135:
515:
261:
564:
353:
315:
55:
119:
236:
process. Dehydrogenative aromatization is the reverse of arene hydrogenation. As such, hydrogenation catalysts are effective for the reverse reaction. Platinum-catalyzed dehydrogenations of cyclohexanes and related feedstocks are the largest scale applications of this reaction (see above).
231:
For cyclohexane, cyclohexene, and cyclohexadiene, dehydrogenation is the conceptually simplest pathway for aromatization. The activation barrier decreases with the degree of unsaturation. Thus, cyclohexadienes are especially prone to aromatization. Formally, dehydrogenation is a
545:
to a dehydrobenzene intermediate diradical, which abstracts hydrogen to aromatize. The enediyne moiety can be included within an existing ring, allowing access to a bicyclic system under mild conditions as a consequence of the
1152:
Mohamed, R. K.; Peterson, P. W.; Alabugin, I. V. (2013). "Concerted
Reactions that Produce Diradicals and Zwitterions: Electronic, Steric, Conformational and Kinetic Control of Cycloaromatization Processes".
1079:
Kündig, E. P.; Garcia, A. E.; Lomberget, T.; Bernardinelli, G. (2005). "Rediscovery, Isolation, and
Asymmetric Reduction of 1,2,3,4-Tetrahydronaphthalene-1,4-dione and Studies of its Complex".
41:
is formed from a single nonaromatic precursor. Typically aromatization is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of
825:
Brown, W.; Turner, A. B. (1971). "Applications of High-Potential
Quinones. Part VII. The Synthesis of Steroidal Phenanthrenes by Double Methyl Migration".
827:
240:
975:
864:
534:
The aromatization of acyclic precursors is rarer in organic synthesis, although it is a significant component of the BTX production in refineries.
1081:
621:
936:
Shimizu, S.; Watanabe, N.; Kataoka, T.; Shoji, T.; Abe, N.; Morishita, S.; Ichimura, H. (2005). "Pyridine and
Pyridine Derivatives".
1136:
1034:
920:
809:
784:
759:
735:
645:
1205:
957:
1050:
Capponi, M.; Gut, I. G.; Hellrung, B.; Persy, G.; Wirz, J. (1999). "Ketonization
Equilibria of Phenol in Aqueous Solution".
1052:
563:
537:
Among acyclic precursors, alkynes are relatively prone to aromatizations since they are partially dehydrogenated. The
66:
is a classic aromatization reaction. This platinum (Pt)-catalyzed process is practiced on scale in the production of
973:
Horning, E. C.; Stromberg, V. L.; Lloyd, H. A. (1952). "Beckmann
Rearrangements. An Investigation of Special Cases".
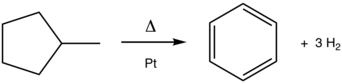
368:. Isomerization of 1,4-naphthalenediol at 200 °C produces a 2:1 mixture with its keto form, 1,4-dioxotetralin.
862:
Bergmann, F.; Szmuszkowicz, J.; Fawaz, G. (1947). "The
Condensation of 1,1-Diarylethylenes with Maleic Anhydride".
385:
243:(DDQ) is often the reagent of choice. DDQ and an acid catalyst has been used to synthesise a steroid with a
1022:
381:
330:
211:
611:
356:
1,4-Dioxotetralin and its aromatized tautomer 1,4-naphthalenediol coexist in equal abundance in solution.
1200:
296:
558:
at 37 °C, the reaction being highly favorable owing to the formation of two new aromatic rings:
514:
276:
Soluble transition metal complexes can induce oxidative aromatization concomitant with complexation.
134:
584:
376:
Classically, aromatization reactions involve changing the C:H ratio of a substrate. When applied to
326:
195:
1128:
904:
538:
260:
105:
converts paraffins (acyclic hydrocarbons) into aromatics. A related aromatization process includes
661:
Ono, Y. (1992). "Transformation of Lower
Alkanes into Aromatic Hydrocarbons over ZSM-5 Zeolites".
1120:
713:
551:
110:
1026:
1015:
93:
into aromatics. The process, which is catalyzed by platinum, is exemplified in the conversion
1195:
1172:
1132:
1098:
1030:
953:
916:
881:
844:
805:
780:
755:
705:
641:
617:
94:
59:
34:
1164:
1155:
1116:
1090:
1061:
1002:
984:
945:
908:
873:
836:
747:
697:
670:
352:
270:
248:
214:
for the enzyme) have been shown to be more effective than anti-estrogen medications such as
804:(in German). Vol. 4 (21st ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag.
377:
334:
159:
86:
779:(in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 656–660.
