231:
710:
619:
685:
696:
172:
716:
Phosphite esters are the least reactive class of reagents used in this reaction. They react to produce phosphonates. They require the most heating for the reaction to occur (120 °C - 160 °C is common). This high temperature allows for fractional distillation to be employed in the removal of
611:
is a competing reaction pathway for α-bromo- and α-chloroketones. Under the reaction conditions a mixture of the Perkow product and the normal
Arbuzov product occur, usually favoring the Perkow product by a significant amount. Using higher temperatures during the reaction can lead to favoring of the
680:
of the reaction. The reaction proceeds smoothly when the R group is aliphatic. When all of A, B and R are aryl groups, a stable phosphonium salt is formed and the reaction proceeds no further under normal conditions. Heating to higher temperatures in the presence of alcohols has been known to give
743:
Phosphinites are the most reactive class of reagents used in this reaction. They react to produce phosphine oxides. They often require very little heating (45 °C) for the reaction to occur and have been known to self-isomerize without the presence of alkyl halides.
270:). These intermediates are occasionally stable enough to be isolated, such as for triaryl phosphites which do not react to form the phosphonate without thermal cleavage of the intermediate (200 °C), or cleavage by alcohols or bases. The displaced
691:
Phosphite salts (Ex: R = Na) can also undergo the reaction with precipitation of the corresponding Na-halide salt. Amidophosphites and silyloxyphosphites have been used before to yield amidophosphonates and phosphinic acids.
515:
675:
groups are known to slow down the rate of the reaction, with electron donating groups increasing the rate of the reaction. This is consistent with initial attack of the phosphorus reagent on the alkyl halide as the
717:
the alkyl halide produced, though excess of the starting alkyl halide can also be used. Solvents are often not used for this reaction, though there is precedent for the improvement of selectivity with its usage.
314:
group initially dissociates from the phosphonium salt followed by attack of the anion. Phosphite esters with tertiary alkyl halide groups can undergo the reaction, which would be unexpected if only an S
4247:
681:
the isomerization product. Cyclic phosphites generally react to eject the non-cyclic OR group, though for some 5-member rings additional heating is required to afford the final cyclic product.
555:
405:
of the anion. There exists many instances of the intermediate phosphonium salts being sufficiently stable that they can be isolated when the anion is weakly nucleophilic, such as with
669:
3363:
3308:
4076:
724:
of the ester to an acid is a common side reaction. The poor availability of substituted phosphonites limits the usage of this class of reagent in the
Arbuzov reaction.
3418:
980:
Jacobsen, H. I.; Griffin, M. J.; Preis, S.; Jensen, E. V. (1957). "Phosphonic Acids. IV. Preparation and
Reactions of β-Ketophosphonate and Enol Phosphate Esters".
3568:
2202:
230:
358:
groups. For example, the triaryl phosphites mentioned above generally do not react because they form stable phosphonium salts. Since aryl groups do not undergo S
4297:
821:
Arbuzov, A. E. (1906). "On the structure of phosphonic acid and its derivates: Isometization and transition of bonds from trivalent to pentavalent phosphorus".
4071:
3173:
1897:
576:
and substituted derivatives have been known to undergo the reaction under photolytic conditions. Secondary alkyl halides often do not react well, producing
2943:
1094:
3743:
1687:
3838:
1942:
3818:
3313:
2480:
2361:
1917:
3908:
3663:
720:
Phosphonites are generally more reactive than phosphite esters. They react to produce phosphinates. Heating is also required for the reaction, but
1130:
2495:
612:
Arbuzov product. The reaction of α-iodoketones give only the
Arbuzov product. Other methods of producing β-ketophosphonates have been developed.
342:
2 reaction is unlikely to be the mechanism for the synthesis of the neopentyl halides in this reaction. Substrates that cannot react through an S
4141:
3598:
4091:
194:. Several reviews have been published. The reaction also occurs for coordinated phosphite ligands, as illustrated by the demethylation of {(C
3703:
3683:
3643:
2450:
4237:
4162:
4046:
2658:
2062:
1393:
4232:
4061:
3718:
3573:
3203:
83:
3048:
2285:
3408:
2898:
2573:
120:
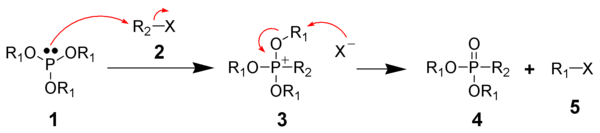
phosphorus species and another alkyl halide. The picture below shows the most common types of substrates undergoing the
Arbuzov reaction;
4312:
4096:
3118:
437:
As a general guideline, the reactivity of the organic halide component can be listed as follows: (from most reactive to least reactive)
3673:
560:
In general, tertiary alkyl halides, aryl halides and vinyl halides do not react. There are notable exceptions to this trend, including
4307:
4021:
3883:
3638:
4197:
3668:
3583:
3553:
3533:
3398:
3393:
2768:
2693:
2336:
2290:
2157:
1418:
4136:
4302:
4262:
4212:
3688:
3438:
3368:
1857:
3898:
3503:
2396:
2117:
3888:
1428:
1066:
1055:
1044:
4056:
3813:
3763:
2553:
2485:
2376:
1952:
1707:
1632:
1413:
615:
The reaction of trivalent phosphorus compounds with alkyl fluorides is abnormal. One example of this reactivity is shown below.
443:
294:
groups experience inversion of configuration at the carbon center attacked by the halide anion. This is what is expected of an S
4396:
3768:
3578:
3053:
2963:
1087:
4342:
4126:
4066:
3468:
3443:
3353:
2933:
2813:
1847:
1343:
4227:
3713:
3508:
1777:
4332:
3918:
3428:
2938:
2883:
2728:
2688:
2520:
2275:
1992:
1842:
763:
4292:
3853:
3808:
3298:
3153:
4327:
4242:
4101:
4016:
3913:
2988:
2643:
2311:
1722:
1283:
4217:
4192:
4177:
3873:
3738:
3693:
3458:
3003:
2853:
2067:
1747:
1692:
4222:
4167:
3698:
3113:
2828:
2823:
2316:
2132:
2122:
1837:
1697:
1647:
1642:
1617:
1523:
4391:
4277:
3878:
3798:
3413:
3378:
3223:
2648:
2608:
2505:
2280:
2032:
1977:
1577:
1288:
1278:
1253:
4252:
3953:
3758:
3193:
2758:
2733:
2673:
2265:
1972:
1622:
1807:
702:
An
Arbuzov type rearrangement can also occur where the O from an OR group acts as the leaving group in the initial S
3543:
3078:
2530:
1752:
1717:
1313:
1248:
1080:
600:
being inert to the reaction conditions. When a halide atom is found in the ester chain off of the phosphorus atom,
76:
4111:
3733:
2793:
2718:
2242:
2077:
1762:
1538:
1498:
1243:
4352:
4257:
3991:
3963:
3933:
3848:
3778:
3708:
3628:
3528:
3488:
3183:
2803:
2102:
2097:
1559:
1423:
526:
331:
4317:
4187:
4051:
3893:
3753:
3273:
2247:
1797:
1757:
1508:
4207:
3803:
3773:
3648:
3603:
3433:
3343:
3158:
3148:
2978:
2535:
2475:
2440:
2227:
2187:
1962:
1832:
1348:
1338:
1268:
3783:
2763:
1787:
1333:
1213:
366:
2 type mechanisms, triaryl phosphites lack a low energy pathway for decomposition of the phosphonium salt. An
3996:
4401:
4287:
4146:
3938:
3863:
3843:
3563:
3513:
3373:
3338:
3278:
3208:
2510:
2490:
2222:
2142:
2037:
1997:
1967:
1902:
1772:
1682:
1672:
1548:
1258:
740:
functional groups cannot be used with phosphonites in the reaction as they all react with the phosphonite.
