614:
the additions and fusions greatly affect the perturbations of the biphenylene system, with many fusions resulting in counter-intuitive stabilization by rings, or destabilization by 6-membered rings. This has led to significant interest in the systems by theoretical chemists and graph theoreticians. Even a complete 2-dimensional carbon sheet with biphenylene-like subunits has been proposed and was in-depth investigated by theoretical means, finding a technologically relevant direct band gap of ca. 1 eV, excitonic binding energies of ca. 500 meV and potential as a gas sensor.
597:
243:
168:
33:
24:
413:
613:
Polycycles containing the biphenylene nucleus have also been prepared, some having considerable antiaromatic character. In general, additional 6-membered rings add further aromatic character, and additional 4-membered and 8-membered rings add antiaromatic character. However, the exact natures of
512:
studies show a considerable alternation of bond lengths, with the bridging bonds between the benzenoid rings having the unusually great length of 1.524 Å. The separation of the rings is also reflected by the absence of the transmission of
1444:
Fan, Qitang; Yan, Linghao; Tripp, Matthias W.; Krejčí, Ondřej; Dimosthenous, Stavrina; Kachel, Stefan R.; Chen, Mengyi; Foster, Adam S.; Koert, Ulrich; Liljeroth, Peter; Gottfried, J. Michael (2021-05-21).
525:
in the central ring. This upfield shift has been interpreted in terms of diminished benzenoid ring currents, either with or without an accompanying paramagnetic ring current in the central ring.
622:
Researchers synthesized a biphenylene sheet consisting of sp2-hybridized carbon atoms that formed four-, six-, and eight-membered rings on a smooth gold surface. A bottom-up two-step interpolymer
1401:
Zhu L., Jin Y.,Xue Q., Li X., Zheng H., Wu T. Ling C. (2016). "Theoretical study of a tunable and strain-controlled nanoporous graphenylene membrane for multifunctional gas separation".
1273:
G. Brunetto, P. A. S. Autreto, L. D. Machado, B. I. Santos, R. P. B. dos Santos, and D. S. Galvao (2012). "Nonzero gap two-dimensional carbon allotrope from porous graphene".
426:
596:
1430:
1387:
1328:
1190:
Wilcox Jr., Charles F.; Farley, Erik N. (1985). "Cyclooctannelated
Biphenylenes. Diagnosis of an Anomalous Bond Length by Analysis of Ring Current Geometric Factors".
492:. The spectral and chemical properties show the influence of the central ring, leading to considerable interest in the system in terms of its degree of lessened
292:
488:), thus forming a 6-4-6 arene system. The resulting planar structure was one of the first π-electronic hydrocarbon systems discovered to show evidence of
553:
Biphenylene was first synthesized by
Lothrop in 1941. The biphenylene structure can also be understood as a dimer of the reactive intermediate
1342:
Lüder J., Puglia C., Ottosson H., Eriksson O., Sanyal B., Brena B. (2016). "Many-body effects and excitonic features in 2D biphenylene carbon".
795:
Yokozeki, A.; Wilcox Jr., C. F.; Bauer, S. H. (1974). "Biphenylene. Internuclear distances and their root mean square amplitudes of vibration".
675:
257:
1134:
Wilcox Jr., Charles F.; Farley, Erik N. (1983). "Dicyclooctabiphenylene. Benzenoid
Atropism in a Highly Antiaromatic Polycycle".
912:
Anet, F. A. L.; Schenck, G. (1971). "Application of solvent effects to the study of diamagnetic and paramagnetic ring currents".
1514:
942:"The electronic characterization of biphenylene—Experimental and theoretical insights from core and valence level spectroscopy"
465:
578:
200:
884:
Dauben Jr., Hyp. J.; Wilson, James D.; Laity, John L. (1969). "Diamagnetic susceptibility exaltation in hydrocarbons".
855:
Fraenkel, G.; Asahi, Y.; Mitchell, M. J.; Cava, M. P. (1964). "NMR spectroscopy of benzocyclobutene and biphenylene".
