2422:
49:
482:
307:
2967:, is the only name for a primary amine retained as a preferred IUPAC name for which full substitution is permitted on the ring and the nitrogen atom. It is a Type 2a retained name; for the rules of substitution see P-15.1.8.2. Substitution is limited to substituent groups cited as prefixes in accordance with the seniority of functional groups explicitly expressed or implied in the functional parent compound name. The name benzenamine may be used in general nomenclature.
1884:
1048:
823:
1723:
1606:
833:
1482:
828:
818:
813:
2013:
1806:
1044:
4299:
59:
2471:
1049:
1306:
2483:
acid (GAA, essentially distilled vinegar) in a 50:50 solution. GAA is a much safer, less reactive acid. This single combined reagent is relatively stable over time. A single spot or line applied to the pileus (or other surface). In my experience the newer formulation works as well as
Schaffer's while being safer and more convenient."
3986:: 186 – 196. (Note: In the case of a metal having two or more distinct oxides (e.g., iron), a "protosalt" is an obsolete term for a salt that is obtained from the oxide containing the lowest proportion of oxygen to metal; e.g., in the case of iron, which has two oxides – iron (II) oxide (FeO) and iron (III) oxide (Fe
2482:
Aniline oil is also used for mushroom identification. Kerrigan's 2016 Agaricus of North
America P45: (Referring to Schaffer's reaction) "In fact I recommend switching to the following modified test. Frank (1988) developed an alternative formulation in which aniline oil is combined with glacial acetic
3431:
Westerhaus, Felix A.; Jagadeesh, Rajenahally V.; Wienhöfer, Gerrit; Pohl, Marga-Martina; Radnik, Jörg; Surkus, Annette-Enrica; Rabeah, Jabor; Junge, Kathrin; Junge, Henrik; Nielsen, Martin; Brückner, Angelika; Beller, Matthias (2013). "Heterogenized Cobalt Oxide
Catalysts for Nitroarene Reduction by
3889:
N. Zinin (1842). "Beschreibung einiger neuer organischer Basen, dargestellt durch die
Einwirkung des Schwefelwasserstoffes auf Verbindungen der Kohlenwasserstoffe mit Untersalpetersäure" (Description of some new organic bases, produced by the action of hydrogen sulfide on compounds of hydrocarbons
1572:
Consistent with these factors, substituted anilines with electron donating groups are more pyramidalized, while those with electron withdrawing groups are more planar. In the parent aniline, the lone pair is approximately 12% s character, corresponding to sp hybridization. (For comparison,
1047:
2008:
Traditionally, the weak basicity of aniline is attributed to a combination of inductive effect from the more electronegative sp carbon and resonance effects, as the lone pair on the nitrogen is partially delocalized into the pi system of the benzene ring. (see the picture below):
1564:
substituent. The observed geometry reflects a compromise between two competing factors: 1) stabilization of the N lone pair in an orbital with significant s character favors pyramidalization (orbitals with s character are lower in energy), while 2)
2911:
pathway was also activated, its activity was not sufficient to prevent the accumulation of 8-OHdG. The accumulation of oxidative DNA damages in the spleen following exposure to aniline may increase mutagenic events that underlie tumorigenesis.
1436:: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Aniline can be diazotized to give a
3417:
841:
793:
4054:
1065:
3365:
Alabugin I. V.; Manoharan, M.; Buck, M.; Clark, R. J. Substituted
Anilines: The Tug-Of-War between Pyramidalization and Resonance Inside and Outside of Crystal Cavities. THEOCHEM, 2007, 813, 21-27.
3859:: 453–457. In a postscript to this article, Erdmann (one of the journal's editors) argues that aniline and the "cristallin", which was found by Unverdorben in 1826, are the same substance; see
2719:, not yet recognized as a bacterium, was still thought to be a parasite, and medical bacteriologists, believing that bacteria were not susceptible to the chemotherapeutic approach, overlooked
1556:. The nitrogen is described as having high p character. The amino group in aniline is flatter (i.e., it is a "shallower pyramid") than that in an aliphatic amine, owing to conjugation of the
1734:
The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution,
963:
2342:, which is known as "ice cold mixture" because the temperature for the reaction was as low as 0.5 °C. The benzene diazonium salt is formed as major product alongside the byproducts
1642:
In commerce, three brands of aniline are distinguished: aniline oil for blue, which is pure aniline; aniline oil for red, a mixture of equimolecular quantities of aniline and ortho- and
3045:
2993:
1190:
1319:
1694:, and many others. They also are usually prepared by nitration of the substituted aromatic compounds followed by reduction. For example, this approach is used to convert
3900:"Beschreibung einiger neuer organischer Basen, dargestellt durch die Einwirkung des Schwefelwasserstoffes auf Verbindungen der Kohlenwasserstoffe mit Untersalpetersäure"
2016:
The lone electron pair on the nitrogen delocalizes into the pi system of the benzene ring. This is responsible for nitrogen's weaker basicity compared to other amines.
1050:
3352:
2408:
Aniline is predominantly used for the preparation of methylenedianiline and related compounds by condensation with formaldehyde. The diamines are condensed with
1569:
of the N lone pair into the aryl ring favors planarity (a lone pair in a pure p orbital gives the best overlap with the orbitals of the benzene ring π system).
3119:
Kahl, Thomas; Schröder, K. W.; Lawrence, F. R.; Elvers, Barbara; Höke, Hartmut; Pfefferkorn, R.; Marshall, W. J. (2007). "Aniline". In
Ullmann, Fritz (ed.).
531:
4319:
2861:
1576:
The pyramidalization angle between the C–N bond and the bisector of the H–N–H angle is 142.5°. For comparison, in more strongly pyramidal amine group in
1600:: Approximately 4B kg are produced annually. Catalysts include nickel, copper, palladium, and platinum, and newer catalysts continue to be discovered.
3976:"De l'action des protosels de fer sur la nitronaphtaline et la nitrobenzine. Nouvelle méthode de formation des bases organiques artificielles de Zinin"
3910:(1): 140–153. Benzidam is named on page 150. Fritzsche, Zinin's colleague, soon recognized that "benzidam" was actually aniline. See: Fritzsche (1842)
959:
2633:
Factory), now the largest chemical supplier, echoes the legacy of the synthetic dye industry, built via aniline dyes and extended via the related
1072:
3265:
G. M. Wójcik "Structural
Chemistry of Anilines" in Anilines (Patai's Chemistry of Functional Groups), S. Patai, Ed. 2007, Wiley-VCH, Weinheim.
1706:. Alternatively, using Buchwald-Hartwig coupling or Ullmann reaction approaches, aryl halides can be aminated with aqueous or gaseous ammonia.
742:
4106:
3598:
3504:
3328:
3138:
2948:
3164:"Electron conjugation versus π-π repulsion in substituted benzenes: why the carbon-nitrogen bond in nitrobenzene is longer than in aniline"
1714:
The chemistry of aniline is rich because the compound has been cheaply available for many years. Below are some classes of its reactions.
2848:, igniting on contact between fuel and oxidizer. It is also dense, and can be stored for extended periods. Aniline was later replaced by
1429:
3415:, Heinrich, Sperber; Guenter, Poehler & Joachim, Pistor Hans et al., "Production of aniline", issued 1964-06-09
4396:
4314:
3653:
2265:
48:
4391:
3716:
3568:
3388:
3300:
3016:
2865:
496:
3219:"On the Harmonic Oscillator Model of Electron Delocalization (HOMED) Index and its Application to Heteroatomic π-Electron Systems"
3978:(On the action of iron protosalts on nitronaphthaline and nitrobenzene. New method of forming Zinin's synthetic organic bases.),
2896:
2413:
774:
4033:
4008:
943:
2024:. Aniline is, for example, more basic than ammonia in the gas phase, but ten thousand times less so in aqueous solution.
1326:
911:
951:
901:
417:
999:
827:
822:
1887:
Aniline can react with bromine even in room temperatures in water. Acetyl chloride is added to prevent tribromination.
935:
460:
2609:. At the time of mauveine's discovery, aniline was expensive. Soon thereafter, applying a method reported in 1854 by
1015:
2334:
amine into diazonium salt is called diazotisation. In this reaction primary aromatic amine is allowed to react with
1058:
832:
4386:
4343:
2932:
2900:
2582:
2558:
1826:
1214:
1201:
657:
2421:
4401:
2546:
2508:
314:
3788:
F. F. Runge (1834) "Ueber einige
Produkte der Steinkohlendestillation" (On some products of coal distillation),
2872:), and it has specifically been linked to bladder cancer. Aniline has been implicated as one possible cause of
2586:
2496:
2373:
Being a standard reagent in laboratories, aniline is used for many niche reactions. Its acetate is used in the
1095:
20:
1730:, a colorless liquid when pure, illustrating the tendency of anilines to air-oxidize to dark-colored products.
889:
2174:
of 193–195 °C and 192 °C, respectively. These derivatives are of importance in the color industry.