4026:
3748:
3498:
3478:
3453:
3403:
3318:
3293:
3248:
3218:
3198:
3168:
3133:
3088:
3063:
3038:
2923:
2848:
2628:
2321:
2257:
2057:
1782:
1702:
1388:
1363:
1140:
1135:
4362:
3108:
1732:
68:
3948:
3903:
3618:
3588:
3558:
3493:
3473:
3388:
3383:
3348:
3303:
3288:
3283:
3263:
3253:
3188:
3178:
3058:
2578:
2381:
1957:
1912:
1742:
1478:
1198:
1160:
853:
677:
569:
367:
1408:
1403:
4001:
618:
4131:
4081:
4031:
4011:
3858:
3833:
3548:
3538:
3423:
3238:
3233:
3163:
2948:
2748:
2708:
2638:
2603:
2558:
2525:
2391:
2366:
2346:
2167:
2127:
2087:
2052:
1982:
1737:
1607:
1582:
1120:
672:
593:
394:
709:
4347:
4337:
4322:
3968:
3943:
3928:
3923:
3653:
3608:
3593:
3483:
3463:
3358:
3243:
3228:
3073:
3018:
3008:
2998:
2973:
2738:
2613:
2588:
2500:
2356:
2341:
2326:
2182:
2147:
2092:
1862:
1712:
1657:
1528:
1443:
1303:
1228:
644:
1373:
957:
Gerrard, W.; Green, W. J. (1951). "568. Mechanism of the formation of dialkyl alkylphosphonates".
684:
4086:
4036:
4006:
3868:
3658:
3448:
3333:
3268:
3258:
3023:
2953:
2918:
2913:
2893:
2888:
2833:
2743:
2593:
2455:
2445:
2351:
2137:
2082:
2012:
1932:
1827:
1727:
1662:
1587:
1433:
1298:
1233:
904:
561:
1218:
389:
Stereochemical experiments on cyclic phosphites have revealed the presence of both pentavalent
3823:
3143:
3028:
2993:
2958:
2903:
2858:
2818:
2773:
2753:
2703:
2698:
2668:
2653:
2563:
2470:
2406:
2371:
2197:
2072:
1947:
1872:
1852:
1767:
1602:
1597:
1543:
1453:
1358:
1318:
1273:
1155:
1150:
1115:
1014:
982:
406:
183:
105:
52:
42:
580:
as side-products. Allyl and propargyl halides are also reactive, but can proceed through an S
4357:
4202:
4172:
4116:
4041:
3973:
3728:
3678:
3523:
3328:
3103:
3098:
3043:
3033:
2808:
2618:
2598:
2568:
2465:
2401:
2386:
2217:
2172:
2162:
2152:
2047:
2027:
2022:
2007:
2002:
1882:
1877:
1817:
1802:
1792:
1637:
1627:
1493:
1483:
1473:
1383:
1378:
1353:
1293:
1145:
1104:
990:
962:
939:
894:
886:
803:
753:
695:
263:
179:
38:
4267:
3958:
3793:
3788:
3083:
3068:
3013:
2968:
2928:
2878:
2843:
2838:
2783:
2778:
2713:
2663:
2583:
2411:
2295:
2270:
2232:
2207:
2192:
2177:
2112:
1987:
1937:
1927:
1907:
1867:
1677:
1667:
1652:
1448:
1368:
1193:
1188:
866:
790:"Ueber das Verhalten der Jodalkyle gegen die sogen. Phosphorigsäureester oder O-Phosphine"
758:
733:
608:
402:
191:
186:
soon thereafter. This reaction is widely used for the synthesis of various phosphonates,
161:
121:
1238:
1208:
604:
to the corresponding
Arbuzov product has been known without addition of an alkyl halide.
4272:
4182:
4121:
3213:
3123:
3093:
2868:
2723:
2460:
2237:
2107:
1922:
1892:
1592:
1488:
1263:
1125:
768:
398:
171:
4385:
4282:
3983:
3828:
3723:
3518:
2908:
2873:
2863:
2798:
2788:
2678:
2515:
2331:
2042:
2017:
1887:
1533:
1518:
1503:
1398:
1328:
1308:
1223:
725:
601:
255:
908:
3323:
2683:
2435:
2212:
1812:
1612:
1463:
1458:
1323:
1178:
641:
The general form of the trivalent phosphorus reagent can be considered as follows:
303:
247:
239:
219:
113:
1822:
1468:
1438:
1203:
1009:
929:
Bhattacharya, A. K.; Thyagarajan, G. (1981). "Michaelis–Arbuzov rearrangement".
589:
573:
390:
379:
351:
322:
1 type mechanism comes from the use of the
Arbuzov reaction in the synthesis of
299:
187:
153:
145:
137:
129:
117:
17:
4106:
3633:
2983:
1072:
931:
807:
597:
706:
2 attack of the phosphorus. This is only known to occur when A and B are Cl.
899:
881:
721:
383:
323:
109:
1513:
1183:
966:
794:
410:
994:
943:
1173:
326:
halides, a class of compounds that are notoriously unreactive towards S
401:. The decomposition of these intermediates is driven primarily by the
596:
interestingly enough, only undergoes the reaction a single time with
577:
565:
282:
carbons, displacing the oxygen atom to give the desired phosphonate (
271:
789:
1065:, Coll. Vol. 10, p. 289 (2004); Vol. 78, p. 169 (2002). (
1054:, Coll. Vol. 8, p. 451 (1993); Vol. 65, p. 119 (1987). (
737:
729:
1043:, Coll. Vol. 4, p. 325 (1963); Vol. 31, p. 33 (1951). (
355:
1557:
1076:
671:
with A and B generally being alkyl, alkoxy or aryloxy groups.
397:
being involved in the dealkylation step of the reaction using
708:
694:
683:
617:
229:
170:
510:{\displaystyle {\ce {RCOX>RCH2X>RR'CHX\gg RR'R''CX}}}
290:). This has been supported by the observation that chiral R
464:
334:, the inert nature of the neopentyl halides towards the S
588:
2` mechanism. Reaction with primary alkyl halides and
568:
halides. Some activated aryl halides, often involving
647:
529:
446:
318:
2 mechanism was operating. Further support for this S
238:
The
Michaelis–Arbuzov reaction is initiated with the
4248:
Erlenmeyer–Plöchl azlactone and amino-acid synthesis
4155:
3982:
3617:
3132:
2627:
2544:
2424:
2304:
2256:
1566:
663:
549:
509:
3309:Divinylcyclopropane-cycloheptadiene rearrangement
1008:Nagata, W.; Wakabayashi, T.; Hayase, Y. (1988).
350:1 pathway generally do not react, which include
1050:Davidsen, S. K.; Phllips, G. W.; Martin, S. F.
234:The mechanism of the Michaelis–Arbuzov reaction
3569:Thermal rearrangement of aromatic hydrocarbons
2203:Thermal rearrangement of aromatic hydrocarbons
302:based mechanism of dealkylation similar to an
4298:Lectka enantioselective beta-lactam synthesis
1088:
1010:"Diethyl 2-(cyclohexylamino)vinylphosphonate"
393:and tetravalent phosphonium intermediates in
8:
4077:Inverse electron-demand Diels–Alder reaction
1898:Heterogeneous metal catalyzed cross-coupling
550:{\displaystyle {\ce {RI > RBr > RCl}}}
3419:Lobry de Bruyn–Van Ekenstein transformation
3979:
2253:
1554:
1095:
1081:
1073:
882:"Michaelis–Arbusow- und Perkow-Reaktionen"
26:
3909:Petrenko-Kritschenko piperidone synthesis
3364:Fritsch–Buttenberg–Wiechell rearrangement
898:
652:
648:
646:
572:have been known to undergo the reaction.
530:
528:
463:
458:
447:
445:
4072:Intramolecular Diels–Alder cycloaddition
1061:Enders, D.; von Berg, S.; Jandeleit, B.
780:
330:2 reactions. Based on the principle of
298:2 reaction. Evidence also exists for a
274:anion then usually reacts via another S
4092:Metal-centered cycloaddition reactions
3744:Debus–Radziszewski imidazole synthesis
1688:Bodroux–Chichibabin aldehyde synthesis
862:
851:
4238:Diazoalkane 1,3-dipolar cycloaddition
4142:Vinylcyclopropane (5+2) cycloaddition
4047:Diazoalkane 1,3-dipolar cycloaddition
3819:Hurd–Mori 1,2,3-thiadiazole synthesis
3314:Dowd–Beckwith ring-expansion reaction
2481:Hurd–Mori 1,2,3-thiadiazole synthesis
1394:LFER solvent coefficients (data page)
7:
3049:Sharpless asymmetric dihydroxylation
2286:Methoxymethylenetriphenylphosphorane
924:
922:
920:
918:
3174:Allen–Millar–Trippett rearrangement
4313:Nitrone-olefin (3+2) cycloaddition
4308:Niementowski quinazoline synthesis
4097:Nitrone-olefin (3+2) cycloaddition
4022:Azide-alkyne Huisgen cycloaddition
3884:Niementowski quinazoline synthesis
3639:Azide-alkyne Huisgen cycloaddition
2944:Meerwein–Ponndorf–Verley reduction
2496:Leimgruber–Batcho indole synthesis
788:Michaelis, A.; Kaehne, R. (1898).
