and
64:
55:
2906:
Short
Summary of IUPAC Nomenclature of Organic Compounds
1381:
that attacks the partially positive carbon atom of the
1047:
The formyl group readily oxidizes to the corresponding
27:
Organic compound containing the functional group R−CH=O
1450:
add to aldehydes to form β-hydroxycarbonyl compounds (
1166:, producing a mixture of alcohol and carboxylic acid.
2968:." (I will describe it in this memoir by the name of
1035:
reductions are also popular, as can be effected with
258:
and often contribute to their favorable odours, e.g.
2611:. Organic Reactions. Vol. 3. pp. 267–306.
73:
61:
58:
3891:
3811:
3788:
3750:
3727:
3622:
3543:
3413:
3390:
3346:
3289:
3212:
3187:
3052:
2668:"Aldehyde and Ketone - NEB Class 12 Chemistry 2080"
1772:associated with some aldehydes that are related to
1544:
If an aldehyde is converted to a simple hydrazone (
67:
52:
2764:The Systematic Identification of Organic Compounds
1768:which metabolize aldehydes in the body. There are
484:catalyze aldehyde formation with a cheaper oxidant
1141:complex ions are reduced to a red-brick-coloured
357:. When a mixture of products may be acceptable,
164:spectra, the formyl hydrogen center absorbs near
2989:Historical Studies in the Language of Chemistry
2952:(On the products of the oxidation of alcohol),
1094:Another oxidation reaction is the basis of the
193:Important aldehydes and related compounds. The
93:. The functional group itself (without the "R"
2449:Ullmann's Encyclopedia of Industrial Chemistry
2337:(6th ed.), New York: Wiley-Interscience,
1745:from which they can be derived. An example is
1154:If the aldehyde cannot form an enolate (e.g.,
3030:
2950:"Sur les produits de l'oxidation de l'alcool"
2711:Organic synthesis: the disconnection approach
1011:The formyl group can be readily reduced to a
462:will activate other pre-oxidized substrates:
8:
2050:. The name is formed by changing the suffix
1749:, which is also called succinaldehyde (from
1098:. In this test, an aldehyde is treated with
2248:
2221:
2189:
1969:because it mainly served as a precursor to
1640:Reagent: dimethyl (diazomethyl)phosphonate
3410:
3209:
3037:
3023:
3015:
2128:If the compound is a natural product or a
1519:
2865:
2816:
2713:(2nd ed.). Wiley. pp. 129–133.
2533:
2522:Bulletin of the Chemical Society of Japan
2757:
2755:
2139:
2108:
2104:
2097:group is attached to a ring, the suffix
2079:
2075:
2071:
2041:
2037:
2033:
1558:
1547:
1333:
1329:
1318:
1314:
1298:
1255:
1145:
1121:
1117:
1102:, which is prepared by adding a drop of
1018:
614:
434:
430:
426:
422:
418:
414:
410:
324:
320:
316:
312:
308:
304:
134:Physical properties and characterization
2322:
2320:
2318:
2316:
2314:
2297:
2257:, and in the simplest carboxylic acid,
2151:If replacing the aldehyde group with a
1577:With reducing agents such as magnesium
1528:
1158:), addition of strong base induces the
965:anion, delocalizes its negative charge.
529:
388:will further oxidize the aldehyde to a
2640:Sutton, Peter; Whittall, John (2012).
2466:
2464:
1365:can add to the carbonyl group to form
1133:A further oxidation reaction involves
1087:will convert the aldehyde to a methyl
771:geminal dihalides to yield aldehydes.
254:Traces of many aldehydes are found in
1259:. These diols are stable when strong
995:nucleophilic attack at the α position
961:the fact that the conjugate base, an
700:The oxidation of primary halide with
201:) is colored red. From the left: (1)
7:
2709:Warren, Stuart; Wyatt, Paul (2008).
2607:Everett, Wallis; Lane, John (1946).
1291:This reaction is catalyzed by acid.
685:nitro compound to form an aldehyde.
509:to two aldehydes or an aldehyde and
337:Aldehydes are commonly generated by
290:. Illustrative is the generation of
1950:. It is the principal precursor to
1625:Phosphine-dibromomethylene reagent
820:Aldehydes via the hydrolysis of an
655:Various reactions, for example the
227:-glucopyranose), (6) the flavorant
2226:"wine", the traditional source of
1761:Some aldehydes are substrates for
789:oxazine hydrolysis with water and
635:methoxymethylenetriphenylphosphine
576:with diisobutylaluminium hydride (
444:hypervalent organoiodine compounds
25:
2793:Journal of Visualized Experiments
2582:Justus Liebigs Annalen der Chemie
1646:Johnson–Corey–Chaykovsky reaction
633:A modified Wittig reaction using
378:chromium(VI) reagents are popular
160:band near 1700 cm. In their
118:. The aldehyde group is somewhat
2954:Annales de Chimie et de Physique
1555:, giving the overall conversion
1277:alkylimino-de-oxo-bisubstitution
891:condensations, e.g., to prepare
722:formed in a reaction variation.
101:but can also be classified as a
48:
2093:In other cases, such as when a
1925:methylene diphenyl diisocyanate
1712:Catalysed by transition metals
1170:Nucleophilic addition reactions
2992:, Courier Dover Publications,
2188:as a contraction of the Latin
2121:. This prefix is preferred to
1931:. The second main aldehyde is
470:), or amine oxides (e.g., the
1:
2986:Crosland, Maurice P. (2004),
2894:10.1002/14356007.a01_321.pub2
2563:10.1016/S0040-4039(00)88578-0
2457:10.1002/14356007.a05_217.pub2
400:) or milder reagents such as
250:Naturally occurring aldehydes
126:bond length is about 120–122
2964: ; ce nom est formé de
2046:is named as a derivative of
1421:Nozaki–Hiyama–Kishi reaction
392:, so either the aldehyde is
351:Oppenauer transfer oxidation
2972:; this name is formed from
2617:10.1002/0471264180.or003.07
2382:Jacob Zabicky, ed. (1970).