4232:(1995), R. J. Flanagan, S. S. Brown, F. A. de Wolff, R. A. Braithwaite, B. Widdop: World Health Organization
3646:
Epoxy Resins
Technology Handbook (Manufacturing Process, Synthesis, Epoxy Resin Adhesives and Epoxy Coatings
2691:
2676:
2541:
979:
686:
4218:
Forest
Decline: Cause-Effect Research in the United States of North America and Federal Republic of Germany
967:
812:
302:
2809:
1767:
1727:
1671:
3954:
3588:
2363:
2845:
2528:
1873:
1553:
1533:
855:
817:
805:
90:
4361:
3542:
1552:
in anilines is a slightly pyramidalized molecule, with hybridization of the nitrogen somewhere between
3484:
1003:
244:
3763:
3441:
3230:
3175:
2908:
2374:
1485:
971:
78:
3217:
Raczyńska, Ewa D.; Hallman, Małgorzata; Kolczyńska, Katarzyna; Stępniewski, Tomasz M. (2010-07-12).
3934:"Organische Salzbasen, aus Nitronaphtalose und Nitrobenzid mittelst Schwefelwasserstoff entstehend"
2817:
2103:
1566:
1406:
1342:
1288:
927:
477:
121:
4220:. Germany: Assessment Group for Biology, Ecology and Energy of the Julich Nuclear Research Center.
4116:
3809:
1883:
1833:, which enhances the electron-donating ability of the ring. For example, reaction of aniline with
174:
4337:
3965:: 37–87. On page 48, Hofmann argues that krystallin, kyanol, benzidam, and aniline are identical.
3698:
3465:
3346:
2784:, chemotherapy of wide effectiveness, propelled the American pharmaceutics industry. In 1939, at
2716:
2594:
2426:
2393:
2297:
1982:
1529:
1437:
1425:
readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
919:
204:
2895:
response. Rats exposed to aniline in drinking water, showed a significant increase in oxidative
2696:
2610:
1621:
983:
915:
192:
3740:
Kerrigan, Richard (2016). Agaricus of North America. NYBG Press. p. 45. ISBN 978-0-89327-536-5.
3720:
931:
4376:
4276:
4199:
4093:
4076:
3933:
3919:
3899:
3860:
3848:
3801:
3751:
3690:
3649:
3619:
3594:
3564:
3500:
3457:
3394:
3384:
3334:
3324:
3296:
3248:
3199:
3191:
3144:
3134:
3012:
2944:
2785:
2747:
2720:
2675:. During the first decade of the 20th century, while trying to modify synthetic dyes to treat
2614:
2339:
2331:
1783:
1687:
1625:
1489:
947:
4245:
Ma, Huaxian; Wang, Jianling; Abdel-Rahman, Sherif Z.; Boor, Paul J.; Khan, M. Firoze (2008).
4176:
Tanaka, Takuji; Miyazawa, Katsuhito; Tsukamoto, Testuya; Kuno, Toshiya; Suzuki, Koji (2011).
3797:
2929:
Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book)
1417:, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten
4266:
4258:
4247:"Oxidative DNA damage and its repair in rat spleen following subchronic exposure to aniline"
4189:
3822:
3771:
3682:
3492:
3449:
3288:
3266:
3238:
3183:
3126:
2936:
2837:
2630:
2516:
2492:
2444:
2318:
2183:
1970:
1877:
1747:
1481:
1444:
1358:
1354:
699:
636:
559:
167:
58:
2768:
azo dyes – already with an expired patent, synthesized in 1908 in Vienna by the researcher
1605:
987:
433:
426:
4111:
3975:
3556:
2456:
2397:
2367:
2347:
2096:
2043:
1838:
1703:
1537:
1506:
1268:
1256:
1252:
1108:
991:
955:
333:
264:
4351:
3936:(Organic bases originating from nitronaphthalene and nitrobenzene via hydrogen sulfide),
2860:
Aniline is toxic by inhalation of the vapour, ingestion, or percutaneous absorption. The
975:
138:
3767:
3445:
3234:
3179:
1722:
1620:). The reduction of nitrobenzene to aniline was also performed as part of reductions by
1007:
481:
306:
224:
131:
4347:, vol. 2 (9th ed.), New York: Charles Scribner's Sons, pp. 47–48 short=x
4271:
4246:
2873:
2735:
2638:
2532:
2335:
2301:
2261:
2206:
2092:
1974:
1892:
1643:
1636:
1617:
1402:
1297:
1272:
1246:
907:
647:
2443:(2%), and dyes and pigments (2%). As additives to rubber, aniline derivatives such as
2012:
1805:
1428:
Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in
4381:
4370:
4310:
4305:
2805:
2789:
2761:
2739:
2448:
2328:
2171:
2039:
1895:
is to protect the amine with acetyl chloride, then hydrolyse back to reform aniline.
1865:
1834:
1791:
1787:
1759:
1699:
1683:
1613:
1593:
1514:
1410:
728:
625:
615:
438:
390:
295:
143:
3702:
3366:
1023:
881:
4138:
3875:
3469:
2892:
2833:
2797:
2777:
2685:
2680:
2668:
2550:
2323:
2257:
1899:
1771:
1743:
1739:
1691:
1655:
1414:
1376:
1276:
923:
869:
724:
3412:
3270:
1674:
of aniline are known where the phenyl group is further substituted. These include
3836:
J. Fritzsche (1840) "Ueber das Anilin, ein neues Zersetzungsproduct des Indigo",
3120:
939:
385:
378:
3614:
3584:
3496:
3059:
2940:
2841:
2656:
2452:
2386:
2073:. At high temperatures aniline and carboxylic acids react to give the anilides.
2070:
1953:
1869:
1809:
1799:
1779:
1577:
1549:
1455:
1391:
1176:
1158:
1132:
1084:
4262:
2617:
enabled the evolution of a massive dye industry in Germany. Today, the name of
4356:
3995:
3292:
2989:
2801:
2793:
2773:
2769:
2724:
2660:
2500:
2475:
2460:
2285:
1898:
The largest scale industrial reaction of aniline involves its alkylation with
1795:
1775:
1735:
1422:
584:
255:
4009:"Proceedings of Chemical Societies: Chemical Society, Thursday, May 16, 1861"
3775:
3694:
3338:
3252:
3195:
3130:
3918:: 352. Reprinted as a postscript to Zinin's article in: J. Fritzsche (1842)
3398:
3163:
3148:
3037:
2849:
2765:
2743:
2712:
2664:
2645:
2440:
2359:
2222:
2021:
1978:
1763:
1675:
1651:
1647:
1581:
1557:
1459:
718:
449:
4280:
4203:
3461:
3203:
885:
4194:
4177:
3084:
1440:
salt, which can then undergo various nucleophilic substitution reactions.
893:
865:
4323:, vol. 2 (11th ed.), Cambridge University Press, pp. 47–48
3321:
Stereoelectronic effects : a bridge between structure and reactivity
2708:
2672:
2606:
2602:
2590:
2512:
2459:). The principal use of aniline in the dye industry is as a precursor to
2409:
2378:
2269:
2187:
2088:
1755:
1751:
1679:
1659:
1597:
1573:
alkylamines generally have lone pairs in orbitals that are close to sp.)
1502:
1466:
1071:
1064:
1057:
1030:
873:
275:
31:
4336:
3285:
Amino, Nitrosco and Nitro Compounds and Their Derivatives: Vol. 1 (1982)
2470:
284:
3754:[On the behaviour of organic substances at high temperatures].
3686:
3453:
3243:
3218:
3187:
2887:
Exposure of rats to aniline can elicit a response that is toxic to the
2829:
2813:
2756:
2751:
2704:
2634:
2598:
2578:
2451:, are antioxidants. Illustrative of the drugs prepared from aniline is
2293:
2277:
2051:
2033:
1830:
1695:
1510:
1465:
Because an early source of the benzene from which they are derived was
605:
365:
315:
27:
3669:
Jung, Woo-Hyuk; Ha, Eun-Ju; Chung, Il doo; Lee, Jang-Oo (2008-08-01).
1628:). These stoichiometric routes remain useful for specialty anilines.
3955:"Chemische Untersuchung der organischen Basen im Steinkohlen-Theeröl"
3670:
2888:
2812:
in 1939, was the first antibiotic, yet its toxicity restricted it to
2700:
2436:
2310:
2268:. Through these intermediates, the amine group can be converted to a
1822:
1632:
235:
3752:"Ueber das Verhalten der organischen Körper in höheren Temperaturen"
3162:
Zhang, Huaiyu; Jiang, Xiaoyu; Wu, Wei; Mo, Yirong (April 28, 2016).