25:
4137:Trimethylenemethane cycloaddition
3839:Johnson–Corey–Chaykovsky reaction
3704:Cadogan–Sundberg indole synthesis
3684:Bohlmann–Rahtz pyridine synthesis
3644:Baeyer–Emmerling indole synthesis
2451:Cadogan–Sundberg indole synthesis
1943:Johnson–Corey–Chaykovsky reaction
182:in 1898, and greatly explored by
4233:Cook–Heilbron thiazole synthesis
4062:Hexadehydro Diels–Alder reaction
3889:Niementowski quinoline synthesis
3719:Cook–Heilbron thiazole synthesis
3664:Bischler–Möhlau indole synthesis
3574:Tiffeneau–Demjanov rearrangement
3204:Baker–Venkataraman rearrangement
2362:Horner–Wadsworth–Emmons reaction
2033:Mizoroki-Heck vs. Reductive Heck
1918:Horner–Wadsworth–Emmons reaction
1429:Neighbouring group participation
1039:Ford-Moore, A. H.; Perry, B. J.
3769:Fiesselmann thiophene synthesis
3599:Westphalen–Lettré rearrangement
3579:Vinylcyclopropane rearrangement
3409:Kornblum–DeLaMare rearrangement
3054:Epoxidation of allylic alcohols
2964:Noyori asymmetric hydrogenation
2899:Kornblum–DeLaMare rearrangement
2574:Gallagher–Hollander degradation
178:The reaction was discovered by
4228:Chichibabin pyridine synthesis
3714:Chichibabin pyridine synthesis
3674:Blum–Ittah aziridine synthesis
3509:Ring expansion and contraction
1778:Cross dehydrogenative coupling
664:{\displaystyle {\ce {ABP-OR}}}
378:) has also been implicated in
338:2 reaction indicates that an S
1:
4198:Bischler–Napieralski reaction
4156:Heterocycle forming reactions
3809:Hemetsberger indole synthesis
3669:Bischler–Napieralski reaction
3584:Wagner–Meerwein rearrangement
3554:Sommelet–Hauser rearrangement
3534:Seyferth–Gilbert homologation
3399:Ireland–Claisen rearrangement
3394:Hofmann–Martius rearrangement
3154:2,3-sigmatropic rearrangement
2769:Corey–Winter olefin synthesis
2694:Barton–McCombie deoxygenation
2337:Corey–Winter olefin synthesis
2291:Seyferth–Gilbert homologation
2158:Seyferth–Gilbert homologation
4303:Lehmstedt–Tanasescu reaction
4263:Gabriel–Colman rearrangement
4218:Bucherer carbazole synthesis
4213:Borsche–Drechsel cyclization
4193:Bernthsen acridine synthesis
4178:Bamberger triazine synthesis
4163:Algar–Flynn–Oyamada reaction
3874:Nazarov cyclization reaction
3739:De Kimpe aziridine synthesis
3694:Bucherer carbazole synthesis
3689:Borsche–Drechsel cyclization
3459:Nazarov cyclization reaction
3439:Meyer–Schuster rearrangement
3369:Gabriel–Colman rearrangement
3119:Wolffenstein–Böters reaction
3004:Reduction of nitro compounds
2854:Grundmann aldehyde synthesis
2659:Algar–Flynn–Oyamada reaction
2068:Olefin conversion technology
2063:Nozaki–Hiyama–Kishi reaction
1858:Gabriel–Colman rearrangement
1748:Claisen-Schmidt condensation
1693:Bouveault aldehyde synthesis
592:generally proceed smoothly.
286:) and another alkyl halide (
4278:Hantzsch pyridine synthesis
4057:Enone–alkene cycloadditions
3879:Nenitzescu indole synthesis
3799:Hantzsch pyridine synthesis
3764:Ferrario–Ackermann reaction
3414:Kowalski ester homologation
3379:Halogen dance rearrangement
3224:Benzilic acid rearrangement
2649:Akabori amino-acid reaction
2609:Von Braun amide degradation
2554:Barbier–Wieland degradation
2506:Nenitzescu indole synthesis
2486:Kharasch–Sosnovsky reaction
2377:Julia–Kocienski olefination
2281:Kowalski ester homologation
1978:Kowalski ester homologation
1953:Julia–Kocienski olefination
1708:Cadiot–Chodkiewicz coupling
1633:Aza-Baylis–Hillman reaction
1578:Acetoacetic ester synthesis
1289:Dynamic binding (chemistry)
1279:Conrotatory and disrotatory
1254:Charge remote fragmentation
30:Michaelis–Arbuzov reaction
4418:
4343:Robinson–Gabriel synthesis
4293:Kröhnke pyridine synthesis
4127:Retro-Diels–Alder reaction
4067:Imine Diels–Alder reaction
3854:Kröhnke pyridine synthesis
3469:Newman–Kwart rearrangement
3444:Mislow–Evans rearrangement
3354:Fischer–Hepp rearrangement
3299:Di-π-methane rearrangement
3079:Stephen aldehyde synthesis
2814:Eschweiler–Clarke reaction
2531:Williamson ether synthesis
1848:Fujiwara–Moritani reaction
1753:Combes quinoline synthesis
1718:Carbonyl olefin metathesis
1419:More O'Ferrall–Jencks plot
1344:Grunwald–Winstein equation
1314:Electron-withdrawing group
1249:Catalytic resonance theory
1024:, vol. 6, p. 448
278:2 reaction on one of the R
98:Michaelis–Arbuzov reaction
4353:Urech hydantoin synthesis
4333:Pomeranz–Fritsch reaction
4258:Fischer oxazole synthesis
3992:1,3-Dipolar cycloaddition
3964:Urech hydantoin synthesis
3934:Reissert indole synthesis
3919:Pomeranz–Fritsch reaction
3849:Knorr quinoline synthesis
3779:Fischer oxazole synthesis
3709:Camps quinoline synthesis
3629:1,3-Dipolar cycloaddition
3529:Semipinacol rearrangement
3504:Ramberg–Bäcklund reaction
3489:Piancatelli rearrangement
3429:McFadyen–Stevens reaction
3184:Alpha-ketol rearrangement
2939:McFadyen–Stevens reaction
2884:Kiliani–Fischer synthesis
2804:Elbs persulfate oxidation
2729:Bouveault–Blanc reduction
2689:Baeyer–Villiger oxidation
2521:Schotten–Baumann reaction
2397:Ramberg–Bäcklund reaction
2276:Kiliani–Fischer synthesis
2118:Ramberg–Bäcklund reaction
2103:Pinacol coupling reaction
2098:Piancatelli rearrangement
1993:Liebeskind–Srogl coupling
1843:Fujimoto–Belleau reaction
1560:List of organic reactions
1424:Negative hyperconjugation
1169:
1111:
808:10.1002/cber.189803101190
764:Michaelis–Becker reaction
332:microscopic reversibility
112:phosphorus ester with an
90:
64:Organic Chemistry Portal
58:
29:
4328:Pictet–Spengler reaction
4243:Einhorn–Brunner reaction
4208:Boger pyridine synthesis
4102:Oxo-Diels–Alder reaction
4017:Aza-Diels–Alder reaction
3914:Pictet–Spengler reaction
3814:Hofmann–Löffler reaction
3804:Hegedus indole synthesis
3774:Fischer indole synthesis
3649:Bartoli indole synthesis
3604:Willgerodt rearrangement
3434:McLafferty rearrangement
3344:Ferrier carbocyclization
3159:2,3-Wittig rearrangement
3149:1,2-Wittig rearrangement
2989:Parikh–Doering oxidation
2979:Oxygen rebound mechanism
2644:Adkins–Peterson reaction
2536:Yamaguchi esterification
2476:Hegedus indole synthesis
2441:Bartoli indole synthesis
2312:Bamford–Stevens reaction
2228:Weinreb ketone synthesis
2188:Stork enamine alkylation
1963:Knoevenagel condensation
1833:Ferrier carbocyclization
1723:Castro–Stephens coupling
1349:Hammett acidity function
1339:Free-energy relationship
1284:Curtin–Hammett principle
1269:Conformational isomerism
823:J. Russ. Phys. Chem. Soc
736:, primary and secondary
254:- A phosphite) with the
4288:Knorr pyrrole synthesis
4223:Bucherer–Bergs reaction
4168:Allan–Robinson reaction
4147:Wagner-Jauregg reaction
3939:Ring-closing metathesis
3864:Larock indole synthesis
3844:Knorr pyrrole synthesis
3699:Bucherer–Bergs reaction
3564:Stieglitz rearrangement
3544:Skattebøl rearrangement
3514:Ring-closing metathesis
3374:Group transfer reaction
3339:Favorskii rearrangement
3279:Cornforth rearrangement
3209:Bamberger rearrangement
3114:Wolff–Kishner reduction
2934:Markó–Lam deoxygenation
2829:Fleming–Tamao oxidation
2824:Fischer–Tropsch process
2511:Oxymercuration reaction
2491:Knorr pyrrole synthesis
2317:Barton–Kellogg reaction
2223:Wagner-Jauregg reaction
2143:Ring-closing metathesis
2133:Reimer–Tiemann reaction
2123:Rauhut–Currier reaction
2038:Nef isocyanide reaction
1998:Malonic ester synthesis
1968:Knorr pyrrole synthesis
1903:High dilution principle
1838:Friedel–Crafts reaction
1773:Cross-coupling reaction
1698:Bucherer–Bergs reaction
1683:Blanc chloromethylation
1673:Blaise ketone synthesis
1648:Baylis–Hillman reaction
1643:Barton–Kellogg reaction
1618:Allan–Robinson reaction
1524:Woodward–Hoffmann rules
1259:Charge-transfer complex
900:10.1351/pac196409020307
880:Arbuzov, B. A. (1964).