1973:, which is now prepared by
1465:occurs when a nucleophilic
1261:electron withdrawing groups
1162:. This reaction results in
876:in the presence of oxygen.
852:of acylsulfonylhydrazides.
618:) is an effective reagent.
603:Acyl chlorides selectively
523:
456:also oxidize the α position
182:Applications and occurrence
97:) can be referred to as an
4010:
2858:10.1152/physrev.00017.2013
2736:Advanced Organic Chemistry
2504:, vol. 6, p. 373
1722:
1708:
1703:
1689:
1684:
1670:
1665:
1649:
1644:
1634:
1629:
1619:
1614:
1604:
1599:
1586:
1581:
1566:
1538:
1533:
1339:2,4-dinitrophenylhydrazine
1004:
916:of the conjugate base, an
861:
856:
836:
831:
803:
797:
780:
775:
758:
753:
731:
728:Stephen aldehyde synthesis
726:
713:
708:
694:
689:
668:
663:
646:
641:
627:
622:
611:-butoxyaluminium hydride (
607:to aldehydes. Lithium tri-
597:
592:
557:
539:
534:
353:avoids overoxidation to a
3932:
2766:. John Wiley & Sons.
2488:Ratcliffe, R. W. (1988).
2425:10.1002/9780470771051.ch1
2014:IUPAC names for aldehydes
1610:Diorganochromium reagent
1568:Pinacol coupling reaction
920:in an aldehyde is weakly
833:McFadyen-Stevens reaction
755:Geminal halide hydrolysis
2692:"Aldehyde Tautomerism".
2594:10.1002/jlac.19134010102
2476:10.1002/14356007.a11_619
2357:Saul Patai, ed. (1966).
2167:in this trivial name by
2062:, so that HCHO is named
1927:("MDI"), a precursor to
1923:). It is a precursor to
1667:Oxo-Diels–Alder reaction
1413:organostannane additions
1393:Organometallic compounds
1187:RCH(Nu)O + H → RCH(Nu)OH
657:Vilsmeier-Haack reaction
582:sodium aluminium hydride
474:). Sterically-hindered
454:), although these often
153:, they display a strong
3943:chemical classification
2695:Encyclopedia Britannica
2451:, Wiley-VCH: Weinheim.
2115:cyclohexanecarbaldehyde
1860:Examples of dialdehydes
1631:Ohira–Bestmann reaction
1535:Wolff–Kishner reduction
1373:. In this reaction the
1311:derivative of the form
914:resonance stabilization
505:) can be oxidized with
452:Dess–Martin periodinane
384:. In this case, excess
294:by hydroformylation of
2974:alcohol dehydrogenatus
2576:Weerman, R.A. (1913).
2516:Ōeda, Haruomi (1934).
2249:
2222:
2190:
1763:aldehyde dehydrogenase
1516:More complex reactions
1397:organolithium reagents
1057:potassium permanganate
1029:transfer hydrogenation
1027:either directly or by
793:to yield an aldehyde.
549:; similar effect with
242:, and (8) the vitamin
190:
35:
3950:chemical nomenclature
2966:alcool dehydrogenatus
2846:Physiological Reviews
2392:10.1002/9780470771228
2367:10.1002/9780470771051
1997:and its derivatives,
1954:, which is used as a
1788:Examples of aldehydes
1616:Corey–Fuchs reactions
1409:nucleophilic addition
1271:Nitrogen nucleophiles
1206:condensation reaction
1071:. The combination of
990:Keto–enol tautomerism
850:thermal decomposition
799:Hofmann rearrangement
749:to form an aldehyde.
704:to form an aldehyde.
643:Formylation reactions
189:
109:Structure and bonding
33:
2609:The Hofmann Reaction
2243:is derived from the
1780:, and some types of
1182:RCHO + Nu → RCH(Nu)O
396:out as it forms (if
382:potassium dichromate
221:(pyranose form as α-
3406:not C, H or O)
2551:Tetrahedron Letters
2327:Smith, Michael B.;
2101:may be used. Thus,
1967: tons per year
1944: tons per year
1905: tons per year
1353:Carbon nucleophiles
1263:are present, as in
1212:Oxygen nucleophiles
1160:Cannizzaro reaction
908:Acid-base reactions
235:, which forms with
205:and (2) its trimer
89:with the structure
3848:Hypervalent iodine
2948:Liebig, J. (1835)
2911:2006-09-01 at the
2417:The Carbonyl Group
2384:The Carbonyl Group
2359:The Carbonyl Group
2146:2-oxoethanoic acid
1477:Bisulfite reaction
1456:aldol condensation
1164:disproportionation
1128:silver-mirror test
1096:silver-mirror test
1065:chromium(VI) oxide
1037:sodium borohydride
1007:Aldehyde reduction
741:salt generated by
702:dimethyl sulfoxide
691:Kornblum oxidation
594:Rosenmund reaction
559:Carbonyl reduction
464:various sulfoxides
191:
178: 190 to 205.
36:
34:Aldehyde structure
3989:Functional groups
3971:
3970:
3909:Sulfenyl chloride
3887:
3886:
3386:
3385:
3205:(only C, H and O)
3046:Functional groups
2773:978-0-471-59748-3
2745:978-0-387-44899-2
2720:978-0-470-71236-8
2557:(31): 3135–3138.