2392:
In addition, aniline is the starting component in the production of
1296:
Except where otherwise noted, data are given for materials in their
3994:) – FeO is the "protoxide" from which protosalts can be made. See:
3671:"Synthesis of aniline-based azopolymers for surface relief grating"
2742:
a red azo dye, introduced in 1935 as the first antibacterial drug,
2561:
showed that these were all the same substance, known thereafter as
353:
4304:
This article incorporates text from a publication now in the
3085:"aniline | Etymology, origin and meaning of aniline by etymonline"
2731:
2626:
2469:
2464:
2420:
2343:
2047:
2011:
1882:
1804:
1721:
1480:
1433:
861:
401:
215:
173:
166:
156:
4090:
The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine
1011:
3590:
Advanced Organic Chemistry: Reactions, Mechanisms, and Structure
2618:
1612:
The reduction of nitrobenzene to aniline was first performed by
1561:
1520:
1418:
1227:
1019:
877:
344:
1413:
synthesis. Its main use is in the manufacture of precursors to
2904:
2820:
introduced the chemotherapeutic approach to cancer treatment.
2382:
2314:
1802:. These polymers exhibit rich redox and acid-base properties.
3648:(2nd ed.). Asia Pacific Business Press Inc. p. 38.
3042:
Immediately Dangerous to Life or Health Concentrations (IDLH)
995:
465:
57:
47:
3957:(Chemical investigation of organic bases in coal tar oil),
3849:"Ueber das Anilin, ein neues Zersetzungsproduct des Indigo"
3823:"Ueber das Anilin, ein neues Zersetzungsproduct des Indigo"
1754:, in the presence of certain metallic salts (especially of
2772:
for his doctoral research. By the 1940s, over 500 related
3525:
3523:
3521:
2655:
In the late 19th century, derivatives of aniline such as
2377:
for carbohydrates, identifying pentoses by conversion to
1042:
2695:
approach to medicine – failed and switched to modifying
2256:
Aniline and its ring-substituted derivatives react with
1977:
such as aniline are, in general, much weaker bases than
1596:(typically at 200–300 °C) in the presence of metal
1580:, this value is ~125°, while that of the amine group in
2792:
developed Fleming's penicillin into the first systemic
1876:
is formed. To generate the mono-substituted product, a
1631:
Aniline can alternatively be prepared from ammonia and
1314:
3383:(7th ed.). Boston: McGraw-Hill Higher Education.
2050:. The amides formed from aniline are sometimes called
1829:
reactions. Its high reactivity reflects that it is an
3920:"Bemerkung zu vorstehender Abhandlung des Hrn. Zinin"
3825:(On aniline, a new decomposition product of indigo),
3046:
National Institute for Occupational Safety and Health
2994:
National Institute for Occupational Safety and Health
2515:
that turned a beautiful blue color when treated with
1981:
amines. Aniline reacts with strong acids to form the
1817:
Electrophilic reactions at ortho- and para- positions
4178:"Pathobiology and Chemoprevention of Bladder Cancer"
2593:. Mauveine quickly became a commercial dye. Other
4045:Wilcox RW, "The treatment of influenza in adults",
4013:
The Chemical News and Journal of Industrial Science
2707:drug, and serendipitously obtained a treatment for
16:
Organic compound (C₆H₅NH₂); simplest aromatic amine
2899:to the spleen, detected as a 2.8-fold increase in
3922:(Comment on the preceding article by Mr. Zinin),
2879:Many methods exist for the detection of aniline.
2776:were produced. Medications in high demand during
1742:produces the violet-coloring matter violaniline.
191:
4216:Krahl-Urban, B., Papke, H.E., Peters, K. (1988)
4166:. 10th ed. (1983), p.96, Rahway: Merck & Co.
4139:http://www.nuclear-weapons.info/cde.htm#Corporal
3639:
3637:
3491:, John Wiley & Sons, Ltd, pp. 455–481,
3367:http://dx.doi.org/10.1016/j.theochem.2007.02.016
2020:Missing in such an analysis is consideration of
1825:, aniline derivatives are highly susceptible to
1762:. Hydrochloric acid and potassium chlorate give
1505:, compared to the C−N bond length of 1.47 Å for
384:
377:
2300:. This diazonium salt can also be reacted with
1409:, as well as a versatile starting material for
1046:
630:184.13 °C (363.43 °F; 457.28 K)
137:
130:
3719:. The Chemical Market Reporter. Archived from
3593:(6th ed.), New York: Wiley-Interscience,
3122:Ullmann's encyclopedia of industrial chemistry
2425:Most aniline is consumed in the production of
3898:(18): 272–285. Reprinted in: N. Zinin (1842)
3880:, ultimately from Sanskrit "nīla", dark-blue.
3432:Pyrolysis of Molecularly Defined Complexes".
3011:(8th ed.). W. H. Freeman. p. 1031.
1458:, although less so than structurally similar
620:−6.30 °C (20.66 °F; 266.85 K)
8:
3543:"Aniline synthesis by amination (Arylation)"
3283:Sorriso, S. (1982). "Structural chemistry".
3066:. US NOAA Office of Response and Restoration
2764:– a colorless intermediate for many, highly
2170:-dimethylaniline are colorless liquids with
4357:CDC - NIOSH Pocket Guide to Chemical Hazrds
4154:, The Royal Institute of Chemistry, London.
4092:(New York: Oxford University Press, 2007),
2715:– the first successful chemotherapy agent.
4067:
4065:
4063:
3351:: CS1 maint: location missing publisher (
1952:The resulting diamine is the precursor to
1864:If bromine water is added to aniline, the
1103:770 °C (1,420 °F; 1,040 K)
480:
305:
263:
36:
4270:
4240:
4238:
4193:
3242:
3114:
3112:
3110:
3108:
3106:
3104:
2828:Some early American rockets, such as the
2788:, seeking an alternative to sulfa drugs,
2734:sought medical applications of its dyes.
1513:between C(aryl) and N. The length of the
1432:reactions. Likewise, it is also prone to
505:InChI=1S/C6H7N/c7-6-4-2-1-3-5-6/h1-5H,7H2
432:
425:
3529:
2988:NIOSH Pocket Guide to Chemical Hazards.
2539:, after an indigo-yielding plant, anil (
2304:
2244:
2240:
2236:
2232:
2228:
2216:
2212:
2201:
2197:
2193:
2151:
2147:
2143:
2139:
2135:
2131:
2127:
2123:
2119:
2065:
2061:
2057:
1992:
1988:
1944:
1940:
1936:
1932:
1928:
1924:
1920:
1916:
1912:
1908:
1856:
1852:
1848:
1844:
1524:
1501:In aniline, the C−N bond length is 1.41
1397:
1386:
1382:
1371:
1367:
1363:
575:
571:
567:
515:InChI=1/C6H7N/c7-6-4-2-1-3-5-6/h1-5H,7H2
4352:International Chemical Safety Card 0011
2920:
2358:It reacts with nitrobenzene to produce
2205:), which may be decomposed into phenyl
1592:Industrial aniline production involves
536:
501:
476:
283:
26:Not to be confused with the amino acid
3344:
3032:
3030:
3028:
2983:
2981:
2979:
2977:
2975:
2667:drugs, with their cardiac-suppressive
2491:Aniline was first isolated in 1826 by
1624:in 1854, using iron as the reductant (
1353:indicating a derived substance) is an
296:
4024:Auerbach G, "Azo and naphthol dyes",
3314:
3312:
2186:, it gives sulfocarbanilide (diphenyl
1782:; in acid solution to aniline black.
1443:Like other amines, aniline is both a
1089:70 °C (158 °F; 343 K)
508:Key: PAYRUJLWNCNPSJ-UHFFFAOYSA-N
243:
223:
7:
3563:(3rd ed.), Belmont: Wadsworth,
3485:"The Zinin Reduction of Nitroarenes"
3007:Vollhardt, P.; Schore, Neil (2018).
2901:8-hydroxy-2'-deoxyguanosine (8-OHdG)
2723:'s report in 1928 on the effects of
2613:, it was prepared "by the ton". The
2577:In 1856, while trying to synthesise
2416:, a precursor to urethane polymers.
1405:. It is an industrially significant
4251:Toxicology and Applied Pharmacology
4007:Perkin, William Henry. 1861-06-08.
3168:Physical Chemistry Chemical Physics
2844:as an oxidizer. The combination is
1812:can form upon oxidation of aniline.
1770:in neutral solution oxidizes it to
1650:, which contains aniline and ortho-
1532:. The C−N bond length is 1.34 Å in
1528:in anilines is highly sensitive to
1430:electrophilic aromatic substitution
518:Key: PAYRUJLWNCNPSJ-UHFFFAOYAP
368:
352:
4152:Hazards in the Chemical Laboratory
2717:Salvarsan's targeted microorganism
2553:and obtained a base that he named
2535:and obtained an oil that he named
2266:benzenediazonium tetrafluoroborate
1902:. An idealized equation is shown:
1880:with acetyl chloride is required:
788:potential occupational carcinogen
14:
3838:Justus Liebigs Annalen der Chemie
2478:, which is prepared from aniline.
4297:
3980:Annales de Chemie et de Physique
3959:Annalen der Chemie und Pharmacie
3938:Annalen der Chemie und Pharmacie
2836:, used a mixture of aniline and
2623:Badische Anilin- und Soda-Fabrik
2531:(1808–1871) treated indigo with
1604:
1304:
831:
826:
821:
816:
811:
2870:Probably carcinogenic to humans
2429:, a precursor to polyurethanes.
