220:
559:
454:
29:
429:, respectively. Betaenone B is therefore not considered toxic to the plant, but will produce leaf spots when present in high concentrations (0.33 μg/μL). While the mechanism of action of betaenone B has yet to be elucidated, betaenone C has been shown to inhibit
287:
513:
Very little work has been done to elucidate the biosynthetic pathways of betaenones with almost no literature references published on the subject since 1988. Their low phytotoxicity and lack of biological significance in
550:. The origin of the subsequent oxidations at positions 1, 2 and 8 have yet to be characterized, but they have been shown not to originate from acetate. It has been theorized that
41:
339:
InChI=1S/C21H36O5/c1-7-12(2)17-20(5,15(23)8-9-22)16-13(3)10-19(4,25)11-14(16)18(24)21(17,6)26/h12-14,16-17,22,25-26H,7-11H2,1-6H3/t12-,13-,14+,16+,17-,19-,20-,21+/m1/s1
437:. Most of the major work on betaenone B, including the initial structure elucidation of betaenone A, B and C as well as the partial elucidation mechanism of
311:
587:
Ichihara A, Oikawa H, Hayashi K, Sakamura S, Furusaki A, Matsumoto T (1983). "Structures of
Betaenones A and B, Novel Phytotoxins from Phoma betae Fr".
558:
649:
Oikawa H (1988). "Biosynthetic Study of
Betaenone B: Origin of the Oxygen Atoms and Accumulation of Deoxygenated Intermediate using P-450 Inhibitor".
804:
460:
771:
Pratt D, Hopkins PB (1988). "Synthesis of (.+-.)-4-De(3-hydroxypropionyl)betaenone B, an advanced model for the betaenones and stamphyloxin I.".
518:
has provoked a fairly small amount of interest in these compounds. The backbone carbon units of betaenone B are known to be synthesized via the
493:
of betaenone B has yet to be reported, Daniel Pratt and Paul
Hopkins in 1988 proposed a method for synthesizing a precursor of betaenone B from
554:
is responsible for the oxidation at these three positions since its inhibition produces probetaenone 1, the non-oxidized form of betaenone B.
453:
674:"Interactive Agricultural Ecological Atlas of Russia and Neighboring Countries. Economic Plants and their Diseases, Pests and Weeds Online"
425:), betaenone B showed the least amount of phytotoxicity showing only 8% inhibition of growth while betaenone A and C showed 73% and 89%
331:
65:
694:"Specific inhibitors of eukaryotic DNA synthesis and DNA polymerase alpha, 3-deoxyaphidicolin and aphidicolin-17-monoacetate"
483:
of this synthetic product was not tested and no further work on total synthesis of betaenones has been published since.
441:, was presented in three short papers published between 1983 and 1988. The compounds were found to inhibit a variety of
179:
464:
199:
819:
471:
by PCC followed by exposure to base, it wasn't until 1988 that a semi-complete total synthesis was reported. Using
824:
498:
522:
synthesis (PKS) pathway. The backbone is synthesized through the addition of two carbon units via addition of
834:
814:
494:
531:
502:
829:
382:
215:
114:
467:(ORD) measurements. While it was also shown that betaenone B could be converted to betaenone A by
839:
479:
synthesis of (+/-)-4-de(3'-hydroxypropionyl) betanenone B was achieved in a 24-step synthesis.
809:
723:
487:
434:
426:
410:
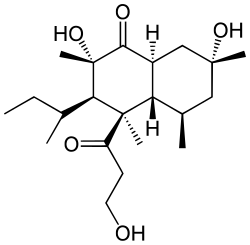
101:)-3-sec-butyl-2,7-dihydroxy-4-(3-hydroxypropanoyl)-2,4,5,7-tetramethyloctahydronaphthalen-1(2
780:
750:
713:
705:
654:
626:
596:
551:
515:
446:
236:
168:
188:
123:
844:
476:
390:
542:(instead of acetate) to the growing compound during biosynthesis. The following internal
219:
741:
Patrick D, Heimbrook D (1996). "Protein kinase inhibitors for the treatment of cancer".
442:
718:
693:
798:
754:
472:
298:
438:
148:
543:
535:
480:
417:
of the leaf tissue. Of the seven phytotoxins isolated in fungal leaf spots from
378:
374:
621:
Oikawa H (1984). "Biosynthesis of
Betaenone B, Phytotoxins of Phoma betae Fr".