2502:Collected Volumes
2495:Organic Syntheses
2344:978-0-471-72091-1
2304:IUPAC Gold Book,
2186:Justus von Liebig
1935:, of which about
1774:neurodegenerative
1716:
1715:
1417:Barbier reactions
1401:Grignard reagents
1289:hexahydrotriazine
1287:or its trimer, a
1135:Fehling's reagent
1073:manganese dioxide
880:
879:
870:cell cultures of
858:Biotransformation
737:Hydrolysis of an
517:Specialty methods
495:oxidized sequelae
339:alcohol oxidation
213:and (4) its enol
40:organic chemistry
16:(Redirected from
4001:
3994:1830s neologisms
3938:
3843:Trifluoromethoxy
3411:
3407:
3210:
3206:
3059:
3039:
3032:
3025:
3016:
3004:
3002:
2983:
2977:
2946:
2940:
2934:
2928:
2922:
2916:
2903:
2897:
2886:
2880:
2879:
2869:
2837:
2831:
2830:
2820:
2784:
2778:
2777:
2759:
2750:
2749:
2731:
2725:
2724:
2706:
2700:
2699:
2689:
2683:
2682:
2680:
2679:
2672:Iswori Education
2664:
2658:
2657:
2637:
2631:
2630:
2604:
2598:
2597:
2573:
2567:
2566:
2546:
2540:
2539:
2537:
2535:10.1246/bcsj.9.8
2513:
2507:
2505:
2498:
2485:
2479:
2468:
2459:
2445:
2439:
2438:
2412:
2406:
2405:
2380:
2354:
2348:
2347:
2324:
2309:
2302:
2252:
2225:
2200:
2158:
2143:
2112:
2096:
2083:
2045:
1993:. These include
1968:
1966:
1963:
1948:hydroformylation
1946:are prepared by
1945:
1943:
1940:
1906:
1904:
1901:
1876:Succindialdehyde
1825:(phenylmethanal)
1818:Isovaleraldehyde
1561:
1553:one-pot reaction
1550:
1520:
1508:
1507:
1506:
1503:
1498:
1497:
1494:
1448:carboxylic acids
1388:
1376:
1372:
1336:
1321:
1302:
1258:
1220:reaction, under
1188:
1183:
1150:
1140:
1125:
1104:sodium hydroxide
1100:Tollens' reagent
1054:
1022:
883:Common reactions
873:Trametes hirsuta
777:Meyers synthesis
743:tin(II) chloride
720:Zincke aldehydes
617:
572:Reduction of an
521:
438:
374:oxidizing agents
361:directly adds a
359:hydroformylation
333:Oxidative routes
328:
288:hydroformylation
226:
225:
125:
92:
87:functional group
83:organic compound
80:
79:
76:
75:
72:
69:
66:
63:
60:
57:
54:
21:
4009:
4008:
4004:
4003:
4002:
4000:
3999:
3998:
3974:
3973:
3972:
3967:
3936:
3928:
3883:
3838:Trichloromethyl
3833:Trifluoromethyl
3807:
3784:
3746:
3723:
3618:
3587:Phosphine oxide
3539:
3405:
3403:
3402:
3400:
3398:
3396:
3394:
3392:
3382:
3342:
3285:
3204:
3203:
3198:
3193:
3183:
3057:
3056:
3048:
3043:
3013:
3008:
3007:
3000:
2985:
2984:
2980:
2947:
2943:
2935:
2931:
2923:
2919:
2913:Wayback Machine
2904:
2900:
2887:
2883:
2839:
2838:
2834:
2786:
2785:
2781:
2774:
2761:
2760:
2753:
2746:
2733:
2732:
2728:
2721:
2708:
2707:
2703:
2691:
2690:
2686:
2677:
2675:
2666:
2665:
2661:
2654:
2639:
2638:
2634:
2627:
2606:
2605:
2601:
2575:
2574:
2570:
2548:
2547:
2543:
2515:
2514:
2510:
2500:
2487:
2486:
2482:
2469:
2462:
2446:
2442:
2435:
2414:
2413:
2409:
2402:
2381:
2377:
2356:
2355:
2351:
2345:
2326:
2325:
2312:
2303:
2299:
2294:
2267:
2230:, cognate with
2207:vinous aldehyde
2205:, for example,
2178:
2156:
2141:
2137:
2130:carboxylic acid
2110:
2106:
2102:
2094:
2081:
2077:
2073:
2069:
2043:
2039:
2035:
2031:
2016:
2011:
1964:
1961:
1959:
1941:
1938:
1936:
1902:
1899:
1897:
1894:
1871:Malondialdehyde
1862:
1806:Propionaldehyde
1790:
1759:
1727:
1721:
1705:Decarbonylation
1583:Wittig reaction
1560:
1556:
1549:
1545:
1518:
1504:
1501:
1500:
1495:
1492:
1491:
1489:
1479:
1386:
1374:
1370:
1355:
1335:
1331:
1327:
1320:
1316:
1312:
1300:
1296:
1273:
1265:chloral hydrate
1257:
1253:
1228:conditions, an
1214:
1186:
1181:
1172:
1147:
1142:
1138:
1137:as a test. The
1123:
1119:
1115:
1052:
1045:
1020:
1016:
1013:primary alcohol
1009:
1003:
975:
952:
942:
932:
910:
885:
710:Zincke reaction
670:Nitro compounds
624:Wittig reaction
616:
612:
586:amide reduction
553:and no work-up