2414:methylene diphenyl diisocyanate
1469:, aniline dyes are also called
1300:(at 25 °C , 100 kPa).
3953:August Wilhelm Hofmann (1843)
2933:The Royal Society of Chemistry
2400:is the other main ingredient.
2091:at elevated temperatures over
1616:in 1842, using sulfide salts (
775:Occupational safety and health
1:
4362:Aniline electropolymerisation
4115:. 15 May 1950. Archived from
3924:Journal für praktische Chemie
3904:Journal für praktische Chemie
3853:Journal für praktische Chemie
3833:(12): 161–165. Reprinted in:
3790:Annalen der Physik und Chemie
3756:Annalen der Physik und Chemie
3271:10.1002/9780470682531.pat0385
2381:. It is used to stain neural
2327:. The reaction of converting
2087:-Methylation of aniline with
1195:(US health exposure limits):
1147:400 mg/kg (guinea pig, oral)
52:Structural formula of aniline
2178:Carbon disulfide derivatives
1868:is decolourised and a white
4335:Baynes, T. S., ed. (1878),
4230:Basic Analytical Toxicology
3497:10.1002/0471264180.or020.04
2941:10.1039/9781849733069-FP001
2935:. 2014. pp. 416, 668.
2439:processing chemicals (9%),
1956:and related diisocyanates.
1666:Related aniline derivatives
1401:), aniline is the simplest
1121:or concentration (LD, LC):
43:
4420:
4263:10.1016/j.taap.2008.08.010
4075:(Houten: Springer, 2009),
4015:. Retrieved on 2007-09-24.
3379:Carey, Francis A. (2008).
3319:Alabugin, Igor V. (2016).
2816:use.) After World War II,
2683:– who had coined the term
2643:
2559:August Wilhelm von Hofmann
2511:isolated a substance from
2031:
1827:electrophilic substitution
1774:; in alkaline solution to
1349: 'indigo shrub', and
25:
18:
4397:IARC Group 2A carcinogens
4107:"Medicine: Spoils of War"
3932:See also: (Anon.) (1842)
3750:Otto Unverdorben (1826).
3629:, vol. 1, p. 82
3293:10.1002/9780470771662.ch1
2677:African sleeping sickness
2547:Nikolay Nikolaevich Zinin
1985:(or phenylammonium) ion (
1654:and is obtained from the
1294:
1287:
1282:
1238:
1189:
1117:
792:
772:
767:
735:
552:
527:
492:
114:
101:
89:
77:
72:
42:
4392:Hazardous air pollutants
3776:10.1002/andp.18260841109
3131:10.1002/14356007.a02_303
2651:Developments in medicine
2637:. The first azo dye was
2497:destructive distillation
1837:at 180 °C produces
1283:Supplementary data page
902:Precautionary statements
642:3.6 g/(100 mL) at 20 °C
21:Aniline (disambiguation)
4344:Encyclopædia Britannica
4320:Encyclopædia Britannica
4073:The History of Oncology
3890:and hyponitric acid ),
3675:Macromolecular Research
3064:cameochemicals.noaa.gov
2780:(1939–45), these first
2597:dyes followed, such as
2542:Indigofera suffruticosa
1143:464 mg/kg (mouse, oral)
687:Magnetic susceptibility
673:4.63 (conjugate acid; H
3483:Porter, H. K. (2011),
2856:Toxicology and testing
2573:Synthetic dye industry
2479:
2430:
2366:. Hydrogenation gives
2321:, in a process called
2017:
1888:
1813:
1768:Potassium permanganate
1731:
1728:2,6-diisopropylaniline
1646:; and aniline oil for
1584:has an angle of 180°.
1493:
1053:
652:0.6 mmHg (20 °C)
63:
53:
4150:Muir, GD (ed.) 1971,
4137:Brian Burnell. 2016.
3996:Wiktionary: protosalt
3912:Bulletin Scientifique
3892:Bulletin Scientifique
3827:Bulletin Scientifique
3613:Carl N. Webb (1941).
2810:Rockefeller Institute
2671:often countered with
2644:Further information:
2529:Carl Julius Fritzsche
2473:
2424:
2015:
1960:Reactions at nitrogen
1891:The reaction to form
1886:
1874:2,4,6-tribromoaniline
1808:
1798:affords a variety of
1725:
1534:2,4,6-trinitroaniline
1509:, indicating partial
1484:
1208:TWA 5 ppm (19 mg/m)
1165:175 ppm (mouse, 7 h)
1145:440 mg/kg (rat, oral)
1141:250 mg/kg (rat, oral)
1139:195 mg/kg (dog, oral)
1052:
91:Systematic IUPAC name
61:
51:
3847:J. Fritzsche (1840)
3821:J. Fritzsche (1840)
3644:Panda, Dr H (2019).
2909:base excision repair
2883:Oxidative DNA damage
2703:, the first organic
2587:William Henry Perkin
2375:aniline acetate test
2038:Aniline reacts with
1698:into toluidines and
1488:of aniline from the
1486:Ball-and-stick model
1159:median concentration
1035:(fire diamond)
79:Preferred IUPAC name
19:For other uses, see
4195:10.1155/2011/528353
4182:Journal of Oncology
3768:1826AnP....84..397U
3583:Smith, Michael B.;
3446:2013NatCh...5..537W
3235:2010Symm....2.1485R
3180:2016PCCP...1811821Z
3174:(17): 11821–11828.
3125:. Wiley: New York.
2818:Cornelius P. Rhoads
2435:Other uses include
2298:Sandmeyer reactions
2162:-Methylaniline and
1670:Many analogues and
1530:substituent effects
1289:Aniline (data page)
1185:180 ppm (cat, 8 h)
637:Solubility in water
592: g·mol
205:Beilstein Reference
39:
3974:A. Béchamp (1854)
3687:10.1007/BF03218555
3454:10.1038/nchem.1645
3323:. Chichester, UK.
3244:10.3390/sym2031485
3188:10.1039/c6cp00471g
3089:www.etymonline.com
2480:
2431:
2427:methylenedianiline
2394:diglycidyl aniline
2264:. One example is
2018:
1969:Aniline is a weak
1889:
1814:
1732:
1688:aminobenzoic acids
1658:(échappés) of the
1494:
1407:commodity chemical
1375:. Consisting of a
1327:Infobox references
1239:Related compounds
1230:(Immediate danger)
1183:250 ppm (rat, 4 h)
1054:
64:
54:
37:
4387:German inventions
3627:Collected Volumes
3620:Organic Syntheses
3600:978-0-471-72091-1
3561:Organic Chemistry
3506:978-0-471-26418-7
3489:Organic Reactions
3381:Organic chemistry
3330:978-1-118-90637-8
3287:. pp. 1–51.
3140:978-3-527-20138-9
3009:Organic Chemistry
2950:978-0-85404-182-4
2786:Oxford University
2748:Pasteur Institute
2738:identified as an
2721:Alexander Fleming
2615:Béchamp reduction
2445:phenylenediamines
2364:Wohl–Aue reaction
2221:), and triphenyl
1794:. Oxidation with
1784:Hypochlorous acid
1746:converts it into
1738:results, whereas
1635:derived from the
1626:Bechamp reduction
1490:crystal structure
1390:) attached to an
1335:Chemical compound
1333:
1332:
1264:Related compounds
856:Hazard statements
693:−62.95·10 cm/mol
600:Colorless liquid
461:CompTox Dashboard
175:Interactive image
168:Interactive image
68:
67:
4409:
4402:Phenyl compounds
4348:
4340:
4324:
4303:
4301:
4300:
4285:
4284:
4274:
4242:
4233:
4227:
4221:
4214:
4208:
4207:
4197:
4173:
4167:
4161:
4155:
4148:
4142:
4135:
4129:
4128:
4126:
4124:
4103:
4097:
4086:
4080:
4071:D J Th Wagener,
4069:
4058:
4043:
4037:
4026:Textile Colorist
4022:
4016:
4005:
3999:
3972:
3966:
3951:
3945:
3887:
3881:
3872:
3866:
3819:
3813:
3786:
3780:
3779:
3747:
3741:
3738:
3732:
3731:
3729:
3728:
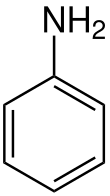
3713:
3707:
3706:
3666:
3660:
3659:
3641:
3632:
3630:
3623:
3610:
3604:
3603:
3580:
3574:
3573:
3557:McMurry, John E.
3553:
3547:
3546:
3539:
3533:
3527:
3516:
3515:
3514:
3513:
3480:
3474:
3473:
3434:Nature Chemistry
3428:
3422:
3421:
3420:
3416:
3409:
3403:
3402:
3376:
3370:
3363:
3357:
3356:
3350:
3342:
3316:
3307:
3306:
3280:
3274:
3263:
3257:
3256:
3246:
3229:(3): 1485–1509.