751:
314:
187:
183:
1189:
1010:
1006:
701:
361:
269:
Sulfur and selenium are traditionally used in aromatization, the leaving group being
179:
175:
717:
277:
252:
244:
191:
151:
82:
439:
arises by the aromatization reaction of cycloheptatriene with hydride acceptors.
607:
547:
203:
171:
42:
38:
912:
674:
302:
Oxidative dehydrogenation of dihydropyridine results in aromatization, giving
202:(which forms a permanent and deactivating bond with the aromatase enzyme) and
199:
949:
941:
579:
215:
207:
155:
143:
90:
1176:
1102:
1094:
1065:
885:
54:
848:
709:
174:
concomitant with aromatization. Such conversions are relevant to estrogen
840:
743:
555:
542:
409:
303:
285:
81:
Although not practiced under the name, aromatization is a cornerstone of
67:
17:
988:
877:
118:
518:
341:
319:
163:
98:
63:
46:
1168:
688:
Lephart, E. D. (1996). "A Review of Brain
Aromatase Cytochrome P450".
365:
167:
147:
899:
Bennett, M. A.; Huang, T. N.; Matheson, T. W.; Smith, A. K. (1982).
166:. Each of these aromatizations involves the oxidation of the C-19
150:
that aromatize rings within steroids. The specific conversions are
49:. Aromatization includes the formation of heterocyclic systems.
513:
351:
337:
313:
233:
53:
613:
Advanced
Organic Chemistry: Reactions, Mechanisms, and Structure
562:
259:
133:
117:
251:. In the process, DDQ is itself reduced into an aromatic
418:
Aromatization can entail removal of hydride. Tropylium,
218:
likely because they prevent the formation of estradiol.
550:
in the reactant. Cyclodeca-3-en-1,5-diyne reacts with
521:
of a pyrrole to a pyridine. The first step involves
380:, proton removal gives the aromatic conjugate base
325:Non-aromatic rings can be aromatized in many ways.
1014:
364:of cyclohexadienones gives the aromatic tautomer
85:. One of the major reforming reactions is the
938:Ullmann's Encyclopedia of Industrial Chemistry
616:(6th ed.), New York: Wiley-Interscience,
8:
638:Petroleum Refining Technology and Economics
284:-propyl-1,3-cyclohexadiene) is oxidised to
828:Journal of the Chemical Society C: Organic
247:core by oxidation accompanied by a double
800:Dinnendahl, V.; Fricke, U., eds. (2007).
729:
727:
525:. The second step involves aromatization.
241:2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
976:Journal of the American Chemical Society
901:(η-Hexamethylbenzene)ruthenium Complexes
865:Journal of the American Chemical Society
1082:Angewandte Chemie International Edition
740:Medicinal Chemistry of Anticancer Drugs
601:
599:
595:
734:Avendaño, C.; Menéndez, J. C. (2008).
170:group to allow for the elimination of
7:
640:(2nd ed.). Marcel Dekker, Inc.
636:Gary, J.H.; Handwerk, G.E. (1984).
752:10.1016/B978-0-444-52824-7.00003-2
25:
519:Ciamician-Dennstedt rearrangement
907:. Vol. 21. pp. 74–78.
318:240pxSemmler-Wolff synthesis of
372:Hydride and proton abstraction
1:
1053:Canadian Journal of Chemistry
567:Scheme 1. Bergman cyclization
1125:Advanced Inorganic Chemistry
702:10.1016/0165-0173(96)00002-1
1222:
913:10.1002/9780470132524.ch16
675:10.1080/01614949208020306
344:under acidic conditions.
227:Oxidative dehydrogenation
950:10.1002/14356007.a22_399
386:sodium cyclopentadienide
1206:Organic redox reactions
1023:Oxford University Press
775:Jasek, W., ed. (2007).
554:to produce benzene and
530:From acyclic precursors
1095:10.1002/anie.200502588
1066:10.1139/cjc-77-5-6-605
736:"Aromatase Inhibitors"
663:Catal. Rev. - Sci. Eng
568:
526:
382:cyclopentadienyl anion
357:
331:Semmler-Wolff reaction
322:
295:with the reduction of
264:
222:Aromatization pathways
178:in the development of
138:
122:
71:
566:
517:
355:
317:
297:ruthenium trichloride
263:
137:
128:Biochemical processes
121:
57:
841:10.1039/J39710002566
585:Aromatic hydrocarbon
196:Aromatase inhibitors
107:dehydroisomerization
1129:John Wiley and Sons
989:10.1021/ja01140a048
905:Inorganic Syntheses
878:10.1021/ja01199a055
802:Arzneistoff-Profile
606:Smith, Michael B.;
539:Bergman cyclization
97:(a naphthene) into
77:Industrial practice
746:. pp. 65–73.