519:
503:α-hydroxy acids
489:Alternatively,
472:Ganem oxidation
468:Swern oxidation
436:
432:
428:
424:
420:
416:
412:
408:
390:carboxylic acid
355:carboxylic acid
335:
326:
322:
318:
314:
310:
306:
302:
284:
252:
223:
222:
184:
177:
170:
159:
151:IR spectroscopy
136:
123:
111:
90:
51:
47:
28:
23:
22:
15:
12:
11:
5:
4007:
4005:
3997:
3996:
3991:
3986:
3976:
3975:
3969:
3968:
3966:
3965:
3964:
3963:
3958:
3946:
3939:
3933:
3930:
3929:
3927:
3926:
3924:Sulfinylamines
3921:
3916:
3911:
3906:
3904:Phosphoramides
3901:
3899:Isothiocyanate
3895:
3893:
3889:
3888:
3885:
3884:
3882:
3881:
3876:
3875:
3874:
3864:
3863:
3862:
3852:
3851:
3850:
3845:
3840:
3835:
3830:
3819:
3817:
3809:
3808:
3806:
3805:
3800:
3794:
3792:
3786:
3785:
3783:
3782:
3777:
3775:Selenenic acid
3772:
3770:Seleninic acid
3767:
3765:Selenonic acid
3762:
3756:
3754:
3748:
3747:
3745:
3744:
3739:
3733:
3731:
3725:
3724:
3722:
3721:
3716:
3711:
3706:
3701:
3696:
3691:
3686:
3681:
3676:
3671:
3666:
3661:
3656:
3651:
3646:
3645:
3644:
3634:
3628:
3626:
3620:
3619:
3617:
3616:
3611:
3606:
3601:
3600:
3599:
3589:
3584:
3579:
3574:
3573:
3572:
3562:
3561:
3560:
3558:Phosphodiester
3549:
3547:
3541:
3540:
3538:
3537:
3532:
3527:
3522:
3517:
3512:
3507:
3502:
3497:
3492:
3487:
3482:
3477:
3472:
3467:
3462:
3457:
3452:
3447:
3442:
3437:
3436:
3435:
3430:
3419:
3417:
3408:
3404:(one element,
3388:
3387:
3384:
3383:
3381:
3380:
3379:
3378:
3368:
3367:
3366:
3361:
3350:
3348:
3344:
3343:
3341:
3340:
3335:
3330:
3329:
3328:
3318:
3317:
3316:
3311:
3306:
3295:
3293:
3287:
3286:
3284:
3283:
3281:Methylenedioxy
3278:
3273:
3272:
3271:
3266:
3256:
3255:
3254:
3249:
3239:
3238:
3237:
3227:
3222:
3216:
3214:
3207:
3185:
3184:
3182:
3181:
3176:
3171:
3170:
3169:
3164:
3154:
3153:
3152:
3147:
3142:
3137:
3132:
3127:
3117:
3116:
3115:
3110:
3100:
3099:
3098:
3093:
3088:
3083:
3078:
3073:
3062:
3060:
3058:(only C and H)
3050:
3049:
3044:
3042:
3041:
3034:
3027:
3019:
3012:
3011:External links
3009:
3006:
3005:
2998:
2978:
2941:
2929:
2917:
2898:
2881:
2832:
2795:(134): 57639.
2779:
2772:
2751:
2744:
2726:
2719:
2701:
2698:. 4 June 2024.
2684:
2659:
2652:
2632:
2625:
2599:
2568:
2541:
2508:
2480:
2460:
2440:
2433:
2407:
2400:
2375:
2349:
2343:
2310:
2296:
2295:
2293:
2290:
2289:
2288:
2283:
2278:
2273:
2266:
2263:
2184:was coined by
2177:
2174:
2173:
2172:
2153:carboxyl group
2149:
2126:
2091:
2054:of the parent
2015:
2012:
2010:
2007:
1995:cinnamaldehyde
1952:2-ethylhexanol
1893:
1890:
1889:
1888:
1886:Phthalaldehyde
1883:
1881:Glutaraldehyde
1878:
1873:
1868:
1861:
1858:
1857:
1856:
1854:Glycolaldehyde
1851:
1846:
1841:
1836:
1831:
1829:Cinnamaldehyde
1826:
1820:
1815:
1809:
1803:
1797:
1789:
1786:
1758:
1755:
1723:Main article:
1720:
1717:
1714:
1713:
1710:
1707:
1701:
1700:
1693:
1688:
1686:Hydroacylation
1682:
1681:
1674:
1669:
1663:
1662:
1653:
1648:
1642:
1641:
1638:
1633:
1627:
1626:
1623:
1618:
1612:
1611:
1608:
1603:
1601:Takai reaction
1597:
1596:
1590:
1585:
1579:
1578:
1575:
1570:
1564:
1563:
1542:
1537:
1531:
1530:
1527:
1524:
1517:
1514:
1510:
1509:
1478:
1475:
1463:Prins reaction
1428:aldol reaction
1383:carbonyl group
1354:
1351:
1272:
1269:
1213:
1210:
1190:
1189:
1184:
1171:
1168:
1108:silver nitrate
1106:solution into
1049:carboxyl group
1044:
1041:
1033:Stoichiometric
1005:Main article:
1002:
999:
974:
971:
967:
966:
959:
950:
940:
930:
909:
906:
905:
904:
900:
884:
881:
878:
877:
865:
860:
854:
853:
840:
835:
829:
828:
818:
802:
795:
794:
784:
779:
773:
772:
767:Hydrolysis of
765:
757:
751:
750:
735:
730:
724:
723:
717:
712:
706:
705:
698:
693:
687:
686:
672:
667:
661:
660:
653:
645:
639:
638:
637:as a reagent.