3214:
3208:
3207:
3159:
3153:
3152:
3116:
3099:
3098:
3096:
3095:
3081:
3075:
3074:
3072:
3071:
3056:
3050:
3049:
3034:
3023:
3022:
3004:
2998:
2997:
2985:
2970:
2969:
2925:
2840:as a fuel, with
2838:furfuryl alcohol
2746:, soon found at
2517:chloride of lime
2493:Otto Unverdorben
2455:(acetaminophen,
2319:benzeneazophenol
2307:
2291:
2283:
2275:
2247:
2220:
2204:
2184:carbon disulfide
2155:
2111:-dimethylaniline
2068:
2004:
2003:
2002:
1999:
1948:
1860:
1608:
1544:Pyramidalization
1527:
1497:Aryl-N distances
1400:
1389:
1374:
1355:organic compound
1317:
1311:
1308:
1307:
1177:lowest published
1133:lowest published
1109:Explosive limits
1074:
1067:
1060:
1045:
1025:
1021:
1017:
1013:
1009:
1005:
1001:
997:
993:
989:
985:
981:
977:
973:
969:
965:
961:
957:
953:
949:
945:
941:
937:
933:
929:
925:
921:
917:
913:
909:
895:
891:
887:
883:
879:
875:
871:
867:
863:
835:
830:
825:
820:
815:
759:
736:Thermochemistry
700:Refractive index
591:
578:
560:Chemical formula
485:
484:
469:
467:
436:
429:
388:
381:
370:
356:
334:Gmelin Reference
317:
309:
298:
287:
267:
247:
227:
195:
177:
170:
141:
134:
44:
40:
4419:
4418:
4412:
4411:
4410:
4408:
4407:
4406:
4367:
4366:
4338:"Aniline"
4334:
4331:
4313:, ed. (1911), "
4309:
4298:
4296:
4293:
4288:
4244:
4243:
4236:
4228:
4224:
4215:
4211:
4175:
4174:
4170:
4164:The Merck Index
4162:
4158:
4149:
4145:
4136:
4132:
4122:
4120:
4119:on 24 June 2013
4105:
4104:
4100:
4087:
4083:
4070:
4061:
4044:
4040:
4023:
4019:
4006:
4002:
3993:
3989:
3973:
3969:
3952:
3948:
3931:
3888:
3884:
3873:
3869:
3820:
3816:
3812:(see page 331).
3800:(see page 65),
3787:
3783:
3762:(11): 397–410.
3749:
3748:
3744:
3739:
3735:
3726:
3724:
3715:
3714:
3710:
3668:
3667:
3663:
3656:
3643:
3642:
3635:
3625:
3612:
3611:
3607:
3601:
3582:
3581:
3577:
3571:
3555:
3554:
3550:
3541:
3540:
3536:
3528:
3519:
3511:
3509:
3507:
3482:
3481:
3477:
3430:
3429:
3425:
3418:
3411:
3410:
3406:
3391:
3378:
3377:
3373:
3364:
3360:
3343:
3331:
3318:
3317:
3310:
3303:
3282:
3281:
3277:
3264:
3260:
3216:
3215:
3211:
3161:
3160:
3156:
3141:
3118:
3117:
3102:
3093:
3091:
3083:
3082:
3078:
3069:
3067:
3058:
3057:
3053:
3036:
3035:
3026:
3019:
3006:
3005:
3001:
2987:
2986:
2973:
2966:
2962:
2958:
2951:
2927:
2926:
2922:
2918:
2907:. Although the
2885:
2858:
2826:
2804:, developed by
2653:
2648:
2611:Antoine Béchamp
2575:
2509:Friedlieb Runge
2503:. He called it
2489:
2406:
2398:Epichlorohydrin
2368:cyclohexylamine
2356:
2354:Other reactions
2348:sodium chloride
2338:and 2 moles of
2306:
2302:
2292:, where X is a
2289:
2281:
2273:
2262:diazonium salts
2254:
2246:
2242:
2238:
2234:
2230:
2226:
2218:
2214:
2210:
2203:
2199:
2195:
2191:
2180:
2153:
2149:
2145:
2141:
2137:
2133:
2129:
2125:
2121:
2117:
2082:
2067:
2063:
2059:
2055:
2044:acetyl chloride
2036:
2030:
2000:
1997:
1996:
1994:
1990:
1986:
1975:Aromatic amines
1967:
1962:
1946:
1942:
1938:
1934:
1930:
1926:
1922:
1918:
1914:
1910:
1906:
1858:
1854:
1850:
1846:
1842:
1839:sulfanilic acid
1819:
1790:and para-amino
1778:, ammonia, and
1720:
1712:
1704:4-chloroaniline
1668:
1644:para-toluidines
1622:Antoine Béchamp
1590:
1546:
1538:3-methylaniline
1526:
1518:
1507:cyclohexylamine
1499:
1479:
1453:
1399:
1395:
1388:
1384:
1380:
1373:
1369:
1365:
1361:
1336:
1329:
1324:
1323:
1322: ?)
1313:
1309:
1305:
1301:
1275:
1271:
1269:Phenylhydrazine
1265:
1257:2-Naphthylamine
1255:
1253:1-Naphthylamine
1249:
1247:aromatic amines
1231:
1218:
1205:
1184:
1180:
1174:
1162:
1156:
1146:
1144:
1142:
1140:
1136:
1130:
1100:
1097:
1079:
1078:
1077:
1076:
1069:
1062:
1055:
1051:
1043:
904:
858:
844:
808:
785:
760:
757:
751:
747:
744:
743:Std enthalpy of
731:at 25 °C)
710:
708:
690:
680:
676:
666:
639:
589:
577:
573:
569:
565:
562:
548:
545:
540:
535:
534:
523:
520:
519:
516:
510:
509:
506:
500:
499:
488:
470:
463:
444:
412:
396:
371:
359:
336:
327:
290:
270:
250:
230:
207:
198:
180:
160:
149:
124:
110:
109:
107:
105:
97:
96:
85:
84:
35:
24:
17:
12:
11:
5:
4417:
4416:
4413:
4405:
4404:
4399:
4394:
4389:
4384:
4379:
4369:
4368:
4365:
4364:
4359:
4354:
4349:
4330:
4329:External links
4327:
4326:
4325:
4311:Chisholm, Hugh
4292:
4289:
4287:
4286:
4257:(2): 247–253.
4234:
4222:
4209:
4168:
4156:
4143:
4130:
4098:
4088:John E Lesch,
4081:
4059:
4049:, 1900 Dec 15;
4038:
4017:
4000:
3991:
3987:
3982:, 3rd series,
3967:
3946:
3882:
3867:
3865:
3864:
3845:
3814:
3781:
3742:
3733:
3708:
3681:(6): 532–538.
3661:
3655:978-8178331829
3654:
3633:
3605:
3599:
3575:
3569:
3548:
3534:
3517:
3505:
3475:
3440:(6): 537–543.