840:Arbuzov, A. E. (1906).
218:}, which is called the
4397:Substitution reactions
4253:Feist–Benary synthesis
4027:Bradsher cycloaddition
3997:4+4 Photocycloaddition
3954:Simmons–Smith reaction
3899:Paternò–Büchi reaction
3759:Feist–Benary synthesis
3749:Dieckmann condensation
3499:Pummerer rearrangement
3479:Oxy-Cope rearrangement
3454:Myers allene synthesis
3404:Jacobsen rearrangement
3319:Electrocyclic reaction
3294:Demjanov rearrangement
3249:Buchner ring expansion
3219:Beckmann rearrangement
3199:Aza-Cope rearrangement
3194:Arndt–Eistert reaction
3169:Alkyne zipper reaction
3089:Transfer hydrogenation
3064:Sharpless oxyamination
3039:Selenoxide elimination
2924:Lombardo methylenation
2849:Griesbaum coozonolysis
2759:Corey–Itsuno reduction
2734:Boyland–Sims oxidation
2674:Angeli–Rimini reaction
2322:Boord olefin synthesis
2266:Arndt–Eistert reaction
2258:Homologation reactions
2058:Nitro-Mannich reaction
1973:Kolbe–Schmitt reaction
1783:Cross-coupling partner
1703:Buchner ring expansion
1623:Arndt–Eistert reaction
1389:Kinetic isotope effect
1136:Rearrangement reaction
713:
699:
688:
665:
622:
551:
511:
235:
175:
4112:Pauson–Khand reaction
3949:Sharpless epoxidation
3904:Pechmann condensation
3784:Friedländer synthesis
3734:Davis–Beirut reaction
3589:Wallach rearrangement
3559:Stevens rearrangement
3494:Pinacol rearrangement
3474:Overman rearrangement
3389:Hofmann rearrangement
3384:Hayashi rearrangement
3349:Ferrier rearrangement
3304:Dimroth rearrangement
3289:Curtius rearrangement
3284:Criegee rearrangement
3264:Claisen rearrangement
3254:Carroll rearrangement
3189:Amadori rearrangement
3179:Allylic rearrangement
3059:Sharpless epoxidation
2794:Dess–Martin oxidation
2719:Bohn–Schmidt reaction
2579:Hofmann rearrangement
2382:Kauffmann olefination
2305:Olefination reactions
2243:Wurtz–Fittig reaction
2078:Palladium–NHC complex
1958:Kauffmann olefination
1913:Homologation reaction
1763:Corey–House synthesis
1743:Claisen rearrangement
1539:Yukawa–Tsuno equation
1499:Swain–Lupton equation
1479:Spherical aromaticity
1414:Möbius–Hückel concept
1199:Aromatic ring current
1161:Substitution reaction
712:
698:
687:
678:rate-determining step
666:
621:
552:
512:
368:allylic rearrangement
233:
174:
4318:Paal–Knorr synthesis
4188:Barton–Zard reaction
4132:Staudinger synthesis
4082:Ketene cycloaddition
4052:Diels–Alder reaction
4032:Cheletropic reaction
4012:Alkyne trimerisation
3894:Paal–Knorr synthesis
3859:Kulinkovich reaction
3834:Jacobsen epoxidation
3754:Diels–Alder reaction
3549:Smiles rearrangement
3539:Sigmatropic reaction
3424:Lossen rearrangement
3274:Corey–Fuchs reaction
3239:Boekelheide reaction
3234:Bergmann degradation
3164:Achmatowicz reaction
2949:Methionine sulfoxide
2749:Clemmensen reduction
2709:Bergmann degradation
2639:Acyloin condensation
2604:Strecker degradation
2559:Bergmann degradation
2526:Ullmann condensation
2392:Peterson olefination
2367:Hydrazone iodination
2347:Elimination reaction
2248:Zincke–Suhl reaction
2168:Sonogashira coupling
2128:Reformatsky reaction
2088:Peterson olefination
2053:Nierenstein reaction
1983:Kulinkovich reaction
1798:Diels–Alder reaction
1758:Corey–Fuchs reaction
1738:Claisen condensation
1608:Alkyne trimerisation
1583:Acyloin condensation
1549:Σ-bishomoaromaticity
1509:Thorpe–Ingold effect
1121:Elimination reaction
967:10.1039/jr9510002550
673:Electron-withdrawing
645:
594:Carbon tetrachloride
527:
444:
395:chemical equilibrium
266:as an intermediate (
250:phosphorus species (
4338:Prilezhaev reaction
4323:Pellizzari reaction
4002:(4+3) cycloaddition
3969:Van Leusen reaction
3944:Robinson annulation
3929:Pschorr cyclization
3924:Prilezhaev reaction
3654:Bergman cyclization
3609:Wolff rearrangement
3594:Weerman degradation
3484:Pericyclic reaction
3464:Neber rearrangement
3359:Fries rearrangement
3244:Brook rearrangement
3229:Bergman cyclization
3074:Staudinger reaction
3019:Rosenmund reduction
3009:Reductive amination
2974:Oppenauer oxidation
2764:Corey–Kim oxidation
2739:Cannizzaro reaction
2614:Weerman degradation
2589:Isosaccharinic acid
2501:Mukaiyama hydration
2357:Hofmann elimination
2342:Dehydrohalogenation
2327:Chugaev elimination
2148:Robinson annulation
2093:Pfitzinger reaction
1863:Gattermann reaction
1808:Wulff–Dötz reaction
1788:Dakin–West reaction
1713:Carbonyl allylation
1658:Bergman cyclization
1444:Kennedy J. P. Orton
1364:Hammond's postulate
1334:Flippin–Lodge angle
1304:Electromeric effect
1229:Beta-silicon effect
1214:Baker–Nathan effect
995:10.1021/ja01567a067
944:10.1021/cr00044a004
626:Phosphorus reactant
466:
4392:Coupling reactions
4087:McCormack reaction
4037:Conia-ene reaction
3869:Madelung synthesis
3659:Biginelli reaction
3449:Mumm rearrangement
3334:Favorskii reaction
3269:Cope rearrangement
3259:Chan rearrangement
3024:Rubottom oxidation
2954:Miyaura borylation
2919:Lipid peroxidation
2914:Lindgren oxidation
2894:Kornblum oxidation
2889:Kolbe electrolysis
2834:Fukuyama reduction
2744:Carbonyl reduction
2594:Marker degradation
2456:Diazonium compound
2446:Boudouard reaction
2425:Carbon-heteroatom
2352:Grieco elimination
2138:Rieche formylation
2083:Passerini reaction
2013:Meerwein arylation
1933:Hydroxymethylation
1828:Favorskii reaction
1728:Chan rearrangement
1663:Biginelli reaction
1588:Aldol condensation
1434:2-Norbornyl cation
1409:Möbius aromaticity
1404:Markovnikov's rule
1299:Effective molarity
1244:Bürgi–Dunitz angle
1234:Bicycloaromaticity
714:
700:
689:
661:
623:
562:1,2-dichloroethene
547:
507:
454:
236:
226:Reaction mechanism
176:
4379:
4378:
4375:
4374:
4371:
4370:
4363:Wohl–Aue reaction
4007:6+4 Cycloaddition
3824:Iodolactonization
3144:1,2-rearrangement
3109:Wohl–Aue reaction
3029:Sabatier reaction
2994:Pinnick oxidation
2959:Mozingo reduction
2904:Leuckart reaction
2859:Haloform reaction
2774:Criegee oxidation
2754:Collins oxidation
2704:Benkeser reaction
2699:Bechamp reduction
2669:Andrussow process
2654:Alcohol oxidation
2564:Edman degradation
2471:Haloform reaction
2420:
2419:
2407:Takai olefination
2372:Julia olefination
2198:Takai olefination
2073:Olefin metathesis
1948:Julia olefination
1873:Grignard reaction
1853:Fukuyama coupling
1768:Coupling reaction
1733:Chan–Lam coupling
1603:Alkyne metathesis
1598:Alkane metathesis
1454:Phosphaethynolate
1359:George S. Hammond
1319:Electronic effect
1274:Conjugated system
1156:Stereospecificity
1151:Stereoselectivity
1116:Addition reaction
1105:organic reactions
1063:Organic Syntheses
1052:Organic Syntheses
1041:Organic Syntheses
1022:Collected Volumes
1015:Organic Syntheses
983:J. Am. Chem. Soc.