631:
626:
620:
619:
601:
599:Acyl chlorides
596:
590:
589:
570:
561:
555:
554:
551:singlet oxygen
543:
538:
532:
531:
528:
525:
518:
515:
511:carbon dioxide
460:Lux-Flood acid
440:
439:
343:Wacker process
334:
331:
330:
329:
283:
280:
260:cinnamaldehyde
256:essential oils
251:
248:
240:photoreceptors
229:cinnamaldehyde
207:1,3,5-trioxane
195:aldehyde group
183:
180:
175:
168:
157:
135:
132:
110:
107:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
4006:
3995:
3992:
3990:
3987:
3985:
3982:
3981:
3979:
3962:
3959:
3957:
3954:
3953:
3952:
3951:
3947:
3945:
3944:
3940:
3935:
3934:
3931:
3925:
3922:
3920:
3917:
3915:
3912:
3910:
3907:
3905:
3902:
3900:
3897:
3896:
3894:
3890:
3880:
3877:
3873:
3870:
3869:
3868:
3865:
3861:
3858:
3857:
3856:
3853:
3849:
3846:
3844:
3841:
3839:
3836:
3834:
3831:
3829:
3826:
3825:
3824:
3821:
3820:
3818:
3816:
3815:
3810:
3804:
3803:Telluroketone
3801:
3799:
3796:
3795:
3793:
3791:
3787:
3781:
3778:
3776:
3773:
3771:
3768:
3766:
3763:
3761:
3758:
3757:
3755:
3753:
3749:
3743:
3740:
3738:
3735:
3734:
3732:
3730:
3726:
3720:
3717:
3715:
3712:
3710:
3707:
3705:
3702:
3700:
3697:
3695:
3692:
3690:
3689:Sulfonic acid
3687:
3685:
3682:
3680:
3679:Sulfinic acid
3677:
3675:
3674:Thiosulfonate
3672:
3670:
3667:
3665:
3664:Thiosulfinate
3662:
3660:
3659:Sulfenic acid
3657:
3655:
3652:
3650:
3647:
3643:
3640:
3639:
3638:
3635:
3633:
3630:
3629:
3627:
3625:
3621:
3615:
3614:Phosphaallene
3612:
3610:
3609:Phosphaalkyne
3607:
3605:
3604:Phosphaalkene
3602:
3598:
3595:
3594:
3593:
3590:
3588:
3585:
3583:
3580:
3578:
3575:
3571:
3568:
3567:
3566:
3563:
3559:
3556:
3555:
3554:
3551:
3550:
3548:
3546:
3542:
3536:
3533:
3531:
3528:
3526:
3523:
3521:
3518:
3516:
3513:
3511:
3508:
3506:
3503:
3501:
3498:
3496:
3493:
3491:
3488:
3486:
3483:
3481:
3478:
3476:
3473:
3471:
3468:
3466:
3463:
3461:
3458:
3456:
3453:
3451:
3448:
3446:
3443:
3441:
3438:
3434:
3431:
3429:
3426:
3425:
3424:
3421:
3420:
3418:
3416:
3412:
3409:
3389:
3377:
3374:
3373:
3372:
3369:
3365:
3362:
3360:
3357:
3356:
3355:
3352:
3351:
3349:
3345:
3339:
3336:
3334:
3331:
3327:
3324:
3323:
3322:
3319:
3315:
3312:
3310:
3307:
3305:
3302:
3301:
3300:
3297:
3296:
3294:
3292:
3288:
3282:
3279:
3277:
3276:Ethylenedioxy
3274:
3270:
3267:
3265:
3262:
3261:
3260:
3257:
3253:
3250:
3248:
3245:
3244:
3243:
3240:
3236:
3233:
3232:
3231:
3228:
3226:
3223:
3221:
3218:
3217:
3215:
3211:
3208:
3202:
3196:
3191:
3186:
3180:
3177:
3175:
3172:
3168:
3165:
3163:
3160:
3159:
3158:
3155:
3151:
3148:
3146:
3143:
3141:
3138:
3136:
3133:
3131:
3128:
3126:
3123:
3122:
3121:
3118:
3114:
3111:
3109:
3106:
3105:
3104:
3101:
3097:
3094:
3092:
3089:
3087:
3084:
3082:
3079:
3077:
3074:
3072:
3069:
3068:
3067:
3064:
3063:
3061:
3055:
3051:
3047:
3040:
3035:
3033:
3028:
3026:
3021:
3020:
3017:
3010:
3001:
2999:9780486438023
2995:
2991:
2990:
2982:
2979:
2975:
2971:
2967:
2963:
2959:
2955:
2951:
2945:
2942:
2938:
2933:
2930:
2926:
2921:
2918:
2914:
2910:
2907:
2902:
2899:
2895:
2891:
2885:
2882:
2877:
2873:
2868:
2863:
2859:
2855:
2851:
2847:
2843:
2836:
2833:
2828:
2824:
2819:
2814:
2810:
2806:
2802:
2801:10.3791/57639
2798:
2794:
2790:
2783:
2780:
2775:
2769:
2765:
2758:
2756:
2752:
2747:
2741:
2737:
2730:
2727:
2722:
2716:
2712:
2705:
2702:
2697:
2696:
2688:
2685:
2673:
2669:
2663:
2660:
2655:
2653:9781119991397
2649:
2645:
2644:
2636:
2633:
2628:
2626:9780471005285
2622:
2618:
2614:
2610:
2603:
2600:
2595:
2591:
2587:
2583:
2579:
2572:
2569:
2564:
2560:
2556:
2552:
2545:
2542:
2536:
2531:
2527:
2523:
2519:
2512:
2509:
2503:
2497:
2496:
2491:
2484:
2481:
2477:
2473:
2467:
2465:
2461:
2458:
2454:
2450:
2444:
2441:
2436:
2434:9780470771051
2430:
2426:
2422:
2418:
2411:
2408:
2403:
2401:9780470771228
2397:
2393:
2389:
2385:
2378:
2376:9780470771051
2372:
2368:
2364:
2360:
2353:
2350:
2346:
2340:
2336:
2335:
2330:
2323:
2321:
2319:
2317:
2315:
2311:
2307:
2301:
2298:
2291:
2287:
2284:
2282:
2279:
2277:
2274:
2272:
2269:
2268:
2264:
2262:
2260:
2256:
2251:
2246:
2242:
2237:
2235:
2234:
2229:
2224:
2220:
2216:
2212:
2208:
2204:
2199:
2197:
2193:
2187:
2183:
2175:
2170:
2166:
2162:
2154:
2150:
2147:
2135:
2132:, the prefix
2131:
2127:
2124:
2120:
2116:
2100:
2099:-carbaldehyde
2092:
2089:
2088:
2067:
2066:
2061:
2057:
2053:
2049:
2029:
2025:
2024:
2023:
2021:
2013:
2008:
2006:
2004:
2000:
1996:
1992:
1988:
1984:
1980:
1976:
1975:carbonylation
1972:
1957:
1953:
1949:
1934:
1933:butyraldehyde