221:
32:
828:
659:
464:. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional
433:
538:
105:
585:. The major product, 1-aminobenzotriazole, forms benzyne in an almost quantitative yield by oxidation with
534:
530:
526:
522:
375:
521:
evidence, and particularly the shifting of proton resonances to high field, does indicate the existence of
163:
477:
826:; Reavill, R. E. (1964). "Nuclear magnetic resonance evidence for partial bond fixation in biphenylene".
1524:
1519:
1424:
1381:
1322:
1246:
857:
501:
23:
1458:
1353:
1294:
957:
767:
509:
45:
1162:
Wilcox Jr., Charles F.; Farley, Erik N. (1984). "Dicyclooctabiphenylene. Synthesis and
Properties".
656:
Nomenclature of
Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book)
623:
562:
238:
71:
1077:(1969). "Reactive intermediates. Part I. Synthesis and oxidation of 1- and 2-aminobenzotriazole".
941:
1490:
1310:
1284:
722:
557:, which in fact serves as a major synthetic route, by heating the benzenediazonium-2-carboxylate
1482:
1474:
1403:
1369:
1164:
1136:
1108:
1039:
1003:
983:
914:
886:
823:
797:
759:
727:
671:
590:
505:
1466:
1412:
1361:
1302:
1275:
1200:
1172:
1144:
1116:
1088:
1048:
1011:
973:
965:
922:
894:
866:
837:
805:
775:
735:
663:
586:
449:
315:
209:
1259:
1079:
489:
145:
242:
167:
81:
1462:
1357:
1298:
1106:
Wilcox Jr., Charles F.; Uetrecht, J. P.; Grohman, K. K. (1972). "Cyclooctabiphenylene".
961:
771:
637:
yielded ultraflat four- and eight-membered rings. The resulting allotrope was metallic.
125:
1344:
949:
634:
514:
404:
395:
1446:
870:
1508:
1494:
1314:
1192:
570:
537:, relative to comparable pure systems, which is also consistent with a reduction of
358:
156:
1074:
189:
1030:
667:
627:
497:
493:
485:
391:
780:
754:
558:
337:
136:
1478:
1052:
841:
1470:
630:
1486:
1373:
987:
1092:
978:
387:
1204:
1176:
1148:
1120:
1015:
926:
898:
809:
739:
1416:
940:
Lüder, Johann; de Simone, Monica; Totani, Roberta; et al. (2015).
554:
481:
383:
176:
1365:
1306:
969:
403:
Except where otherwise noted, data are given for materials in their
1031:"Benzenediazonium-2-Carboxylate and Biphenylene (Benzenediazonium,
541:. The electronic structure of biphenylene in the gas phase has the
1289:
116:
104:
94:
1029:
Logullo, Francis M.; Seitz, Arnold M.; Friedman, Lester (1968).
542:
518:
595:
226:
484:
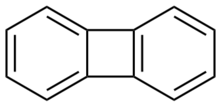
rings joined by two bridging bonds (as opposed to a normal
363:
109 to 111 °C (228 to 232 °F; 382 to 384 K)
266:
InChI=1S/C12H8/c1-2-6-10-9(5-1)11-7-3-4-8-12(10)11/h1-8H
421:
276:
InChI=1/C12H8/c1-2-6-10-9(5-1)11-7-3-4-8-12(10)11/h1-8H
1447:"Biphenylene network: A nonbenzenoid carbon allotrope"
517:
through the central ring. However, more sensitive
706:Barton, J. W. (1969). "2". In J. P. Snyder (ed.).
725:(1944). "The Crystal Structure of Biphenylene".
188:
80:
755:"A refinement of the structure of biphenylene"
710:. Vol. 1. Academic Press. pp. 32–62.
529:measurements also show a diminishing of both
8:
1429:: CS1 maint: multiple names: authors list (
1386:: CS1 maint: multiple names: authors list (
1327:: CS1 maint: multiple names: authors list (
691:Cava, M. P.; Mitchell, M. J. (1967). "10".