569:
552:1,3-cyclohexadiene
527:
358:
323:
265:
139:
123:
111:methylcyclopentane
103:Dehydrocyclization
72:
58:The conversion of
1169:10.1021/cr4000682
1017:Organic Chemistry
983:(20): 5153–5155.
623:978-0-471-72091-1
95:methylcyclohexane
60:methylcyclohexane
35:chemical reaction
27:Chemical reaction
16:(Redirected from
1213:
1181:
1180:
1163:(9): 7089–7129.
1156:Chemical Reviews
1149:
1143:
1142:
1127:(6th ed.).
1113:
1107:
1106:
1076:
1070:
1069:
1060:(5–6): 605–613.
1047:
1041:
1040:
1021:(1st ed.).
1020:
999:
993:
992:
970:
964:
963:
933:
927:
926:
896:
890:
889:
872:(7): 1773–1777.
859:
853:
852:
822:
816:
815:
797:
791:
790:
772:
766:
765:
731:
722:
721:
685:
679:
678:
658:
652:
651:
633:
627:
626:
603:
541:is converts an
510:
506:
505:
504:
495:
494:
493:
485:
484:
474:
473:
472:
462:
461:
460:
452:
451:
438:
437:
436:
428:
427:
271:hydrogen sulfide
249:methyl migration
21:
1221:
1220:
1216:
1215:
1214:
1212:
1211:
1210:
1186:
1185:
1184:
1151:
1150:
1146:
1139:
1115:
1114:
1110:
1078:
1077:
1073:
1049:
1048:
1044:
1037:
1005:; Greeves, N.;
1001:
1000:
996:
972:
971:
967:
960:
935:
934:
930:
923:
898:
897:
893:
861:
860:
856:
824:
823:
819:
812:
799:
798:
794:
787:
774:
773:
769:
762:
733:
732:
725:
687:
686:
682:
660:
659:
655:
648:
635:
634:
630:
624:
605:
604:
597:
593:
576:
532:
523:dearomatization
508:
503:
501:
500:
499:
497:
492:
489:
488:
487:
483:
480:
479:
478:
476:
471:
468:
467:
466:
464:
459:
456:
455:
454:
450:
447:
446:
445:
443:
435:
432:
431:
430:
426:
423:
422:
421:
419:
413:
407:
403:
399:
395:
378:cyclopentadiene
374:
350:
348:Tautomerization
335:2-cyclohexenone
312:
229:
224:
160:androstenedione
130:
101:(an aromatic).
87:dehydrogenation
79:
70:from petroleum.
39:aromatic system
28:
23:
22:
15:
12:
11:
5:
1219:
1217:
1209:
1208:
1203:
1198:
1188:
1187:
1183:
1182:
1144:
1137:
1108:
1071:
1042:
1035:
994:
965:
958:
928:
921:
891:
854:
817:
810:
792:
785:
767:
760:
723:
690:Brain Res. Rev
680:
669:(3): 179–226.
653:
646:
628:
622:
594:
592:
589:
588:
587:
582:
575:
572:
571:
570:
531:
528:
512:
511:
502:
490:
481:
469:
457:
448:
433:
424:
416:
415:
411:
405:
401:
397:
393:
384:, isolable as
373:
370:
349:
346:
311:
308:
293:-propyltoluene
278:α-Phellandrene
267:
266:
228:
225:
223:
220:
188:postmenopausal
184:ovarian cancer
141:
140:
129:
126:
125:
124:
78:
75:
74:
73:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1218:
1207:
1204:
1202:
1199:
1197:
1194:
1193:
1191:
1178:
1174:
1170:
1166:
1162:
1158:
1157:
1148:
1145:
1140:
1138:9780471199571
1134:
1130:
1126:
1122:
1121:Wilkinson, G.
1118:
1117:Cotton, F. A.
1112:
1109:
1104:
1100:
1096:
1092:
1089:(1): 98–101.
1088:
1084:
1083:
1075:
1072:
1067:
1063:
1059:
1055:
1054:
1046:
1043:
1038:
1036:9780198503460
1032:
1028:
1024:
1019:
1018:
1012:
1008:
1004:
998:
995:
990:
986:
982:
978:
977:
969:
966:
961:
955:
951:
947:
943:
939:
932:
929:
924:
922:9780470132524
918:
914:
910:
906:
902:
895:
892:
887:
883:
879:
875:
871:
867:
866:
858:
855:
850:
846:
842:
838:
835:: 2566–2572.