3423:
3404:
3389:
3371:
3358:
3329:
3308:
3301:
3275:
3258:
3209:
3154:
3139:
3100:
3076:
3051:
3024:
3017:
2999:
2971:
2964:
2960:
2956:
2955:Aniline, for C
2949:
2919:
2917:
2914:
2891:, including a
2884:
2881:
2874:forest dieback
2857:
2854:
2825:
2822:
2736:Gerhard Domagk
2652:
2649:
2639:aniline yellow
2574:
2571:
2533:caustic potash
2519:. He named it
2488:
2485:
2463:, the blue of
2433:
2432:
2405:
2402:
2355:
2352:
2336:sodium nitrite
2253:
2250:
2207:isothiocyanate
2179:
2176:
2172:boiling points
2157:
2156:
2100:-methylaniline
2093:acid catalysts
2081:
2075:
2054:, for example
2040:acyl chlorides
2032:Main article:
2029:
2026:
1966:
1963:
1961:
1958:
1950:
1949:
1893:4-bromoaniline
1818:
1815:
1719:
1716:
1711:
1708:
1684:chloroanilines
1667:
1664:
1637:cumene process
1618:Zinin reaction
1610:
1609:
1589:
1586:
1567:delocalization
1545:
1542:
1498:
1495:
1478:
1475:
1451:
1403:aromatic amine
1334:
1331:
1330:
1325:
1303:
1302:
1298:standard state
1295:
1292:
1291:
1285:
1284:
1280:
1279:
1273:Nitrosobenzene
1266:
1263:
1260:
1259:
1250:
1244:
1241:
1240:
1236:
1235:
1232:
1226:
1223:
1222:
1219:
1213:
1210:
1209:
1206:
1200:
1197:
1196:
1187:
1186:
1181:
1172:
1170:
1167:
1166:
1163:
1154:
1152:
1149:
1148:
1137:
1128:
1126:
1123:
1122:
1115:
1114:
1111:
1105:
1104:
1101:
1094:
1091:
1090:
1087:
1081:
1080:
1070:
1063:
1056:
1041:
1040:
1039:
1038:
1036:
1027:
1026:
964:P305+P351+P338
905:
900:
897:
896:
859:
854:
851:
850:
845:
840:
837:
836:
809:
804:
801:
800:
790:
789:
786:
783:
780:
779:
770:
769:
765:
764:
761:
755:
749:
741:
738:
737:
733:
732:
721:
715:
714:
711:
706:
698:
695:
694:
691:
685:
682:
681:
679:
678:
674:
670:
668:
664:
654:
653:
650:
648:Vapor pressure
644:
643:
640:
635:
632:
631:
628:
622:
621:
618:
612:
611:
608:
602:
601:
598:
594:
593:
587:
581:
580:
563:
558:
555:
554:
550:
549:
547:
546:
543:
541:
538:
530:
529:
528:
525:
524:
522:
521:
517:
514:
513:
511:
507:
504:
503:
495:
494:
493:
490:
489:
487:
486:
473:
471:
459:
456:
455:
452:
446:
445:
443:
442:
430:
422:
420:
414:
413:
411:
410:
406:
404:
398:
397:
395:
394:
382:
374:
372:
364:
361:
360:
358:
357:
349:
347:
341:
340:
337:
332:
329:
328:
326:
325:
321:
319:
311:
310:
300:
292:
291:
289:
288:
280:
278:
272:
271:
269:
268:
260:
258:
252:
251:
249:
248:
240:
238:
232:
231:
229:
228:
220:
218:
212:
211:
208:
203:
200:
199:
197:
196:
188:
186:
182:
181:
179:
178:
171:
163:
161:
154:
151:
150:
148:
147:
135:
127:
125:
120:
117:
116:
112:
111:
103:
99:
98:
94:
93:
87:
86:
82:
81:
75:
74:
70:
69:
66:
65:
55:
15:
13:
10:
9:
6:
4:
3:
2:
4415:
4414:
4403:
4400:
4398:
4395:
4393:
4390:
4388:
4385:
4383:
4380:
4378:
4375:
4374:
4372:
4363:
4360:
4358:
4355:
4353:
4350:
4346:
4345:
4339:
4333:
4332:
4328:
4322:
4321:
4316:
4312:
4307:
4306:public domain
4295:
4294:
4290:
4282:
4278:
4273:
4268:
4264:
4260:
4256:
4252:
4248:
4241:
4239:
4235:
4231:
4226:
4223:
4219:
4213:
4210:
4205:
4201:
4196:
4191:
4187:
4183:
4179:
4172:
4169:
4165:
4160:
4157:
4153:
4147:
4144:
4140:
4134:
4131:
4118:
4114:
4113:
4108:
4102:
4099:
4095:
4091:
4085:
4082:
4078:
4074:
4068:
4066:
4064:
4060:
4056:
4052:
4048:
4042:
4039:
4035:
4031:
4027:
4021:
4018:
4014:
4010:
4004:
4001:
3997:
3985:
3981:
3977:
3971:
3968:
3964:
3960:
3956:
3950:
3947:
3943:
3939:
3935:
3929:
3925:
3921:
3917:
3913:
3909:
3905:
3901:
3897:
3893:
3886:
3883:
3879:
3878:
3871:
3868:
3862:
3861:pages 457–459
3858:
3854:
3850:
3846:
3843:
3839:
3835:
3834:
3832:
3828:
3824:
3818:
3815:
3811:
3807:
3803:
3799:
3795:
3791:
3785:
3782:
3777:
3773:
3769:
3765:
3761:
3757:
3753:
3746:
3743:
3737:
3734:
3723:on 2002-02-19
3722:
3718:
3712:
3709:
3704:
3700:
3696:
3692:
3688:
3684:
3680:
3676:
3672:
3665:
3662:
3657:
3651:
3647:
3640:
3638:
3634:
3628:
3622:
3621:
3616:
3615:"Benzanilide"
3609:
3606:
3602:
3596:
3592:
3591:
3586:
3579:
3576:
3572:
3570:0-534-16218-5
3566:
3562:
3558:
3552:
3549:
3544:
3538:
3535:
3532:, p. 48.
3531:
3530:Chisholm 1911
3526:
3524:
3522:
3518:
3508:
3502:
3498:
3494:
3490:
3486:
3479:
3476:
3471:
3467:
3463:
3459:
3455:
3451:
3447:
3443:
3439:
3435:
3427:
3424:
3414:
3408:
3405:
3400:
3396:
3392:
3390:9780073047874
3386:
3382:
3375:
3372:
3368:
3362:
3359:
3354:
3348:
3340:
3336:
3332:
3326:
3322:
3315:
3313:
3309:
3304:
3302:9780470771662
3298:
3294:
3290:
3286:
3279:
3276:
3272:
3268:
3262:
3259:
3254:
3250:
3245:
3240:
3236:
3232:
3228:
3224:
3220:
3213:
3210:
3205:
3201:
3197:
3193:
3189:
3185:
3181:
3177:
3173:
3169:
3165:
3158:
3155:
3150:
3146:
3142:
3136:
3132:
3128:
3124:
3123:
3115:
3113:
3111:
3109:
3107:
3105:
3101:
3090:
3086:
3080:
3077:
3065:
3061:
3055:
3052:
3047:
3043:
3039:
3033:
3031:
3029:
3025:
3020:
3018:9781319079451
3014:
3010:
3003:
3000:
2995:
2991:
2984:
2982:
2980:
2978:
2976:
2972:
2968:
2952:
2946:
2942:
2938:
2934:
2931:. Cambridge:
2930:
2924:
2921:
2915:
2913:
2910:
2906:
2902:
2898:
2894:
2890:
2882:
2880:
2877:
2875:
2871:
2867:
2863:
2855:
2853:
2851:
2847:
2843:
2839:
2835:
2831:
2823:
2821:
2819:
2815:
2811:
2807:
2803:
2799:
2795:
2791:
2790:Howard Florey
2787:
2783:
2782:miracle drugs
2779:
2775:
2771:
2767:
2763:
2762:sulfanilamide
2759:
2758:
2753:
2749:
2745:
2741:
2740:antibacterial
2737:
2733:
2728:
2726:
2722:
2718:
2714:
2710:
2706:
2702:
2698:
2694:
2693:
2688:
2687:
2682:
2678:
2674:
2670:
2666:
2662:
2658:
2650:
2647:
2642:
2640:
2636:
2632:
2628:
2624:
2621:, originally
2620:
2616:
2612:
2608:
2604:
2600:
2596:
2592:
2588:
2584:
2580:
2572:
2570:
2568:
2564:
2560:
2556:
2552:
2548:
2544:
2543:
2538:
2534:
2530:
2526:
2522:
2518:
2514:
2510:
2506:
2502:
2498:
2494:
2486:
2484:
2477:
2472:
2468:
2466:
2462:
2458:
2454:
2450:
2449:diphenylamine
2446:
2442:
2438:
2428:
2423:
2419:
2418:
2417:
2415:
2411:
2403:
2401:
2399:
2395:
2390:
2388:
2384:
2380:
2376:
2371:
2369:
2365:
2361:
2353:
2351:
2349:
2345:
2341:
2337:
2333:
2330:
2326:
2325:
2320:
2316:
2313:to produce a
2312:
2308:
2299:
2295:
2287:
2279:
2271:
2267:
2263:
2259:
2252:Diazotization
2251:
2249:
2224:
2208:
2189:
2185:
2177:
2175:
2173:
2169:
2165:
2161:
2116:
2115:
2114:
2112:
2110:
2106:
2101:
2099:
2094:
2090:
2086:
2079:
2076:
2074:
2072:
2053:
2049:
2045:
2041:
2035:
2027:
2025:
2023:
2014:
2010:
2006:
1984:
1980:
1976:
1972:
1964:
1959:
1957:
1955:
1905:
1904:
1903:
1901:
1896:
1894:
1885:
1881:
1879:
1875:
1871:
1867:
1866:bromine water
1862:
1840:
1836:
1835:sulfuric acid
1832:
1828:
1824:
1816:
1811:
1807:
1803:
1801:
1797:
1793:
1792:diphenylamine
1789:
1788:4-aminophenol
1785:
1781:
1777:
1773:
1769:
1765:
1761:
1760:aniline black
1757:
1753:
1749:
1745:
1741:
1737:
1729:
1724:
1717:
1715:
1709:
1707:
1705:
1701:
1700:chlorobenzene
1697:
1693:
1692:nitroanilines
1689:
1685:
1681:
1677:
1673:
1665:
1663:
1661:
1657:
1653:
1649:
1645:
1640:
1638:
1634:
1629:
1627:
1623:
1619:
1615:
1614:Nikolay Zinin
1607:
1603:
1602:
1601:
1599:
1595:
1587:
1585:
1583:
1579:
1574:
1570:
1568:
1563:
1559:
1555:
1551:
1543:
1541:
1539:
1536:vs 1.44 Å in
1535:
1531:
1522:
1516:
1515:chemical bond
1512:
1508:
1504:
1496:
1491:
1487:
1483:
1476:
1474:
1472:
1471:coal tar dyes
1468:
1463:
1461:
1457:
1454:= 4.6) and a
1450:
1446:
1441:
1439:
1435:
1431:
1426:
1424:
1420:
1416:
1412:
1411:fine chemical
1408:
1404:
1393:
1378:
1360:
1356:
1352:
1348:
1344:
1340:
1328:
1321:
1316:
1299:
1293:
1290:
1286:
1281:
1278:
1274:
1270:
1267:
1262:
1261:
1258:
1254:
1251:
1248:
1243:
1242:
1237:
1233:
1229:
1225:
1224:
1220:
1217:(Recommended)
1216:
1212:
1211:
1207:
1204:(Permissible)
1203:
1199:
1198:
1194:
1193:
1188:
1182:
1178:
1169:
1168:
1164:
1160:
1151:
1150:
1138:
1134:
1125:
1124:
1120:
1116:
1112:
1110:
1107:
1106:
1102:
1099:
1093:
1092:
1088:
1086:
1083:
1082:
1075:
1068:
1061:
1037:
1034:
1033:
1029:
1028:
906:
903:
899:
898:
860:
857:
853:
852:
849:
846:
843:
839:
838:
834:
829:
824:
819:
814:
810:
807:
803:
802:
798:
796:
791:
787:
782:
781:
777:
776:
771:
766:
763:−3394 kJ/mol
762:
754:
746:
740:
739:
734:
730:
726:
722:
720:
717:
716:
712:
705:
701:
697:
696:
692:
688:
684:
683:
672:
671:
669:
663:
659:
656:
655:
651:
649:
646:
645:
641:
638:
634:
633:
629:
627:
626:Boiling point
624:
623:
619:
617:
616:Melting point
614:
613:
609:
607:
604:
603:
599:
596:
595:
588:
586:
583:
582:
564:
561:
557:
556:
551:
542:
537:
533:
526:
512:
502:
498:
491:
483:
479:
478:DTXSID8020090
475:
474:
472:
462:
458:
457:
453:
451:
448:
447:
440:
435:
431:
428:
424:
423:
421:
419:
416:
415:
408:
407:
405:
403:
400:
399:
392:
387:
383:
380:
376:
375:
373:
367:
363:
362:
355:
351:
350:
348:
346:
343:
342:
338:
335:
331:
330:
323:
322:
320:
318:
313:
312:
308:
304:
301:
299:
297:ECHA InfoCard
294:
293:
286:
282:
281:
279:
277:
274:
273:
266:
262:
261:
259:
257:
254:
253:
246:
242:
241:
239:
237:
234:
233:
226:
222:
221:
219:
217:
214:
213:
209:
206:
202:
201:
194:
190:
189:
187:
184:
183:
176:
172:
169:
165:
164:
162:
158:
153:
152:
145:
140:
136:
133:
129:
128:
126:
123:
119:
118:
113:
100:
92:
88:
80:
76:
71:
60:
56:
50:
46:
45:
41:
33:
29:
22:
4342:
4318:
4254:
4250:
4229:
4225:
4217:
4212:
4185:
4181:
4171:
4163:
4159:
4151:
4146:
4133:
4121:. Retrieved
4117:the original
4110:
4101:
4089:
4084:
4072:
4050:
4047:Medical News
4046:
4041:
4032:(17):137-9,
4029:
4025:
4020:
4012:
4003:
3983:
3979:
3970:
3962:
3958:
3949:
3941:
3937:
3927:
3923:
3915:
3911:
3907:
3903:
3895:
3891:
3885:
3876:
3870:
3856:
3852:
3841:
3837:
3830:
3826:
3817:
3805:
3793:
3789:
3784:
3759:
3755:
3745:
3736:
3725:. Retrieved
3721:the original
3711:
3678:
3674:
3664:
3645:
3626:
3618:
3608:
3589:
3585:March, Jerry
3578:
3560:
3551:
3537:
3510:, retrieved
3488:
3478:
3437:
3433:
3426:
3407:
3380:
3374:
3361:
3320:
3284:
3278:
3261:
3226:
3222:
3212:
3171:
3167:
3157:
3121:
3092:. Retrieved
3088:
3079:
3068:. Retrieved
3063:
3054:
3041:
3008:
3002:
2954:
2928:
2923:
2886:
2878:
2869:
2864:lists it in
2859:
2834:WAC Corporal
2827:
2798:penicillin G
2781:
2778:World War II
2755:
2729:
2692:magic bullet
2690:
2686:chemotherapy
2684:
2681:Paul Ehrlich
2669:side effects
2654:
2629:Aniline and
2622:
2576:
2566:
2562:
2554:
2551:nitrobenzene
2545:). In 1842,
2540:
2536:
2524:
2520:
2504:
2490:
2481:
2434:
2407:
2391:
2385:blue in the
2372:
2357:
2322:
2258:nitrous acid
2255:
2182:Boiled with
2181:
2167:
2163:
2159:
2158:
2108:
2104:
2097:
2084:
2083:
2077:
2037:
2019:
2007:
1968:
1951:
1900:formaldehyde
1897:
1890:
1863:
1820:
1810:Polyanilines
1800:polyanilines
1772:nitrobenzene
1744:Chromic acid
1740:arsenic acid
1733:
1713:
1669:
1641:
1630:
1611:
1594:hydrogenated
1591:
1575:
1571:
1547:
1500:
1470:
1464:
1448:
1442:
1427:
1415:polyurethane
1377:phenyl group
1350:
1346:
1338:
1337:
1277:Nitrobenzene
1191:
1118:
1096:Autoignition
1031:
847:
794:
784:Main hazards
773:
752:
703:
661:
610:1.0297 g/mL
402:RTECS number
115:Identifiers
106:Aminobenzene
102:Other names
4123:20 November
4028:, 1880 May;
3844:(1): 84–90.
2893:tumorigenic
2842:nitric acid
2824:Rocket fuel
2774:sulfa drugs
2663:emerged as
2657:acetanilide
2589:discovered
2585:'s student
2583:von Hofmann
2563:phenylamine
2557:. In 1843,
2527:. In 1840,
2507:. In 1834,
2453:paracetamol
2387:Nissl stain
2080:-Alkylation
2071:acetanilide
2060:−C(=O)−NH−C
1870:precipitate
1780:oxalic acid
1672:derivatives
1578:methylamine
1550:amine group
1456:nucleophile
1392:amino group
1119:Lethal dose
1098:temperature
1085:Flash point
842:Signal word
778:(OHS/OSH):
727:(3.71
597:Appearance
553:Properties
544:c1ccc(cc1)N
303:100.000.491
225:CHEBI:17296
104:Phenylamine
95:Benzenamine
4371:Categories
4291:References
4053:():931-2,
3944:: 283–287.
3727:2007-12-21
3512:2022-02-01
3413:US3136818A
3094:2022-02-15
3070:2016-06-16
2897:DNA damage
2846:hypergolic
2806:René Dubos
2802:Gramicidin
2794:antibiotic
2770:Paul Gelmo
2725:penicillin
2661:phenacetin
2625:(English:
2505:Crystallin
2476:indigo dye
2465:blue jeans
2441:herbicides
2235:−N=C(−NH−C
1878:protection
1796:persulfate
1776:azobenzene
1750:, whereas
1736:azobenzene
1726:Sample of
1676:toluidines
1656:distillate
1588:Production
1343:Portuguese
1341:(from
806:Pictograms
745:combustion
585:Molar mass
434:576R1193YL
427:SIR7XX2F1K
256:ChemSpider
155:3D model (
122:CAS Number
3930:(1): 153.
3717:"Aniline"
3695:2092-7673
3347:cite book
3339:957525299
3253:2073-8994
3196:1463-9084
3060:"Aniline"
3038:"Aniline"
2903:in their
2850:hydrazine
2766:colorfast
2754:degraded
2744:prontosil
2730:In 1932,
2713:salvarsan
2705:arsenical
2665:analgesic
2646:Nigrosene
2595:synthetic
2360:phenazine
2317:known as
2223:guanidine
2192:S=C(−NH−C
2028:Acylation
2022:solvation
1983:anilinium
1979:aliphatic
1764:chloranil
1752:chlorates
1718:Oxidation
1710:Reactions
1680:xylidines
1652:toluidine
1648:safranine
1598:catalysts
1582:formamide
1560:with the
1558:lone pair
1554:sp and sp
1511:π-bonding
1477:Structure
1460:aliphatic
1438:diazonium
1434:oxidation
1357:with the
1016:P403+P233
1000:P333+P313
968:P308+P313
960:P304+P340
956:P302+P352
952:P301+P310
797:labelling
719:Viscosity
539:Nc1ccccc1
450:UN number
409:BW6650000
324:200-539-3
316:EC Number
245:ChEMBL538
108:Benzamine
4377:Anilines
4281:18793663
4204:21941546
4188:: 1–23.
4094:pp 202–3
4077:pp 150–1
3874:synonym
3703:94372490
3587:(2007),
3559:(1992),
3462:23695637
3399:71790138
3223:Symmetry
3204:26852720
3149:11469727
3048:(NIOSH).
2996:(NIOSH).