600:Synthesis of Benzyne and Biphenylene from 1
241:
166:
144:
15:
1288:
977:
779:
504:have been investigated repeatedly. Both
208:
646:
297:
262:
237:
1422:
1379:
1320:
1255:
1244:
1001:Lothrop, W. C. (1941). "Biphenylene".
157:
269:Key: IFVTZJHWGZSXFD-UHFFFAOYSA-N
124:
7:
753:Fawcett, J. K.; Trotter, J. (1966).
693:Cyclobutadiene and Related Compounds
695:. Academic Press. pp. 255–316.
279:Key: IFVTZJHWGZSXFD-UHFFFAOYAR
179:
1035:-carboxy-, hydroxide, inner salt)"
14:
411:
327:
31:
22:
593:to biphenylene in good yields.
545:at a binding energy of 7.8 eV.
466:polycyclic aromatic hydrocarbon
407:(at 25 °C , 100 kPa).
660:The Royal Society of Chemistry
321:
1:
1222:(Thesis). Cornell University.
871:10.1016/s0040-4020(01)98985-9
668:10.1039/9781849733069-FP001
1541:
1218:Farley, Erik Neil (1984).
829:Recl. Trav. Chim. Pays-Bas
565:. Another approach is by
1061:, vol. 5, p. 54
781:10.1107/s0365110x66000161
539:ring current diamagnetism
401:
367:
308:
288:
253:
64:
56:
44:
39:
30:
21:
1235:Revue Roumaine de Chimie
1053:10.15227/orgsyn.048.0012
842:10.1002/recl.19640831203
1471:10.1126/science.abg4509
1233:Balaban, A. T. (1968).
1220:Dicyclooctabiphenylenes
527:Magnetic susceptibility
523:electron delocalization
515:NMR substituent effects
1515:Antiaromatic compounds
708:Nonbenzenoid Aromatics
605:
535:diamagnetic anisotropy
531:diamagnetic exaltation
478:polycyclic hydrocarbon
300:c1ccc-2c(c1)-c3c2cccc3
662:. 2014. p. 209.
599:
1093:10.1039/J39690000742
510:electron diffraction
46:Preferred IUPAC name
1463:2021Sci...372..852F
1411:(39): 15015–15021.
1358:2016JChPh.144b4702L
1299:2012arXiv1205.6838B
1283:(23): 12810–12813.
1205:10.1021/jo00203a013
1177:10.1021/ja00335a055
1149:10.1021/ja00362a040
1121:10.1021/ja00762a068
1016:10.1021/ja01850a007
962:2015JChPh.142g4305L
927:10.1021/ja00731a061
899:10.1021/ja01036a022
810:10.1021/ja00811a014
772:1966AcCry..20...87F
740:10.1021/ja01240a012
624:dehydrofluorination
609:Higher biphenylenes
563:2-aminobenzoic acid
452:with the formula (C
345: g·mol
18:
1417:10.1039/C6TA04456E
606:
480:, composed of two
434:Infobox references
368:Related compounds
16:
1457:(6544): 852–856.
1404:J. Mater. Chem. A
1366:10.1063/1.4939273
1307:10.1021/jp211300n
1276:J. Phys. Chem. C
1254:Missing or empty
1171:(23): 7195–7200.
1165:J. Am. Chem. Soc.
1143:(24): 7191–7192.
1137:J. Am. Chem. Soc.
1109:J. Am. Chem. Soc.
1059:Collected Volumes
1040:Organic Syntheses
1004:J. Am. Chem. Soc.
970:10.1063/1.4907723
915:J. Am. Chem. Soc.
887:J. Am. Chem. Soc.
836:(12): 1230–1232.
798:J. Am. Chem. Soc.
760:Acta Crystallogr.
734:(12): 2035–2042.
728:J. Am. Chem. Soc.