1930:
1929:polyurethanes
1926:
1922:
1918:
1914:
1910:
1891:
1887:
1884:
1882:
1879:
1877:
1874:
1872:
1869:
1867:
1864:
1863:
1859:
1855:
1852:
1850:
1849:Retinaldehyde
1847:
1845:
1842:
1840:
1837:
1835:
1832:
1830:
1827:
1824:
1821:
1819:
1816:
1813:
1812:Butyraldehyde
1810:
1807:
1804:
1801:
1798:
1795:
1792:
1791:
1787:
1785:
1783:
1779:
1778:heart disease
1775:
1771:
1767:
1764:
1756:
1754:
1752:
1751:succinic acid
1748:
1744:
1740:
1737:or sometimes
1736:
1732:
1726:
1718:
1711:
1706:
1702:
1698:
1694:
1692:
1687:
1683:
1679:
1678:cycloaddition
1675:
1673:
1668:
1664:
1661:
1658:
1654:
1652:
1647:
1643:
1639:
1637:
1632:
1628:
1624:
1622:
1617:
1613:
1609:
1607:
1602:
1598:
1595:
1591:
1589:
1584:
1580:
1576:
1574:
1571:
1569:
1565:
1557:RCH=O → RCH
1554:
1543:
1541:
1536:
1532:
1525:
1523:Reaction name
1522:
1521:
1515:
1513:
1488:
1487:
1486:
1484:
1476:
1474:
1472:
1468:
1464:
1459:
1457:
1453:
1449:
1445:
1441:
1437:
1433:
1429:
1424:
1422:
1418:
1414:
1410:
1406:
1402:
1398:
1394:
1390:
1384:
1380:
1368:
1364:
1360:
1352:
1350:
1348:
1344:
1340:
1325:
1310:
1306:
1294:
1293:Hydroxylamine
1290:
1286:
1282:
1281:carbinolamine
1278:
1270:
1268:
1266:
1262:
1251:
1247:
1243:
1239:
1235:
1231:
1227:
1223:
1219:
1218:acetalisation
1211:
1209:
1207:
1203:
1199:
1195:
1185:
1180:
1179:
1178:
1176:
1169:
1167:
1165:
1161:
1157:
1152:
1151:precipitate.
1149:
1136:
1131:
1129:
1113:
1109:
1105:
1101:
1097:
1092:
1090:
1086:
1082:
1078:
1074:
1070:
1066:
1062:
1058:
1050:
1042:
1040:
1038:
1034:
1030:
1026:
1025:hydrogenation
1014:
1008:
1000:
998:
996:
991:
987:
984:
980:
972:
970:
964:
960:
957:
956:
955:
953:
949:
943:
939:
933:
929:
923:
919:
915:
907:
901:
898:
894:
890:
889:
888:
882:
875:
874:
869:
866:
864:
859:
855:
851:
848:
844:
841:
839:
834:
830:
826:
823:
819:
817:
814:
810:
806:
800:
796:
792:
788:
785:
783:
778:
774:
770:
766:
764:
761:
756:
752:
748:
744:
740:
736:
734:
729:
725:
721:
718:
716:
711:
707:
703:
699:
697:
692:
688:
684:
680:
677:
673:
671:
666:
662:
658:
654:
652:
649:
644:
640:
636:
632:
630:
625:
621:
610:
606:
602:
600:
595:
591:
587:
583:
579:
575:
571:
569:
565:
562:
560:
556:
552:
548:
544:
542:
537:
533:
526:
524:Reaction name
522:
516:
514:
512:
508:
504:
500:
496:
492:
491:vicinal diols
487:
485:
481:
477:
473:
469:
465:
461:
457:
453:
449:
445:
407:
406:
405:
403:
399:
395:
391:
387:
383:
379:
375:
370:
368:
364:
360:
356:
352:
348:
344:
340:
332:
301:
300:
299:
297:
293:
292:butyraldehyde
289:
281:
279:
277:
273:
269:
265:
261:
257:
249:
247:
245:
241:
238:
234:
230:
220:
216:
215:vinyl alcohol
212:
208:
204:
200:
196:
188:
181:
179:
174:
167:
163:
156:
152:
147:
145:
141:
133:
131:
129:
121:
117:
108:
106:
104:
100:
96:
88:
85:containing a
84:
78:
45:
41:
32:
19:
3948:
3941:
3855:Vinyl halide
3812:
3742:Borinic acid
3737:Boronic acid
3714:Thioxanthate
3320:
3054:Hydrocarbons
2988:
2981:
2973:
2969:
2965:
2961:
2957:
2953:
2944:
2932:
2920:
2901:
2884:
2849:
2845:
2835:
2792:
2782:
2763:
2735:
2729:
2710:
2704:
2694:
2687:
2676:. Retrieved
2674:. 2023-07-29
2671:
2662:
2642:
2635:
2608:
2602:
2585:
2581:
2571:
2554:
2550:
2544:
2525:
2521:
2511:
2501:
2493:
2483:
2448:
2443:
2416:
2410:
2383:
2358:
2352:
2333:
2329:March, Jerry
2300:
2281:Semialdehyde
2255:formaldehyde
2241:formyl group
2240:
2238:
2231:
2214:
2211:acetaldehyde
2206:
2195:
2191:
2181:
2179:
2168:
2164:
2160:
2145:
2133:
2122:
2118:
2114:
2113:is known as
2098:
2085:
2063:
2059:
2051:
2017:
2009:Nomenclature
1991:Chanel No. 5
1983:oxo alcohols
1895:
1839:Tolualdehyde
1823:Benzaldehyde
1800:Acetaldehyde
1794:Formaldehyde
1760:
1757:Biochemistry
1738:
1734:
1730:
1728:
1592:Reagent: an
1511:
1480:
1460:
1430:, the metal
1425:
1391:
1367:cyanohydrins
1356:
1274:
1215:
1191:
1175:Nucleophiles
1173:
1156:benzaldehyde
1153:
1132:
1127:
1095:
1093:
1069:chromic acid
1046:
1010:
976:
968:
947:
937:
927:
911:
893:plasticizers
886:
871:
822:intermediate
665:Nef reaction
648:Nucleophilic
608:
488:
441:
409:[O] + CH
371:
336:
285:
253:
211:acetaldehyde
203:formaldehyde
199:formyl group
198:
194:
192:
172:
165:
154:
148:
144:acetaldehyde
140:formaldehyde
137:
112:
103:formyl group
102:
98:
43:
37:
3919:Thiocyanate
3914:Sulfonamide
3879:Perchlorate
3867:Acyl halide
3828:Fluoroethyl
3709:Thionoester
3597:Phosphonium
3582:Phosphinate
3577:Phosphonous
3565:Phosphonate
3264:Hydroperoxy
3086:Cyclopropyl
2852:(1): 1–34.