2866:Group 2A
2750:to be a
2709:syphilis
2689:for his
2673:caffeine
2635:azo dyes
2607:induline
2603:safranin
2591:mauveine
2555:benzidam
2549:reduced
2513:coal tar
2474:Cake of
2412:to give
2410:phosgene
2379:furfural
2332:aromatic
2324:coupling
2270:hydroxyl
2260:to form
2188:thiourea
2089:methanol
2052:anilides
2046:to give
2042:such as
1965:Basicity
1954:4,4'-MDI
1758:), give
1756:vanadium
1662:fusion.
1660:fuchsine
1492:at 252 K
1467:coal tar
1462:amines.
1245:Related
1234:100 ppm
1113:1.3–11%
1032:NFPA 704
768:Hazards
713:1.58364
689:(χ)
276:DrugBank
139:142-04-1
38:Aniline
32:annulene
4315:Aniline
4308::
4272:2614128
3810:308–332
3802:513–524
3764:Bibcode
3470:3273484
3442:Bibcode
3231:Bibcode
3176:Bibcode
2990:"#0033"
2830:Aerobee
2814:topical
2757:in vivo
2752:prodrug
2697:Béchamp
2599:fuchsin
2579:quinine
2567:aniline
2537:aniline
2487:History
2457:Tylenol
2362:in the
2329:primary
2294:halogen
2288:group (
2278:cyanide
2034:Anilide
1831:enamine
1823:phenols
1748:quinone
1696:toluene
1423:ignites
1359:formula
1339:Aniline
1320:what is
1318: (
658:Acidity
606:Density
579:
437: (
389: (
366:PubChem
285:DB06728
210:605631
142: (
132:62-53-3
83:Aniline
62:Aniline
28:alanine
4302:
4279:
4269:
4202:
3877:I anil
3804:; and
3701:
3693:
3652:
3597:
3567:
3503:
3468:
3460:
3419:
3397:
3387:
3337:
3327:
3299:
3251:
3202:
3194:
3147:
3137:
3015:
2947:
2889:spleen
2796:drug,
2701:atoxyl
2605:, and
2525:cyanol
2521:kyanol
2501:indigo
2461:indigo
2437:rubber
2311:phenol
2296:) via
2286:halide
2284:), or
2219:−N=C=S
2134:OH → C
2130:+ 2 CH
2095:gives
2048:amides
1923:O → CH
1786:gives
1633:phenol
1315:verify
1312:
848:Danger
590:93.129
532:SMILES
354:C00292
236:ChEMBL
193:B00082
185:3DMet
73:Names
4055:p 932
4034:p 138
3798:65–77
3699:S2CID
3466:S2CID
2916:Notes
2760:into
2732:Bayer
2627:Baden
2344:water
1821:Like
1702:into
1421:. It
1345:
1192:NIOSH
729:mPa·s
723:3.71
497:InChI
454:1547
339:2796
216:ChEBI
157:JSmol
30:, or
4382:Dyes
4277:PMID
4200:PMID
4186:2011
4125:2020
4112:Time
3691:ISSN
3650:ISBN
3595:ISBN
3565:ISBN
3501:ISBN
3458:PMID
3395:OCLC
3385:ISBN
3353:link
3335:OCLC
3325:ISBN
3297:ISBN
3249:ISSN
3200:PMID
3192:ISSN
3145:OCLC
3135:ISBN
3013:ISBN
2945:ISBN
2862:IARC
2832:and
2659:and
2631:Soda
2619:BASF
2447:and
2404:Uses
2346:and
2309:and
2303:NaNO
2150:+ 2H
2142:N(CH
2102:and
1971:base
1919:+ CH
1562:aryl
1548:The
1523:)−NH
1521:aryl
1445:base
1419:fish
1351:-ine
1347:anil
1228:IDLH
1221:Ca
1024:P501
1020:P405
1012:P391
1008:P363
1004:P361
996:P330
992:P322
988:P321
984:P314
980:P312
976:P311
972:P310
948:P281
944:P280
940:P273
936:P272
932:P271
928:P270
924:P264
920:P261
916:P260
912:P202
908:P201
894:H400
890:H372
886:H351
882:H341
878:H331
874:H318
870:H317
866:H311
862:H301
418:UNII
386:8870
379:6115
345:KEGG
265:5889
4317:",
4267:PMC
4259:doi
4255:233
4190:doi
4141:SSM
3772:doi
3683:doi
3493:doi
3450:doi
3289:doi
3267:doi
3239:doi
3184:doi
3127:doi
2963:-NH
2937:doi
2905:DNA
2808:at
2800:. (
2699:'s
2565:or
2523:or
2499:of
2495:by
2383:RNA
2340:HCl
2315:dye
2282:−CN
2276:),
2274:−OH
2248:).
2190:) (
2069:is
2005:).
1995:−NH
1943:+ H
1907:2 C
1872:of
1517:of
1396:−NH
1215:REL
1202:PEL
795:GHS
756:298
466:EPA
439:HCl
391:HCl
369:CID
144:HCl
4373::
4341:,
4275:.
4265:.
4253:.
4249:.
4237:^
4198:.
4184:.
4180:.
4109:.
4062:^
4051:77
4011:.
3998:.)
3984:42
3963:47
3961:,
3942:44
3940:,
3928:27
3926:,
3916:10
3914:,
3908:27
3906:,
3902:,
3896:10
3894:,
3857:20
3855:,
3851:,
3842:36
3840:,
3829:,
3808::
3806:32
3796::
3794:31
3792:,
3770:.
3758:.
3697:.
3689:.
3679:16
3677:.
3673:.
3636:^
3624:;
3617:.
3520:^
3499:,
3487:,
3464:.
3456:.
3448:.
3436:.
3393:.
3349:}}
3345:{{
3333:.
3311:^
3295:.
3247:.
3237:.
3225:.
3221:.
3198:.
3190:.
3182:.
3172:18
3170:.
3166:.
3143:.
3133:.
3103:^
3087:.
3062:.
3044:.
3040:.
3027:^
2992:.
2974:^
2953:.
2943:.
2876:.
2852:.
2727:.
2711:–
2679:,
2641:.
2601:,
2581:,
2569:.
2467:.
2396:.
2389:.
2370:.
2350:.
2290:−X
2126:NH
2113::
2056:CH
1973:.
1935:NH
1927:(C
1915:NH
1861:.
1855:SO
1847:NC
1841:,
1766:.
1690:,
1686:,
1682:,
1678:,
1639:.
1540:.
1519:C(
1473:.
1452:aH
1447:(p
1381:−C
1370:NH
1173:Lo
1171:LC
1155:50
1153:LC
1129:Lo
1127:LD
1022:,
1018:,
1014:,
1010:,
1006:,
1002:,
998:,
994:,
990:,
986:,
982:,
978:,
974:,
970:,
966:,
962:,
958:,
954:,
950:,
946:,
942:,
938:,
934:,
930:,
926:,
922:,
918:,
914:,
910:,
892:,
888:,
884:,
880:,
876:,
872:,
868:,
864:,
799::
748:(Δ
725:cP
677:O)
667:)
660:(p
574:NH
4283:.
4261::
4206:.
4192::
4127:.
4096:.
4079:.
4057:.
4036:.
4030:2
3992:3
3990:O
3988:2
3863:.
3831:7
3778:.
3774::
3766::
3760:8
3730:.
3705:.
3685::
3658:.
3631:.
3545:.
3495::
3472:.
3452::
3444::
3438:5
3401:.
3369:.
3355:)
3341:.
3305:.
3291::
3273:.
3269::
3255:.
3241::
3233::
3227:2
3206:.
3186::
3178::
3151:.
3129::
3097:.
3073:.
3021:.
2965:2
2961:5
2959:H
2957:6
2939::
2868:(
2305:2
2280:(
2272:(
2245:2
2243:)
2241:5
2239:H
2237:6
2233:5
2231:H
2229:6
2227:C
2225:(
2217:5
2215:H
2213:6
2211:C
2209:(
2202:2
2200:)
2198:5
2196:H
2194:6
2168:N
2166:,
2164:N
2160:N
2154:O
2152:2
2148:2
2146:)
2144:3
2140:5
2138:H
2136:6
2132:3
2128:2
2124:5
2122:H
2120:6
2118:C
2109:N
2107:,
2105:N
2098:N
2085:N
2078:N
2066:5
2064:H
2062:6
2058:3
2001:3
1998:+
1993:5
1991:H
1989:6
1987:C
1947:O
1945:2
1941:2
1939:)
1937:2
1933:4
1931:H
1929:6
1925:2
1921:2
1917:2
1913:5
1911:H
1909:6
1859:H
1857:3
1853:4
1851:H
1849:6
1845:2
1843:H
1525:2
1503:Å
1449:K
1398:2
1394:(
1387:5
1385:H
1383:6
1379:(
1372:2
1368:5
1366:H
1364:6
1362:C
1310:N
1179:)
1175:(
1161:)
1157:(
1135:)
1131:(
1073:0
1066:2
1059:3
758:)
753:H
750:c
709:)
707:D
704:n
702:(
675:2
665:a
662:K
576:2
572:5
570:H
568:6
566:C
468:)
464:(
441:)
393:)
159:)
146:)
34:.
23:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.