834:
830:
829:
821:
818:
813:
811:9783774198463
807:
803:
796:
793:
788:
786:9783852001814
782:
778:
777:Austria-Codex
771:
768:
763:
761:9780080559629
757:
753:
749:
745:
741:
737:
730:
728:
724:
719:
715:
711:
707:
703:
699:
695:
691:
684:
681:
676:
672:
668:
664:
657:
654:
649:
647:0-8247-7150-8
643:
639:
632:
629:
625:
619:
615:
614:
609:
602:
600:
596:
590:
586:
583:
581:
578:
577:
573:
565:
561:
560:
559:
557:
553:
549:
544:
540:
535:
529:
524:
520:
516:
442:
441:
440:
414:
391:
390:
389:
387:
383:
379:
371:
369:
367:
363:
362:isomerization
354:
347:
345:
343:
339:
336:
332:
328:
321:
316:
309:
307:
305:
300:
298:
294:
292:
288:
283:
279:
274:
272:
262:
258:
257:
256:
254:
250:
246:
242:
238:
235:
226:
221:
219:
217:
213:
209:
205:
201:
197:
193:
189:
185:
181:
180:breast cancer
177:
176:tumorogenesis
173:
169:
165:
161:
157:
153:
149:
145:
136:
132:
131:
127:
120:
116:
115:
114:
112:
108:
104:
100:
96:
92:
88:
84:
76:
69:
65:
61:
56:
52:
51:
50:
48:
44:
40:
36:
32:
31:Aromatization
19:
1201:Oil refining
1160:
1154:
1147:
1124:
1111:
1086:
1080:
1074:
1057:
1051:
1045:
1016:
997:
980:
974:
968:
937:
931:
900:
894:
869:
863:
857:
832:
826:
820:
801:
795:
776:
770:
739:
693:
689:
683:
666:
662:
656:
637:
631:
612:
608:March, Jerry
536:
533:
522:
417:
375:
359:
324:
301:
290:
286:
281:
280:(2-methyl-5-
275:
268:
253:hydroquinone
245:phenanthrene
239:
230:
192:gynecomastia
152:testosterone
142:
113:to benzene:
106:
102:
83:oil refining
80:
37:in which an
30:
29:
1011:Wothers, P.
1003:Clayden, J.
696:(1): 1–26.
548:ring strain
329:allows the
327:Dehydration
310:Dehydration
204:anastrozole
172:formic acid
43:cyclohexane
1190:Categories
1025:. p.
1007:Warren, S.
959:3527306730
591:References
392:2 Na + 2 C
200:exemestane
190:women and
144:Aromatases
91:naphthenes
942:Wiley-VCH
580:Aromatase
255:product.
216:tamoxifen
208:letrozole
156:estradiol
18:Aromatize
1196:Hydrogen
1177:23600723
1123:(1999).
1103:16304647
1013:(2001).
886:20251415
744:Elsevier
718:11987113
610:(2007),
574:See also
556:tetralin
543:enediyne
400:→ 2 NaC
304:pyridine
194:in men.
68:gasoline
849:5167256
710:8871783
342:aniline
320:aniline
212:compete
210:(which
164:estrone
148:enzymes
99:toluene
64:toluene
47:benzene
1175:
1135:
1101:
1033:
956:
919:
884:
847:
808:
783:
758:
716:
708:
644:
620:
366:phenol
168:methyl
714:S2CID
338:oxime
234:redox
198:like
45:into
33:is a
1173:PMID
1133:ISBN
1099:PMID
1031:ISBN
954:ISBN
917:ISBN
882:PMID
845:PMID
806:ISBN
781:ISBN
756:ISBN
706:PMID
642:ISBN
618:ISBN
360:The
206:and
182:and
158:and
146:are
1165:doi
1161:113
1091:doi
1062:doi
1027:531
985:doi
946:doi
909:doi
874:doi
837:doi
748:doi
698:doi
671:doi
509:HBr
408:+
340:to
333:of
291:iso
282:iso
186:in
162:to
154:to
109:of
89:of
62:to
1192::
1171:.
1159:.
1131:.
1119:;
1097:.
1087:45
1085:.
1058:77
1056:.
1029:.
1009:;
981:74
979:.
952:.
944:.
940:.
915:.
903:.
880:.
870:69
868:.
843:.
833:14
831:.
754:.
742:.
738:.
726:^
712:.
704:.
694:22
692:.
667:34
665:.
598:^
507:+
498:Br
496:+
475:→
465:Br
463:+
388::
306:.
299:.
273:.
1179:.
1167::
1141:.
1105:.
1093::
1068:.
1064::
1039:.
991:.
987::
962:.
948::
925:.
911::
888:.
876::
851:.
839::
814:.
789:.
764:.
750::
720:.
700::
677:.
673::
650:.
491:7
486:H
482:7
477:C
470:2
458:8
453:H
449:7
444:C
434:7
429:H
425:7
420:C
412:2
410:H
406:5
404:H
402:5
398:6
396:H
394:5
289:-
287:p
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.