2588:(1): 1–20.
2528:(1): 8–14.
2259:formic acid
1971:acetic acid
1956:plasticizer
1739:-dialdehyde
1719:Dialdehydes
1655:Reagent: a
1499:→ RCH(OH)SO
1379:nucleophile
1377:ion is the
1198:elimination
1081:acetic acid
1061:nitric acid
973:Enolization
912:Because of
868:Lyophilized
805:Unsaturated
791:oxalic acid
696:Haloalkanes
584:; see also
276:hemiacetals
3978:Categories
3823:Haloalkane
3694:Thioketone
3649:Persulfide
3545:Phosphorus
3510:Isocyanate
3500:Isonitrile
3401:or oxygen
3399:hydrogen,
3395:not being
3376:Orthoester
3269:Dioxiranes
3247:Enol ether
3135:1-Propenyl
2962:d'aldehyde
2678:2023-07-29
2292:References
2276:Pseudoacid
2123:methanoyl-
1808:(propanal)
1796:(methanal)
1770:toxicities
1747:butanedial
1731:dialdehyde
1725:Dicarbonyl
1490:RCHO + HSO
1483:bisulfites
1419:, and the
1407:, undergo
1405:acetylides
1395:, such as
1371:R−CH(OH)CN
1234:hemiacetal
1116:[Ag(NH
918:α-hydrogen
838:Hydrazides
801:variation
787:Hemiaminal
679:hydrolysis
613:LiAlH(OBu)
545:Reductive
536:Ozonolysis
466:(e.g. the
404:are used.
386:dichromate
128:picometers
116:hybridized
95:side chain
3984:Aldehydes
3956:inorganic
3790:Tellurium
3704:Thioester
3669:Sulfoxide
3654:Disulfide
3642:Sulfonium
3592:Phosphine
3570:Phosphite
3553:Phosphate
3485:Carbamate
3460:Hydrazone
3393:element,
3391:Only one
3364:Anhydride
3103:Methylene
2809:1940-087X
2306:aldehydes
2239:The term
2198:rogenatus
2180:The word
2176:Etymology
2169:-aldehyde
2165:-oic acid
2144:is named
2084:is named
2028:aliphatic
1814:(butanal)
1802:(ethanal)
1776:disease,
1657:sulfonium
1361:group in
1343:hydrazone
1324:hydrazine
1043:Oxidation
1001:Reduction
847:catalyzed
825:carbamate
763:dihalides
715:Pyridines
527:Substrate
493:or their
476:nitroxyls
394:distilled
307:+ CO + CH
282:Synthesis
274:exist as
244:pyridoxal
18:Aldehydes
3937:See also
3872:Chloride
3798:Tellurol
3752:Selenium
3719:Xanthate
3433:Ammonium
3415:Nitrogen
3397:carbon,
3354:Carboxyl
3321:Aldehyde
3309:Acryloyl
3291:carbonyl
3195:hydrogen
3150:Cumulene
2970:aldehyde
2909:Archived
2876:24382882
2827:29658940
2331:(2007),
2265:See also
2217:is from
2203:alcohols
2182:aldehyde
2161:-ic acid
2065:methanal
2026:Acyclic
1989:such as
1987:perfumes
1979:methanol
1921:Bakelite
1913:melamine
1844:Furfural
1834:Vanillin
1546:RCH=NHNH
1529:Comment
1432:enolates
1322:such as
1254:R−CH(OH)
1250:hydrates
1236:. Under
1202:addition
1194:addition
1085:methanol
986:tautomer
903:sugars).
782:Oxazines
733:Nitriles
530:Comment
507:cleavage
499:acyloins
448:IBX acid
398:volatile
363:carbonyl
268:vanillin
264:cilantro
99:aldehyde
81:) is an
44:aldehyde
3961:organic
3760:Selenol
3684:Sulfone
3637:Sulfide
3535:NONOate
3530:Nitroso
3520:Nitrite
3515:Nitrate
3505:Cyanate
3495:Nitrile
3480:Amidine
3475:Imidate
3445:Nitrene
3440:Hydrazo
3428:Enamine
3359:Acetoxy
3347:carboxy
3314:Benzoyl
3252:Epoxide
3235:Methoxy
3225:Alcohol
3179:Carbene
3113:Methine
2867:3929114
2818:5933314
2250:formica
2228:ethanol
2119:formyl-
2087:butanal
1919:(e.g.,
1866:Glyoxal
1766:enzymes
1709:Alkane
1651:Epoxide
1526:Product
1436:ketones
1426:In the
1347:ketones
1309:ammonia
1246:glucose
1230:alcohol
1216:In the
1112:ammonia
1077:cyanide
981:or the
963:enolate
924:with a
897:polyols
863:Alkenes
813:hydroxy
769:primary
760:Geminal
739:iminium
683:primary
629:Ketones
605:reduced
578:DIBAL-H
547:work-up
541:Alkenes
478:(i.e.,
446:(i.e.,
433:CHO + H
421:OH → CH
296:propene
272:aldoses
233:retinal
219:glucose
3860:Iodide
3780:Selone
3624:Sulfur
3333:Ketone
3326:Ketene
3304:Acetyl
3259:Peroxy
3230:Alkoxy
3220:Acetal
3201:oxygen
3190:carbon
3174:Alkyne