539:
527:
519:
418:
399:
395:
263:
159:
709:
468:
402:
370:
20:
727:
658:
630:
414:
406:
784:
692:
Haraguchi T, Oguro M, Nagano H, Ichihara A, Sakamura S (February 1983).
600:
28:
523:
134:
547:
386:
286:
277:
546:
proceeds through a Diels–Alder reaction catalyzed by an unknown
430:
204:
673:
356:
297:
275:
262:
235:
230:
198:
178:
158:
133:
113:
56:
40:
35:
459:The structure of betaenone B was determined via
147:
122:
303:103.5 to 108 °C (218.3 to 226.4 °F)
8:
766:
764:
687:
685:
683:
644:
642:
640:
616:
614:
612:
610:
582:
580:
578:
576:
574:
319:O=C(CCO)2(((O)(C(=O)12(C(O)(C1)C)C)C)(C)CC)C
19:
218:
167:
717:
187:
570:
461:nuclear magnetic resonance spectroscopy
336:
316:
214:
70:
18:
7:
530:. The 5 methyl groups are added via
398:properties have been shown to cause
405:, which is characterized by black,
138:
14:
538:, as opposed to incorporation of
773:The Journal of Organic Chemistry
557:
452:
247:
27:
344:Key:PUZNAAVWFXQUDM-HBKHSIGZSA-N
805:Drugs not assigned an ATC code
445:signifying a possible role in
253:
241:
1:
755:10.1016/1359-6446(96)10030-1
651:J. Chem. Soc. Chem. Commun.
623:J. Chem. Soc. Chem. Commun.
465:optical rotatory dispersion
861:
475:as a starting material, a
231:Chemical and physical data
352:
327:
307:
61:
26:
497:and 1,3-butadiene via a
394:, a plant pathogen. Its
698:Nucleic Acids Research
413:eventually leading to
710:10.1093/nar/11.4.1197
532:S-adenosyl methionine
743:Drug Discovery Today
659:10.1039/c39880000600
631:10.1039/c39840000814
499:Diels–Alder reaction
383:secondary metabolite
785:10.1021/jo00260a017
601:10.1021/ja00347a070
495:methoxybenzoquinone
23:
672:Afonin AN (2008).
411:concentric circles
385:isolated from the
820:Tertiary alcohols
779:(25): 5885–5894.
503:Claisen chemistry
486:While a complete
435:protein synthesis
427:growth inhibition
364:
363:
288:Interactive image
200:CompTox Dashboard
16:Chemical compound
852:
825:Primary alcohols
789:
788:
768:
759:
758:
738:
732:
731:
721:
689:
678:
677:
669:
663:
662:
646:
635:
634:
618:
605:
604:
595:(9): 2907–2908.
589:J. Am. Chem. Soc
584:
561:
552:cytochrome P-450
516:human physiology
456:
447:cancer treatment
360:
359:
290:
270:
255:
249:
243:
223:
222:
208:
206:
191:
171:
151:
141:
140:
126:
31:
24:
22:
860:
859:
855:
854:
853:
851:
850:
849:
795:
794:
793:
792:
770:
769:
762:
740:
739:
735:
704:(4): 1197–209.
691:
690:
681:
671:
670:
666:
648:
647:
638:
625:(13): 814–815.
620:
619:
608:
586:
585:
572:
567:
511:
477:stereoselective
443:protein kinases
391:Pleospora betae
355:
353:
348:
345:
340:
335:
334:
323:
320:
315:
314:
293:
268:
258:
252:
246:
226:
202:
194:
174:
154:
137:
129:
109:
106:
69:
68:
52:
17:
12:
11:
5:
858:
856:
848:
847:
842:
837:
835:Hydroxyketones
832:
827:
822:
817:
815:Cyclic ketones
812:
807:
797:
796:
791:
790:
760:
749:(8): 325–330.
733:
679:
664:
653:(9): 600–602.