861:Missing or empty
659:
651:
545:
539:
533:
505:
498:
490:
483:
476:
469:
457:
450:
407:tetrafluoroborate
346:2 pathway or an S
184:Aleksandr Arbuzov
106:chemical reaction
100:(also called the
94:
93:
53:Coupling reaction
43:Aleksandr Arbuzov
16:(Redirected from
4409:
4358:Wenker synthesis
4348:Stollé synthesis
4203:Bobbitt reaction
4173:Auwers synthesis
4117:Povarov reaction
4042:Cyclopropanation
3980:
3974:Wenker synthesis
3729:Darzens reaction
3679:Bobbitt reaction
3524:Schmidt reaction
3329:Enyne metathesis
3104:Whiting reaction
3099:Wharton reaction
3044:Shapiro reaction
3034:Sarett oxidation
2999:Prévost reaction
2809:Emde degradation
2619:Wohl degradation
2599:Ruff degradation
2569:Emde degradation
2466:Grignard reagent
2402:Shapiro reaction
2387:McMurry reaction
2254:
2218:Ullmann reaction
2183:Stollé synthesis
2173:Stetter reaction
2163:Shapiro reaction
2153:Sakurai reaction
2048:Negishi coupling
2028:Minisci reaction
2023:Michael reaction
2008:McMurry reaction
2003:Mannich reaction
1883:Hammick reaction
1878:Grignard reagent
1818:Enyne metathesis
1803:Doebner reaction
1793:Darzens reaction
1638:Barbier reaction
1628:Auwers synthesis
1555:
1529:Woodward's rules
1494:Superaromaticity
1484:Spiroaromaticity
1384:Inductive effect
1379:Hyperconjugation
1354:Hammett equation
1294:Edwards equation
1146:Regioselectivity
1097:
1090:
1083:
1074:
1027:
1025:
1018:
1005:
999:
998:
977:
971:
970:
954:
948:
947:
926:
913:
912:
902:
887:Pure Appl. Chem.
877:
871:
870:
864:
859:
857:
849:
837:
831:
830:
818:
812:
811:
785:
754:Abramov reaction
670:
668:
667:
662:
660:
657:
656:
649:
638:
637:
633:
556:
554:
553:
548:
546:
543:
537:
531:
516:
514:
513:
508:
506:
503:
502:
496:
494:
488:
481:
480:
474:
467:
465:
462:
455:
448:
434:
433:
429:
264:phosphonium salt
192:phosphine oxides
180:August Michaelis
162:phosphine oxides
160:) react to form
144:) react to form
128:) react to form
122:phosphite esters
102:Arbuzov reaction
86:
71:
69:arbuzov-reaction
39:August Michaelis
27:
21:
18:Arbuzov reaction
4417:
4416:
4412:
4411:
4410:
4408:
4407:
4406:
4382:
4381:
4380:
4367:
4268:Gewald reaction
4151:
3978:
3959:Skraup reaction
3794:Graham reaction
3789:Gewald reaction
3620:
3613:
3135:
3128:
3084:Swern oxidation
3069:Stahl oxidation
3014:Riley oxidation
2969:Omega oxidation
2929:Luche reduction
2879:Jones oxidation
2844:Glycol cleavage
2839:Ganem oxidation
2784:Davis oxidation
2779:Dakin oxidation
2714:Birch reduction
2664:Amide reduction
2630:
2623:
2584:Hooker reaction
2546:
2540:
2428:
2426:
2416:
2412:Wittig reaction
2300:
2296:Wittig reaction
2271:Hooker reaction
2252:
2233:Wittig reaction
2208:Thorpe reaction
2193:Suzuki reaction
2178:Stille reaction
2113:Quelet reaction
1988:Kumada coupling
1938:Ivanov reaction
1928:Hydrovinylation
1908:Hiyama coupling
1868:Glaser coupling
1678:Blaise reaction
1668:Bingel reaction
1653:Benary reaction
1570:
1568:
1562:
1553:
1449:Passive binding
1369:Homoaromaticity
1219:Baldwin's rules
1194:Antiaromaticity
1189:Anomeric effect
1165:
1107:
1101:
1036:
1031:
1030:
1020:
1007:
1006:
1002:
979:
978:
974:
956:
955:
951:
928:
927:
916:
879:
878:
874:
860:
850:
839:
838:
834:
820:
819:
815:
787:
786:
782:
777:
759:Perkow reaction
750:
734:carboxylic acid
705:
643:
642:
639:
635:
631:
629:
628:
609:Perkow reaction
587:
583:
525:
524:
495:
487:
473:
442:
441:
435:
431:
427:
425:
424:
419:
403:nucleophilicity
375:
365:
361:
349:
345:
341:
337:
329:
321:
317:
313:
307:
297:
293:
281:
277:
243:
228:
217:
213:
209:
205:
201:
197:
82:
67:
41:
23:
22:
15:
12:
11:
5:
4415:
4413:
4405:
4404:
4402:Name reactions
4399:
4394:
4384:
4383:
4377:
4376:
4373:
4372:
4369:
4368:
4366:
4365:
4360:
4355:
4350:
4345:
4340:
4335:
4330:
4325:
4320:
4315:
4310:
4305:
4300:
4295:
4290:
4285:
4280:
4275:
4273:Hantzsch ester
4270:
4265:
4260:
4255:
4250:
4245:
4240:
4235:
4230:
4225:
4220:
4215:
4210:
4205:
4200:
4195:
4190:
4185:
4183:Banert cascade
4180:
4175:
4170:
4165:
4159:
4157:
4153:
4152:
4150:
4149:
4144:
4139:
4134:
4129:
4124:
4122:Prato reaction
4119:
4114:
4109:
4104:
4099:
4094:
4089:
4084:
4079:
4074:
4069:
4064:
4059:
4054:
4049:
4044:
4039:
4034:
4029:
4024:
4019:
4014:
4009:
4004:
3999:
3994:
3988:
3986:
3977:
3976:
3971:
3966:
3961:
3956:
3951:
3946:
3941:
3936:
3931:
3926:
3921:
3916:
3911:
3906:
3901:
3896:
3891:
3886:
3881:
3876:
3871:
3866:
3861:
3856:
3851:
3846:
3841:
3836:
3831:
3826:
3821:
3816:
3811:
3806:
3801:
3796:
3791:
3786:
3781:
3776:
3771:
3766:
3761:
3756:
3751:
3746:
3741:
3736:
3731:
3726:
3721:
3716:
3711:
3706:
3701:
3696:
3691:
3686:
3681:
3676:
3671:
3666:
3661:
3656:
3651:
3646:
3641:
3636:
3631:
3625:
3623:
3615:
3614:
3612:
3611:
3606:
3601:
3596:
3591:
3586:
3581:
3576:
3571:
3566:
3561:
3556:
3551:
3546:
3541:
3536:
3531:
3526:
3521:
3516:
3511:
3506:
3501:
3496:
3491:
3486:
3481:
3476:
3471:
3466:
3461:
3456:
3451:
3446:
3441:
3436:
3431:
3426:
3421:
3416:
3411:
3406:
3401:
3396:
3391:
3386:
3381:
3376:
3371:
3366:
3361:
3356:
3351:
3346:
3341:
3336:
3331:
3326:
3321:
3316:
3311:
3306:
3301:
3296:
3291:
3286:
3281:
3276:
3271:
3266:
3261:
3256:
3251:
3246:
3241:
3236:
3231:
3226:
3221:
3216:
3214:Banert cascade
3211:
3206:
3201:
3196:
3191:
3186:
3181:
3176:
3171:
3166:
3161:
3156:
3151:
3146:
3140:
3138:
3134:Rearrangement
3130:
3129:
3127:
3126:
3124:Zinin reaction
3121:
3116:
3111:
3106:
3101:
3096:
3094:Wacker process
3091:
3086:
3081:
3076:
3071:
3066:
3061:
3056:
3051:
3046:
3041:
3036:
3031:
3026:
3021:
3016:
3011:
3006:
3001:
2996:
2991:
2986:
2981:
2976:
2971:
2966:
2961:
2956:
2951:
2946:
2941:
2936:
2931:
2926:
2921:
2916:
2911:
2906:
2901:
2896:
2891:
2886:
2881:
2876:
2871:
2869:Hydrogenolysis
2866:
2861:
2856:
2851:
2846:
2841:
2836:
2831:
2826:
2821:
2819:Étard reaction
2816:
2811:
2806:
2801:
2796:
2791:
2786:
2781:
2776:
2771:
2766:
2761:
2756:
2751:
2746:
2741:
2736:
2731:
2726:
2724:Bosch reaction
2721:
2716:
2711:
2706:
2701:
2696:
2691:
2686:
2681:
2676:
2671:
2666:
2661:
2656:
2651:
2646:
2641:
2635:
2633:
2629:Organic redox
2625:
2624:
2622:
2621:
2616:
2611:
2606:
2601:
2596:
2591:
2586:
2581:
2576:
2571:
2566:
2561:
2556:
2550:
2548:
2542:
2541:
2539:
2538:
2533:
2528:
2523:
2518:
2513:
2508:
2503:
2498:
2493:
2488:
2483:
2478:
2473:
2468:
2463:
2461:Esterification
2458:
2453:
2448:
2443:
2438:
2432:
2430:
2422:
2421:
2418:
2417:
2415:
2414:
2409:
2404:
2399:
2394:
2389:
2384:
2379:
2374:
2369:
2364:
2359:
2354:
2349:
2344:
2339:
2334:
2329:
2324:
2319:
2314:
2308:
2306:
2302:
2301:
2299:
2298:
2293:
2288:
2283:
2278:
2273:
2268:
2262:
2260:
2251:
2250:
2245:
2240:
2238:Wurtz reaction
2235:
2230:
2225:
2220:
2215:
2210:
2205:
2200:
2195:
2190:
2185:
2180:
2175:
2170:
2165:
2160:
2155:
2150:
2145:
2140:
2135:
2130:
2125:
2120:
2115:
2110:
2108:Prins reaction
2105:
2100:
2095:
2090:
2085:
2080:
2075:
2070:
2065:
2060:
2055:
2050:
2045:
2040:
2035:
2030:
2025:
2020:
2015:
2010:
2005:
2000:
1995:
1990:
1985:
1980:
1975:
1970:
1965:
1960:
1955:
1950:
1945:
1940:
1935:
1930:
1925:
1923:Hydrocyanation
1920:
1915:
1910:
1905:
1900:
1895:
1893:Henry reaction
1890:
1885:
1880:
1875:
1870:
1865:
1860:
1855:
1850:
1845:
1840:
1835:
1830:
1825:
1820:
1815:
1810:
1805:
1800:
1795:
1790:
1785:
1780:
1775:
1770:
1765:
1760:
1755:
1750:
1745:
1740:
1735:
1730:
1725:
1720:
1715:
1710:
1705:
1700:
1695:
1690:
1685:
1680:
1675:
1670:
1665:
1660:
1655:
1650:
1645:
1640:
1635:
1630:
1625:
1620:
1615:
1610:
1605:
1600:
1595:
1593:Aldol reaction
1590:
1585:
1580:
1574:
1572:
1567:Carbon-carbon
1564:
1563:
1558:
1552:
1551:
1546:
1544:Zaitsev's rule
1541:
1536:
1531:
1526:
1521:
1516:
1511:
1506:
1501:
1496:
1491:
1489:Steric effects
1486:
1481:
1476:
1471:
1466:
1461:
1456:
1451:
1446:
1441:
1436:
1431:
1426:
1421:
1416:
1411:
1406:
1401:
1396:
1391:
1386:
1381:
1376:
1371:
1366:
1361:
1356:
1351:
1346:
1341:
1336:
1331:
1326:
1321:
1316:
1311:
1306:
1301:
1296:
1291:
1286:
1281:
1276:
1271:
1266:
1261:
1256:
1251:
1246:
1241:
1236:
1231:
1226:
1221:
1216:
1211:
1206:
1201:
1196:
1191:
1186:
1181:
1176:
1170:
1167:
1166:
1164:
1163:
1158:
1153:
1148:
1143:
1141:Redox reaction
1138:
1133:
1128:
1126:Polymerization
1123:
1118:
1112:
1109:
1108:
1102:
1100:
1099:
1092:
1085:
1077:
1071:
1070:
1059:
1048:
1035:
1034:External links
1032:
1029:
1028:
1000:
972:
949:
938:(4): 415–430.
914:
893:(2): 307–353.
872:
832:
813:
779:
778:
776:
773:
772:
771:
769:Hirao coupling
766:
761:
756:
749:
746:
703:
655:
627:
624:
585:
581:
558:
557:
542:
536:
518:
517:
501:
493:
486:
479:
472:
461:
453:
423:
420:
418:
415:
373:
363:
359:
347:
343:
339:
335:
327:
319:
315:
311:
305:
295:
291:
279:
275:
258:alkyl halide (
241:
227:
224:
215:
211:
207:
203:
199:
195:
92:
91:
88:
87:
80:
73:
72:
65:
61:
60:
56:
55:
50:
49:Reaction type
46:
45:
36:
32:
31:
24:
14:
13:
10:
9:
6:
4:
3:
2:
4414:
4403:
4400:
4398:
4395:
4393:
4390:
4389:
4387:
4364:
4361:
4359:
4356:
4354:
4351:
4349:
4346:
4344:
4341:
4339:
4336:
4334:
4331:
4329:
4326:
4324:
4321:
4319:
4316:
4314:
4311:
4309:
4306:
4304:
4301:
4299:
4296:
4294:
4291:
4289:
4286:
4284:
4283:Herz reaction
4281:
4279:
4276:
4274:
4271:
4269:
4266:
4264:
4261:
4259:
4256:
4254:
4251:
4249:
4246:
4244:
4241:
4239:
4236:
4234:
4231:
4229:
4226:
4224:
4221:
4219:
4216:
4214:
4211:
4209:
4206:
4204:
4201:
4199:
4196:
4194:
4191:
4189:
4186:
4184:
4181:
4179:
4176:
4174:
4171:
4169:
4166:
4164:
4161:
4160:
4158:
4154:
4148:
4145:
4143:
4140:
4138:
4135:
4133:
4130:
4128:
4125:
4123:
4120:
4118:
4115:
4113:
4110:
4108:
4105:
4103:
4100:
4098:
4095:
4093:
4090:
4088:
4085:
4083:
4080:
4078:
4075:
4073:
4070:
4068:
4065:
4063:
4060:
4058:
4055:
4053:
4050:
4048:
4045:
4043:
4040:
4038:
4035:
4033:
4030:
4028:
4025:
4023:
4020:
4018:
4015:
4013:
4010:
4008:
4005:
4003:
4000:
3998:
3995:
3993:
3990:
3989:
3987:
3985:
3984:Cycloaddition
3981:
3975:
3972:
3970:
3967:
3965:
3962:
3960:
3957:
3955:
3952:
3950:
3947:
3945:
3942:
3940:
3937:
3935:
3932:
3930:
3927:
3925:
3922:
3920:
3917:
3915:
3912:
3910:
3907:
3905:
3902:
3900:
3897:
3895:
3892:
3890:
3887:
3885:
3882:
3880:
3877:
3875:
3872:
3870:
3867:
3865:
3862:
3860:
3857:
3855:
3852:
3850:
3847:
3845:
3842:
3840:
3837:
3835:
3832:
3830:
3829:Isay reaction
3827:
3825:
3822:
3820:
3817:
3815:
3812:
3810:
3807:
3805:
3802:
3800:
3797:
3795:
3792:
3790:
3787:
3785:
3782:
3780:
3777:
3775:
3772:
3770:
3767:
3765:
3762:
3760:
3757:
3755:
3752:
3750:
3747:
3745:
3742:
3740:
3737:
3735:
3732:
3730:
3727:
3725:
3724:Cycloaddition
3722:
3720:
3717:
3715:
3712:
3710:
3707:
3705:
3702:
3700:
3697:
3695:
3692:
3690:
3687:
3685:
3682:
3680:
3677:
3675:
3672:
3670:
3667:
3665:
3662:
3660:
3657:
3655:
3652:
3650:
3647:
3645:
3642:
3640:
3637:
3635:
3632:
3630:
3627:
3626:
3624:
3622:
3619:Ring forming
3616:
3610:
3607:
3605:
3602:
3600:
3597:
3595:
3592:
3590:
3587:
3585:
3582:
3580:
3577:
3575:
3572:
3570:
3567:
3565:
3562:
3560:
3557:
3555:
3552:
3550:
3547:
3545:
3542:
3540:
3537:
3535:
3532:
3530:
3527:
3525:
3522:
3520:
3519:Rupe reaction
3517:
3515:
3512:
3510:
3507:
3505:
3502:
3500:
3497:
3495:
3492:
3490:
3487:
3485:
3482:
3480:
3477:
3475:
3472:
3470:
3467:
3465:
3462:
3460:
3457:
3455:
3452:
3450:
3447:
3445:
3442:
3440:
3437:
3435:
3432:
3430:
3427:
3425:
3422:
3420:
3417:
3415:
3412:
3410:
3407:
3405:
3402:
3400:
3397:
3395:
3392:
3390:
3387:
3385:
3382:
3380:
3377:
3375:
3372:
3370:
3367:
3365:
3362:
3360:
3357:
3355:
3352:
3350:
3347:
3345:
3342:
3340:
3337:
3335:
3332:
3330:
3327:
3325:
3322:
3320:
3317:
3315:
3312:
3310:
3307:
3305:
3302:
3300:
3297:
3295:
3292:
3290:
3287:
3285:
3282:
3280:
3277:
3275:
3272:
3270:
3267:
3265:
3262:
3260:
3257:
3255:
3252:
3250:
3247:
3245:
3242:
3240:
3237:
3235:
3232:
3230:
3227:
3225:
3222:
3220:
3217:
3215:
3212:
3210:
3207:
3205:
3202:
3200:
3197:
3195:
3192:
3190:
3187:
3185:
3182:
3180:
3177:
3175:
3172:
3170:
3167:
3165:
3162:
3160:
3157:
3155:
3152:
3150:
3147:
3145:
3142:
3141:
3139:
3137:
3131:
3125:
3122:
3120:
3117:
3115:
3112:
3110:
3107:
3105:
3102:
3100:
3097:
3095:
3092:
3090:
3087:
3085:
3082:
3080:
3077:
3075:
3072:
3070:
3067:
3065:
3062:
3060:
3057:
3055:
3052:
3050:
3047:
3045:
3042:
3040:
3037:
3035:
3032:
3030:
3027:
3025:
3022:
3020:
3017:
3015:
3012:
3010:
3007:
3005:
3002:
3000:
2997:
2995:
2992:
2990:
2987:
2985:
2982:
2980:
2977:
2975:
2972:
2970:
2967:
2965:
2962:
2960:
2957:
2955:
2952:
2950:
2947:
2945:
2942:
2940:
2937:
2935:
2932:
2930:
2927:
2925:
2922:
2920:
2917:
2915:
2912:
2910:
2909:Ley oxidation
2907:
2905:
2902:
2900:
2897:
2895:
2892:
2890:
2887:
2885:
2882:
2880:
2877:
2875:
2874:Hydroxylation
2872:
2870:
2867:
2865:
2864:Hydrogenation
2862:
2860:
2857:
2855:
2852:
2850:
2847:
2845:
2842:
2840:
2837:
2835:
2832:
2830:
2827:
2825:
2822:
2820:
2817:
2815:
2812:
2810:
2807:
2805:
2802:
2800:
2799:DNA oxidation
2797:
2795:
2792:
2790:
2789:Deoxygenation
2787:
2785:
2782:
2780:
2777:
2775:
2772:
2770:
2767:
2765:
2762:
2760:
2757:
2755:
2752:
2750:
2747:
2745:
2742:
2740:
2737:
2735:
2732:
2730:
2727:
2725:
2722:
2720:
2717:
2715:
2712:
2710:
2707:
2705:
2702:
2700:
2697:
2695:
2692:
2690:
2687:
2685:
2682:
2680:
2679:Aromatization
2677:
2675:
2672:
2670:
2667:
2665:
2662:
2660:
2657:
2655:
2652:
2650:
2647:
2645:
2642:
2640:
2637:
2636:
2634:
2632:
2626:
2620:
2617:
2615:
2612:
2610:
2607:
2605:
2602:
2600:
2597:
2595:
2592:
2590:
2587:
2585:
2582:
2580:
2577:
2575:
2572:
2570:
2567:
2565:
2562:
2560:
2557:
2555:
2552:
2551:
2549:
2543:
2537:
2534:
2532:
2529:
2527:
2524:
2522:
2519:
2517:
2516:Reed reaction
2514:
2512:
2509:
2507:
2504:
2502:
2499:
2497:
2494:
2492:
2489:
2487:
2484:
2482:
2479:
2477:
2474:
2472:
2469:
2467:
2464:
2462:
2459:
2457:
2454:
2452:
2449:
2447:
2444:
2442:
2439:
2437:
2434:
2433:
2431:
2427:bond forming
2423:
2413:
2410:
2408:
2405:
2403:
2400:
2398:
2395:
2393:
2390:
2388:
2385:
2383:
2380:
2378:
2375:
2373:
2370:
2368:
2365:
2363:
2360:
2358:
2355:
2353:
2350:
2348:
2345:
2343:
2340:
2338:
2335:
2333:
2332:Cope reaction
2330:
2328:
2325:
2323:
2320:
2318:
2315:
2313:
2310:
2309:
2307:
2303:
2297:
2294:
2292:
2289:
2287:
2284:
2282:
2279:
2277:
2274:
2272:
2269:
2267:
2264:
2263:
2261:
2259:
2255:
2249:
2246:
2244:
2241:
2239:
2236:
2234:
2231:
2229:
2226:
2224:
2221:
2219:
2216:
2214:
2211:
2209:
2206:
2204:
2201:
2199:
2196:
2194:
2191:
2189:
2186:
2184:
2181:
2179:
2176:
2174:
2171:
2169:
2166:
2164:
2161:
2159:
2156:
2154:
2151:
2149:
2146:
2144:
2141:
2139:
2136:
2134:
2131:
2129:
2126:
2124:
2121:
2119:
2116:
2114:
2111:
2109:
2106:
2104:
2101:
2099:
2096:
2094:
2091:
2089:
2086:
2084:
2081:
2079:
2076:
2074:
2071:
2069:
2066:
2064:
2061:
2059:
2056:
2054:
2051:
2049:
2046:
2044:
2043:Nef synthesis
2041:
2039:
2036:
2034:
2031:
2029:
2026:
2024:
2021:
2019:
2018:Methylenation
2016:
2014:
2011:
2009:
2006:
2004:
2001:
1999:
1996:
1994:
1991:
1989:
1986:
1984:
1981:
1979:
1976:
1974:
1971:
1969:
1966:
1964:
1961:
1959:
1956:
1954:
1951:
1949:
1946:
1944:
1941:
1939:
1936:
1934:
1931:
1929:
1926:
1924:
1921:
1919:
1916:
1914:
1911:
1909:
1906:
1904:
1901:
1899:
1896:
1894:
1891:
1889:
1888:Heck reaction
1886:
1884:
1881:
1879:
1876:
1874:
1871:
1869:
1866:
1864:
1861:
1859:
1856:
1854:
1851:
1849:
1846:
1844:
1841:
1839:
1836:
1834:
1831:
1829:
1826:
1824:
1821:
1819:
1816:
1814:
1811:
1809:
1806:
1804:
1801:
1799:
1796:
1794:
1791:
1789:
1786:
1784:
1781:
1779:
1776:
1774:
1771:
1769:
1766:
1764:
1761:
1759:
1756:
1754:
1751:
1749:
1746:
1744:
1741:
1739:
1736:
1734:
1731:
1729:
1726:
1724:
1721:
1719:
1716:
1714:
1711:
1709:
1706:
1704:
1701:
1699:
1696:
1694:
1691:
1689:
1686:
1684:
1681:
1679:
1676:
1674:
1671:
1669:
1666:
1664:
1661:
1659:
1656:
1654:
1651:
1649:
1646:
1644:
1641:
1639:
1636:
1634:
1631:
1629:
1626:
1624:
1621:
1619:
1616:
1614:
1611:
1609:
1606:
1604:
1601:
1599:
1596:
1594:
1591:
1589:
1586:
1584:
1581:
1579:
1576:
1575:
1573:
1569:bond forming
1565:
1561:
1556:
1550:
1547:
1545:
1542:
1540:
1537:
1535:
1534:Y-aromaticity
1532:
1530:
1527:
1525:
1522:
1520:
1519:Walsh diagram
1517:
1515:
1512:
1510:
1507:
1505:
1504:Taft equation
1502:
1500:
1497:
1495:
1492:
1490:
1487:
1485:
1482:
1480:
1477:
1475:
1474:Σ-aromaticity
1472:
1470:
1467:
1465:
1462:
1460:
1457:
1455:
1452:
1450:
1447:
1445:
1442:
1440:
1437:
1435:
1432:
1430:
1427:
1425:
1422:
1420:
1417:
1415:
1412:
1410:
1407:
1405:
1402:
1400:
1399:Marcus theory
1397:
1395:
1392:
1390:
1387:
1385:
1382:
1380:
1377:
1375:
1374:Hückel's rule
1372:
1370:
1367:
1365:
1362:
1360:
1357:
1355:
1352:
1350:
1347:
1345:
1342:
1340:
1337:
1335:
1332:
1330:
1329:Evelyn effect
1327:
1325:
1322:
1320:
1317:
1315:
1312:
1310:
1309:Electron-rich
1307:
1305:
1302:
1300:
1297:
1295:
1292:
1290:
1287:
1285:
1282:
1280:
1277:
1275:
1272:
1270:
1267:
1265:
1262:
1260:
1257:
1255:
1252:
1250:
1247:
1245:
1242:
1240:
1237:
1235:
1232:
1230:
1227:
1225:
1224:Bema Hapothle
1222:
1220:
1217:
1215:
1212:
1210:
1207:
1205:
1202:
1200:
1197:
1195:
1192:
1190:
1187:
1185:
1182:
1180:
1177:
1175:
1172:
1171:
1168:
1162:
1159:
1157:
1154:
1152:
1149:
1147:
1144:
1142:
1139:
1137:
1134:
1132:
1129:
1127:
1124:
1122:
1119:
1117:
1114:
1113:
1110:
1106:
1098:
1093:
1091:
1086:
1084:
1079:
1078:
1075:
1068:
1064:
1060:
1057:
1053:
1049:
1046:
1042:
1038:
1037:
1033:
1023:
1017:
1016:
1011:
1004:
1001:
996:
992:
988:
985:
984:
976:
973:
968:
964:
960:
959:J. Chem. Soc.
953:
950:
945:
941:
937:
934:
933:
925:
923:
921:
919:
915:
910:
906:
901:
896:
892:
889:
888:
883:
876:
873:
868:
855:
847:
843:
836:
833:
828:
824:
817:
814:
809:
805:
802:: 1048–1055.
801:
797:
796:
791:
784:
781:
774:
770:
767:
765:
762:
760:
757:
755:
752:
751:
747:
745:
741:
739:
735:
731:
727:
723:
718:
711:
707:
697:
693:
686:
682:
679:
674:
653:
634:
625:
620:
616:
613:
610:
605:
603:
602:isomerization
599:
595:
591:
579:
575:
571:
567:
563:
540:
534:
523:
522:
521:
499:
491:
484:
477:
470:
459:
451:
440:
439:
438:
430:
421:
416:
414:
412:
408:
404:
400:
396:
392:
387:
385:
381:
377:
369:
357:
353:
333:
325:
310:, where the R
309:
301:
289:
285:
273:
269:
265:
261:
257:
256:electrophilic
253:
249:
245:
232:
225:
223:
221:
206:} to give {(C
193:
189:
185:
181:
173:
169:
167:
163:
159:
155:
151:
147:
143:
139:
135:
131:
127:
123:
119:
115:
111:
107:
103:
99:
89:
85:
81:
78:
75:
74:
70:
66:
63:
62:
57:
54:
51:
48:
47:
44:
40:
37:
34:
33:
28:
19:
3324:Ene reaction
2684:Autoxidation
2545:Degradation
2436:Azo coupling
2213:Ugi reaction
1813:Ene reaction
1613:Alkynylation
1464:Polyfluorene
1459:Polar effect
1324:Electrophile
1239:Bredt's rule
1209:Baird's rule
1179:Alpha effect
1062:
1051:
1040:
1021:
1013:
1003:
989:(10): 2608.
986:
981:
975:
958:
952:
935:
930:
890:
885:
875:
863:|title=
854:cite journal
845:
841:
835:
826:
822:
816:
799:
793:
783:
742:
719:
715:
701:
690:
640:
614:
606:
590:acyl halides
570:heterocycles
559:
519:
436:
422:Alkyl halide
391:phosphoranes
388:
371:
287:
283:
267:
262:) to give a
259:
251:
248:nucleophilic
237:
220:Klaui ligand
188:phosphinates
177:
165:
157:
154:phosphinites
149:
146:phosphinates
141:
138:phosphonites
133:
130:phosphonates
125:
114:alkyl halide
101:
97:
95:
84:RXNO:0000060
79:ontology ID
59:Identifiers
35:Named after
1823:Ethenolysis
1469:Ring strain
1439:Nucleophile
1264:Clar's rule
1204:Aromaticity
842:Chem. Zentr
574:Iodobenzene
370:mechanism (
300:carbocation
118:pentavalent
4386:Categories
4107:Ozonolysis
3634:Annulation
2984:Ozonolysis
1103:Topics in
932:Chem. Rev.
775:References
598:chloroform
308:1 reaction
116:to form a
3621:reactions
3136:reactions
2631:reactions
2547:reactions
2429:reactions
1571:reactions
722:pyrolysis
654:−
584:2 or an S
485:≫
386:halides.
384:propargyl
324:neopentyl
110:trivalent
104:) is the
1514:Vinylogy
1184:Annulene
1131:Reagents
961:: 2550.
909:93719226
795:Berichte
748:See also
500:″
492:′
478:′
413:anions.
411:triflate
244:2 attack
1174:A value
1067:Article
1056:Article
1045:Article
848:: 1639.
726:Hydroxy
578:alkenes
362:1 and S
246:of the
907:
829:: 687.
630:": -->
566:trityl
426:": -->
272:halide
190:, and
152:) and
905:S2CID
738:amine
730:thiol
417:Scope
399:P NMR
380:allyl
352:vinyl
108:of a
867:help
632:edit
607:The
564:and
541:>
535:>
520:and
471:>
452:>
449:RCOX
428:edit
382:and
356:aryl
354:and
136:),
96:The
991:doi
963:doi
940:doi
895:doi
804:doi
650:ABP
544:RCl
538:RBr
482:CHX
456:RCH
409:or
214:)Co
202:)Co
168:).
77:RSC
4388::
1019:;
1012:.
987:79
936:81
917:^
903:.
884:.
858::
856:}}
852:{{
846:II
844:.
827:38
825:.
800:31
798:.
792:.
732:,
728:,
658:OR
532:RI
504:CX
489:RR
475:RR
376:2'
222:.
1096:e
1089:t
1082:v
1069:)
1058:)
1047:)
1026:.
997:.
993::
969:.
965::
946:.
942::
911:.
897::
891:9
869:)
865:(
810:.
806::
704:N
636:]
586:N
582:N
497:R
468:X
460:2
432:]
374:N
372:S
364:N
360:N
348:N
344:N
340:N
336:N
328:N
320:N
316:N
312:1
306:N
304:S
296:N
292:1
288:5
284:4
280:1
276:N
268:3
260:2
252:1
242:N
240:S
216:3
212:5
210:H
208:5
204:3
200:5
198:H
196:5
166:6
164:(
158:5
156:(
150:4
148:(
142:3
140:(
134:2
132:(
126:1
124:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.