677:978-0-85404-182-4
506:X-ray diffraction
476:Biphenylene is a
442:Chemical compound
440:
439:
222:CompTox Dashboard
106:Interactive image
1532:
1499:
1498:
1441:
1435:
1434:
1428:
1420:
1398:
1392:
1391:
1385:
1377:
1339:
1333:
1332:
1326:
1318:
1292:
1270:
1264:
1263:
1257:
1252:
1250:
1242:
1230:
1224:
1223:
1215:
1209:
1208:
1187:
1181:
1180:
1159:
1153:
1152:
1131:
1125:
1124:
1103:
1097:
1096:
1073:Campbell, C.D.;
1070:
1064:
1062:
1055:
1026:
1020:
1019:
1010:(5): 1187–1191.
998:
992:
991:
981:
946:
937:
931:
930:
909:
903:
902:
893:(8): 1991–1998.
881:
875:
874:
865:(5): 1179–1184.
852:
846:
845:
824:Katritzky, A. R.
820:
814:
813:
804:(4): 1026–1032.
792:
786:
785:
783:
750:
744:
743:
718:
712:
711:
703:
697:
696:
688:
682:
681:
654:"Front Matter".
651:
589:, which rapidly
587:lead(IV) acetate
498:bond alternation
450:organic compound
424:
418:
415:
414:
344:
329:
323:
316:Chemical formula
246:
245:
230:
228:
212:
192:
181:
170:
159:
148:
128:
108:
84:
35:
26:
19:
1540:
1539:
1535:
1534:
1533:
1531:
1530:
1529:
1505:
1504:
1503:
1502:
1443:
1442:
1438:
1421:
1400:
1399:
1395:
1378:
1345:J. Chem. Phys.
1341:
1340:
1336:
1319:
1272:
1271:
1267:
1253:
1243:
1232:
1231:
1227:
1217:
1216:
1212:
1189:
1188:
1184:
1161:
1160:
1156:
1133:
1132:
1128:
1105:
1104:
1100:
1080:J. Chem. Soc. C
1072:
1071:
1067:
1057:
1028:
1027:
1023:
1000:
999:
995:
944:
939:
938:
934:
911:
910:
906:
883:
882:
878:
854:
853:
849:
822:
821:
817:
794:
793:
789:
752:
751:
747:
720:
719:
715:
705:
704:
700:
690:
689:
685:
678:
653:
652:
648:
643:
626:of an adsorbed
620:
611:
551:
496:. Questions of
490:antiaromaticity
474:
463:
459:
455:
443:
436:
431:
430:
429: ?)
420:
416:
412:
408:
394:
390:
386:
380:
377:
342:
332:
326:
318:
304:
301:
296:
295:
284:
281:
280:
277:
271:
270:
267:
261:
260:
249:
231:
224:
215:
195:
182:
151:
131:
111:
98:
87:
74:
60:
52:
51:
12:
11:
5:
1538:
1536:
1528:
1527:
1522:
1517:
1507:
1506:
1501:
1500:
1436:
1393:
1334:
1265:
1225:
1210:
1199:(3): 351–356.
1182:
1154:
1126:
1098:
1087:(5): 742–747.
1065:
1021:
993:
950:J. Chem. Phys.
932:
921:(2): 556–557.
904:
876:
847:
815:
787:
745:
713:
698:
683:
676:
645:
644:
642:
639:
635:polymerization
619:
616:
610:
607:
604:-Benzotriazole
583:-sulfonic acid
579:hydroxylamine-
575:-benzotriazole
569:-amination of
561:prepared from
550:
547:
473:
470:
461:
457:
453:
441:
438:
437:
432:
410:
409:
405:standard state
402:
399:
398:
396:cyclobutadiene
381:
373:
370:
369:
365:
364:
361:
355:
354:
351:
347:
346:
340:
334:
333:
330:
324:
319:
314:
311:
310:
306:
305:
303:
302:
299:
291:
290:
289:
286:
285:
283:
282:
278:
275:
274:
272:
268:
265:
264:
256:
255:
254:
251:
250:
248:
247:
234:
232:
220:
217:
216:
214:
213:
205:
203:
197:
196:
194:
193:
185:
183:
175:
172:
171:
161:
153:
152:
150:
149:
141:
139:
133:
132:
130:
129:
121:
119:
113:
112:
110:
109:
101:
99:
92:
89:
88:
86:
85:
77:
75:
70:
67:
66:
62:
61:
58:
54:
53:
49:
48:
42:
41:
37:
36:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1537:
1526:
1523:
1521:
1518:
1516:
1513:
1512:
1510:
1496:
1492:
1488:
1484:
1480:
1476:
1472:
1468:
1464:
1460:
1456:
1452:
1448:
1440:
1437:
1432:
1426:
1418:
1414:
1410:
1406:
1405:
1397:
1394:
1389:
1383:
1375:
1371:
1367:
1363:
1359:
1355:
1352:(2): 024702.