636:
606:
569:
568:
566:
563:
510:
507:
463:(NMR), CD and
362:
361:
350:
349:
347:
346:
343:
341:
338:
330:
329:
328:
325:
324:
322:
321:
318:
310:
309:
308:
305:
304:
301:
295:
294:
292:
291:
283:
281:
273:
272:
266:
260:
259:
256:
250:
244:
239:
233:
232:
228:
227:
225:
224:
216:DTXSID40234463
211:
209:
196:
195:
193:
192:
184:
182:
176:
175:
173:
172:
164:
162:
156:
155:
153:
152:
144:
142:
131:
130:
128:
127:
119:
117:
111:
110:
108:
107:
72:
64:
63:
62:
59:
58:
54:
53:
51:
50:
46:
44:
38:
37:
33:
32:
15:
13:
10:
9:
6:
4:
3:
2:
857:
846:
843:
841:
838:
836:
833:
831:
828:
826:
823:
821:
818:
816:
813:
811:
808:
806:
803:
802:
800:
786:
782:
778:
774:
767:
765:
761:
756:
752:
748:
744:
737:
734:
729:
725:
720:
715:
711:
707:
703:
699:
695:
688:
686:
684:
680:
675:
668:
665:
660:
656:
652:
645:
643:
641:
637:
632:
628:
624:
617:
615:
613:
611:
607:
602:
598:
594:
590:
583:
581:
579:
577:
575:
571:
564:
562:
560:
555:
553:
549:
545:
541:
537:
533:
529:
525:
521:
517:
508:
506:
504:
500:
496:
492:
490:
484:
482:
478:
474:
473:1,3-butadiene
470:
466:
462:
457:
455:
450:
448:
444:
440:
436:
432:
428:
424:
423:Beta vulgaris
420:
416:
412:
408:
404:
401:
397:
393:
392:
388:
384:
380:
376:
372:
369:, like other
368:
358:
351:
342:
337:
333:
326:
317:
313:
306:
302:
300:
299:Melting point
296:
289:
285:
284:
282:
279:
274:
267:
265:
261:
240:
238:
234:
229:
221:
217:
213:
212:
210:
201:
197:
190:
186:
185:
183:
181:
177:
170:
166:
165:
163:
161:
157:
150:
146:
145:
143:
136:
132:
125:
121:
120:
118:
116:
112:
104:
100:
96:
92:
88:
84:
80:
76:
71:
67:
60:
55:
48:
47:
45:
43:
39:
36:Clinical data
34:
30:
25:
776:
772:
746:
742:
736:
701:
697:
667:
650:
622:
592:
588:
556:
512:
509:Biosynthesis
488:
485:
458:
451:
439:biosynthesis
422:
409:containing,
389:
366:
365:
354:
102:
98:
94:
90:
86:
82:
78:
74:
830:Polyketides
544:cyclization
536:methylation
526:units from
481:Bioactivity
367:Betaenone B
271: g·mol
57:Identifiers
21:Betaenone B
799:Categories
565:References
540:propionate
528:acetyl-CoA
520:polyketide
419:sugar beet
403:leaf spots
400:sugar beet
396:phytotoxic
371:betaenones
276:3D model (
264:Molar mass
189:C9873620HS
160:ChemSpider
124:85269-23-4
115:CAS Number
66:IUPAC name
840:Diketones
491:synthesis
469:oxidation
810:Decalins
415:necrosis
407:pycnidia
381:), is a
357:(verify)
42:ATC code
728:6402759
524:acetate
489:de novo
269:368.514
237:Formula
135:PubChem
845:Triols
726:
719:325786
716:
548:enzyme
534:(SAM)
387:fungus
312:SMILES
169:139645
149:158750
332:InChI
278:JSmol
105:)-one
724:PMID
501:and
433:and
377:and
180:UNII
49:none
781:doi
751:doi
714:PMC
706:doi
655:doi
627:doi
597:doi
593:105
431:RNA
205:EPA
139:CID
97:,8a
85:,4a
801::
777:53
775:.
763:^
745:.
722:.
712:.
702:11
700:.
696:.
682:^
639:^
609:^
591:.
573:^
505:.
449:.
251:36
245:21
93:,7
89:,5
81:,4
77:,3
73:(2
787:.
783::
757:.
753::
747:1
730:.
708::
676:.
661:.
657::
633:.
629::
603:.
599::
421:(
379:C
375:A
373:(
280:)
257:5
254:O
248:H
242:C
207:)
203:(
103:H
99:S
95:R
91:R
87:S
83:R
79:R
75:S
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.