3167:Benzyl
3162:Phenyl
3145:Allene
3140:Crotyl
3120:Alkene
3108:Bridge
3096:Pentyl
3081:Propyl
3071:Methyl
2996:
2874:
2864:
2825:
2815:
2807:
2770:
2742:
2717:
2650:
2623:
2431:
2398:
2373:
2341:
2286:Ketone
2215:Vinous
2194:cohol
2068:, and
2056:alkane
2048:butane
2003:lilial
2001:, and
1999:citral
1917:phenol
1915:, and
1782:cancer
1743:diacid
1697:ketone
1691:Ketone
1636:Alkyne
1621:Alkyne
1606:Alkene
1588:Alkene
1540:Alkane
1471:alkyne
1467:alkene
1452:aldols
1446:, and
1444:amides
1440:esters
1242:acetal
1238:acidic
1222:acidic
1067:, and
922:acidic
816:amides
651:arenes
568:amides
564:Esters
482:) can
458:. A
367:olefin
266:, and
237:opsins
231:, (7)
217:, (5)
209:, (3)
122:. The
91:R−CH=O
3892:Other
3729:Boron
3699:Thial
3632:Thiol
3525:Nitro
3490:Imide
3470:Amide
3455:Oxime
3450:Imine
3423:Amine
3371:Ester
3338:Ynone
3242:Ether
3213:R-O-R
3188:Only
3130:Allyl
3125:Vinyl
3091:Butyl
3076:Ethyl
3066:Alkyl
2247:word
2245:Latin
2233:vinyl
2223:vinum
2219:Latin
2196:dehyd
2157:−COOH
2138:CHOCH
2020:IUPAC
1735:-dial
1672:Pyran
1660:ylide
1594:ylide
1403:, or
1359:cyano
1337:) or
1307:. An
1305:oxime
1285:imine
1226:basic
1089:ester
1053:−COOH
899:, and
681:of a
580:) or
574:ester
480:TEMPO
365:to a
347:Green
311:CH=CH
162:H NMR
120:polar
42:, an
3814:Halo
3299:Acyl
3199:and
3157:Aryl
2994:ISBN
2872:PMID
2823:PMID
2805:ISSN
2768:ISBN
2740:ISBN
2715:ISBN
2648:ISBN
2621:ISBN
2429:ISBN
2396:ISBN
2371:ISBN
2339:ISBN
2271:Enol
2209:for
2142:COOH
2134:oxo-
2095:−CHO
1909:urea
1892:Uses
1573:Diol
1461:The
1357:The
1083:and
983:enol
979:keto
895:and
843:Base
745:and
676:acid
674:The
315:→ CH
197:(or
142:and
3465:Azo
2890:doi
2862:PMC
2854:doi
2813:PMC
2797:doi
2613:doi
2590:doi
2586:401
2559:doi
2530:doi
2472:doi
2453:doi
2421:doi
2388:doi
2363:doi
2236:.)
2213:. (
2163:or
2111:CHO
2082:CHO
2060:-al
2058:to
2044:CHO
1977:of
1965:000
1962:000
1942:000
1939:500
1903:000
1900:000
1753:).
1469:or
1434:of
1363:HCN
1332:NNH
1317:NNR
1275:In
1224:or
1200:or
1017:−CH
807:or
747:HCl
501:or
425:(CH
413:(CH
402:PCC
327:CHO
124:C=O
114:sp-
38:In
3980::
3197:,
3192:,
2976:.)
2958:59
2956:,
2870:.
2860:.
2850:94
2848:.
2844:.
2821:.
2811:.
2803:.
2791:.
2754:^
2670:.
2619:.
2584:.
2580:.
2555:23
2553:.
2524:.
2520:.
2499:;
2492:.
2463:^
2427:.
2394:.
2369:.
2313:^
2261:.
2192:al
2109:11
2078:CH
2074:CH
2070:CH
2052:-e
2040:CH
2036:CH
2032:CH
2005:.
1911:,
1784:.
1729:A
1699:.
1562:.
1485::
1458:.
1442:,
1438:,
1423:.
1415:,
1399:,
1375:CN
1369:,
1349:.
1301:OH
1297:NH
1252:,
1144:Cu
1139:Cu
1091:.
1079:,
1075:,
1063:,
1059:,
1039:.
1031:.
1021:OH
997:.
988:.
827:.
659:.
588:.
566:,
513:.
486:.
450:,
376:;
369:.
323:CH
319:CH
298::
262:,
246:.
158:CO
130:.
71:aɪ
3038:e
3031:t
3024:v
3003:.
2896:.
2892::
2878:.
2856::
2829:.
2799::
2776:.
2748:.
2723:.
2681:.
2656:.
2629:.
2615::
2596:.
2592::
2565:.
2561::
2538:.
2532::
2526:9
2506:.
2478:.
2474::
2455::
2437:.
2423::
2404:.
2390::
2379:.
2365::
2308:.
2171:.
2155:(
2148:.
2140:2
2125:.
2107:H
2105:6
2103:C
2090:.
2080:2
2076:2
2072:3
2042:2
2038:2
2034:3
1960:1
1937:2
1898:6
1559:3
1548:2
1505:3
1502:−
1496:3
1493:−
1387:H
1334:2
1330:2
1328:H
1326:(
1319:2
1315:2
1313:H
1299:2
1295:(
1256:2
1204:–
1196:–
1148:O
1146:2
1124:]
1122:2
1120:)
1118:3
1051:(
1019:2
1015:(
951:a
948:K
946:p
941:a
938:K
936:p
931:a
928:K
926:p
845:-
811:-
809:α
615:3
609:t
497:(
437:O
435:2
431:8
429:)
427:2
423:3
419:9
417:)
415:2
411:3
325:2
321:2
317:3
313:2
309:3
305:2
303:H
224:D
176:C
173:δ
169:H
166:δ
155:ν
77:/
74:d
68:h
65:ɪ
62:d
59:l
56:æ
53:ˈ
50:/
46:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.