1351:
1347:
1346:
1338:
1335:
1330:
1324:
1316:
1312:
1308:
1304:
1300:
1296:
1291:
1286:
1282:
1278:
1277:
1269:
1266:
1261:
1248:
1240:
1236:
1229:
1226:
1221:
1214:
1211:
1206:
1202:
1198:
1195:
1194:
1193:J. Org. Chem.
1186:
1183:
1178:
1174:
1170:
1167:
1166:
1158:
1155:
1150:
1146:
1142:
1139:
1138:
1130:
1127:
1122:
1118:
1114:
1111:
1110:
1102:
1099:
1094:
1090:
1086:
1082:
1081:
1076:
1069:
1066:
1060:
1054:
1050:
1046:
1042:
1041:
1036:
1034:
1025:
1022:
1017:
1013:
1009:
1006:
1005:
997:
994:
989:
985:
980:
979:11368/2842819
975:
971:
967:
963:
959:
956:(7): 074305.
955:
952:
951:
943:
936:
933:
928:
924:
920:
917:
916:
908:
905:
900:
896:
892:
889:
888:
880:
877:
872:
868:
864:
860:
859:
851:
848:
843:
839:
835:
831:
830:
825:
819:
816:
811:
807:
803:
800:
799:
791:
788:
782:
777:
773:
769:
765:
762:
761:
756:
749:
746:
741:
737:
733:
730:
729:
724:
721:Waser, Jurg;
717:
714:
709:
702:
699:
694:
687:
684:
679:
673:
669:
665:
661:
658:. Cambridge:
657:
650:
647:
640:
638:
636:
632:
629:
625:
617:
615:
608:
603:
598:
594:
592:
588:
584:
582:
576:
574:
568:
564:
560:
556:
548:
546:
544:
540:
536:
532:
528:
524:
520:
516:
511:
507:
503:
502:ring currents
499:
495:
491:
487:
483:
479:
471:
469:
467:
451:
447:
435:
428:
423:
406:
400:
397:
393:
389:
385:
382:
379:
372:
371:
366:
362:
360:
359:Melting point
357:
356:
352:
349:
348:
341:
339:
336:
335:
320:
317:
313:
312:
307:
298:
294:
287:
273:
263:
259:
252:
244:
240:
239:DTXSID3059765
236:
235:
233:
223:
219:
218:
211:
207:
206:
204:
202:
199:
198:
191:
187:
186:
184:
178:
174:
173:
169:
165:
162:
160:
158:ECHA InfoCard
155:
154:
147:
143:
142:
140:
138:
135:
134:
127:
123:
122:
120:
118:
115:
114:
107:
103:
102:
100:
96:
91:
90:
83:
79:
78:
76:
73:
69:
68:
63:
55:
47:
43:
38:
34:
29:
25:
20:
1525:Biphenylenes
1520:Hydrocarbons
1454:
1450:
1439:
1425:cite journal
1408:
1402:
1396:
1382:cite journal
1349:
1343:
1337:
1323:cite journal
1280:
1274:
1268:
1256:|title=
1247:cite journal
1238:
1234:
1228:
1219:
1213:
1196:
1191:
1185:
1168:
1163:
1157:
1140:
1135:
1129:
1112:
1107:
1101:
1084:
1078:
1068:
1058:
1044:
1038:
1032:
1024:
1007:
1002:
996:
953:
948:
935:
918:
913:
907:
890:
885:
879:
862:
856:
850:
833:
827:
818:
801:
796:
790:
766:(1): 87–93.
763:
758:
748:
731:
726:
716:
707:
701:
692:
686:
655:
649:
621:
612:
601:
580:
572:
566:
552:
475:
445:
444:
378:hydrocarbons
65:Identifiers
57:Other names
17:Biphenylene
1115:(7): 2532.
858:Tetrahedron
723:Lu, Chia-Si
628:halogenated
549:Preparation
494:aromaticity
486:ring fusion
446:Biphenylene
392:cyclobutene
376:unsaturated
350:Appearance
309:Properties
164:100.217.287
126:CHEBI:33079
59:Diphenylene
50:Biphenylene
1509:Categories
1075:Rees, C.W.
641:References
559:zwitterion
338:Molar mass
210:0Z64I7D5M2
137:ChemSpider
93:3D model (
72:CAS Number
1495:234794559
1479:0036-8075
1315:103548116
1290:1205.6838
633:molecule
631:terphenyl
591:dimerises
1487:34016779
1374:26772582
988:25702013
388:biphenyl
374:Related
82:259-79-0
1459:Bibcode
1451:Science
1354:Bibcode
1295:Bibcode
958:Bibcode
768:Bibcode
618:Network
555:benzyne
482:benzene
472:Bonding
427:what is
425: (
384:benzene
343:152.196
177:PubChem
1493:
1485:
1477:
1372:
1313:
1241:: 231.
1047:: 12.
986:
674:
448:is an
422:verify
419:
353:Solid
293:SMILES
40:Names
1491:S2CID
1311:S2CID
1285:arXiv
945:(PDF)
577:with
258:InChI
117:ChEBI
95:JSmol
1483:PMID
1475:ISSN
1431:link
1388:link
1370:PMID
1329:link
1260:help
1085:1969
984:PMID
672:ISBN
543:HOMO
533:and
508:and
500:and
201:UNII
190:9214
146:8859
1467:doi
1455:372
1413:doi
1362:doi
1350:144
1303:doi
1281:116
1201:doi
1173:doi
1169:106
1145:doi
1141:105
1117:doi
1089:doi
1049:doi
1012:doi
974:hdl
966:doi
954:142
923:doi
895:doi
867:doi
838:doi
806:doi
776:doi
736:doi
664:doi
519:NMR
468:.
227:EPA
180:CID
1511::
1489:.
1481:.
1473:.
1465:.
1453:.
1449:.
1427:}}
1423:{{
1407:.
1384:}}
1380:{{
1368:.
1360:.
1348:.
1325:}}
1321:{{
1309:.
1301:.
1293:.
1279:.
1251::
1249:}}
1245:{{
1239:13
1237:.
1197:50
1113:94
1083:.
1056:;
1045:48
1043:.
1037:.
1008:63
982:.
972:.
964:.
947:.
919:93
891:91
863:20
861:.
834:83
832:.
802:96
774:.
764:20
757:.
732:66
670:.
325:12
1497:.
1469::
1461::
1433:)
1419:.
1415::
1409:4
1390:)
1376:.
1364::
1356::
1331:)
1317:.
1305::
1297::
1287::
1262:)
1258:(
1207:.
1203::
1179:.
1175::
1151:.
1147::
1123:.
1119::
1095:.
1091::
1063:.
1051::
1033:o
1018:.
1014::
990:.
976::
968::
960::
929:.
925::
901:.
897::
873:.
869::
844:.
840::
812:.
808::
784:.
778::
770::
742:.
738::
680:.
666::
602:H
581:O
573:H
571:1
567:N
462:2
460:)
458:4
456:H
454:6
417:N
331:8
328:H
322:C
229:)
225:(
97:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.