172:
27:
377:
668:
608:
545:
675:
The rapid development of borepin stabilization and functionalization since the 2000s has catapulted studies of complex and versatile molecules. Like many other main group compounds, borepins have been in the field since the mid-late 1900s yet lay dormant until more modern methods could utilize them.
624:
Spectroscopic data, DFT calculations, and thermochemical data have shown that borepin is weakly aromatic when compared to the tropylium cation. This reduction in aromaticity leads to increased reactivity and instability at the boron center as there is less electron density being donated to boron's
693:
in a borepin was published in 1975 by Eisch and Galle and described how heptaphenylborepin was fluorescent green when probed. Little photophysical phenomena were recorded for many years, until Piers's group published the first example of a blue-fluorescent borepin species in 2009. They discovered
643:
A complication that arises with fusion of the phenyl rings is their positioning. When synthesizing dibenzoborepins (b is the carbon next to the boron atom) they are perfectly aligned for conjugation of the borocycloheptatriene ring. However, if the phenyls are positioned in a fashion (see below)
496:
While direct functionalization of the boron atom is possible due to its vacant p-orbital, most simple borepins are simply too reactive with air and moisture to be isolated. Therefore, borepins have been stabilized by two general methods: bulky, kinetically stabilizing ligands bound to the boron
448:
Previous synthetic methods yielded heavily substituted and bulky borepin compounds such as heptaphenyl borepin. These routes, while generating very stable complexes, made it difficult to analyze the properties of the borepin ring. Minimal substitution allowed scientists like Ashe to confirm the
1108:
Yang, Wenlong; Krantz, Kelsie E.; Freeman, Lucas A.; Dickie, Diane A.; Molino, Andrew; Kaur, Aishvaryadeep; Wilson, David J. D.; Gilliard, Robert J. (25 September 2019). "Stable
Borepinium and Borafluorenium Heterocycles: A Reversible Thermochromic "Switch" Based on Boron–Oxygen Interactions".
484:
528:
Chemists like Ashe were able to utilize this knowledge in the 1990s to functionalize borepins as a compound, leading to the formation of many Lewis acid-base adducts. The most common borepin precursor used by chemists is a borepin-halide complex as halides are a good
409:
521:
721:. Because of borepins’ low-lying LUMO, it can act as an electron acceptor to participate in electron transport. The Wagner group as well as Toscano and co-workers showed computationally and experimentally the potential applications for these complexes.
1358:
Kelch, Hauke; Kachel, Stephanie; Wahler, Johannes; Celik, Mehmet Ali; Stoy, Andreas; Krummenacher, Ivo; Kramer, Thomas; Radacki, Krzysztof; Braunschweig, Holger (12 October 2018). "Borabicycloheptadiene: A Fused
Bicyclic Isomer of Borepin".
577:
636:
698:
of their compounds from around 250 nanometers (nm) to upwards of 450 nm. The rationale behind this shift is that the presence of boron in the aromatic system decreases the energy gap between the HOMO and LUMO, resulting in changing
433:
440:
More recently a method for a minimally substituted borepin was developed by Ashe and Drone. They proceeded from 1,2-dibromocyclopentene and performed a van der Kerk method for boron heterocycle preparation. Next, they initiated a
428:
by the bulky phenyl groups bound to all seven positions on the ring, protecting it from reactions with moisture in the air. However, like most borepins, this compound reacted with oxygen, turning from fluorescent green to purple.
1047:
Eisch, John J.; Galle, James E. (July 1975). "Rearrangements of organometallic compounds. XIII. Boraaromatic systems. IV. Synthesis of heptaphenylborepin via the thermal rearrangement of heptaphenyl-7-borabicycloheptadiene".
1402:
Hollister, Kimberly K.; Yang, Wenlong; Mondol, Ranajit; Wentz, Kelsie E.; Molino, Andrew; Kaur, Aishvaryadeep; Dickie, Diane A.; Frenking, Gernot; Pan, Sudip; Wilson, David J. D.; Gilliard, Robert J. (7 June 2022).
1268:
Eisch, John J.; Galle, James E.; Shafii, Babak; Rheingold, Arnold L. (August 1990). "Bora-aromatic systems. 12. Thermal generation and transformation of the borepin ring system: a paradigm of pericyclic processes".
1159:
Li, Chenglong; Shi, Yafei; Li, Pengfei; Zhang, Niu; Wang, Nan; Yin, Xiaodong; Chen, Pangkuan (17 September 2021). "Access to Highly
Luminescent N-Doped Diazaborepins with Penta-, Hexa-, and Heptagon Substructures".
755:
In contrast to that example, upon addition of cyanide to one of their borepin analogues to tetrathienoanthracence, Adachi and
Ohshita saw a loss of fluorescence. However, upon cooling, there was a noticeable
714:). If the fluorescence “switch” could be controlled, in addition to having stable borepin complexes, then it would be relatively easy and cheap to achieve bright fluorescent lights, potentially of any color.
663:
and long-chain alkanes, and even introducing electron-rich heteroatoms such as nitrogen or sulfur in order to further stabilize the borepins. Some examples of these compounds can be seen in the image below:
456:
As more modern methods appeared, the tin-boron exchange reaction has become more commonly used as tin can act as a placeholder in the seven-membered ring, reacting with boryl halides quite easily.
1585:
Caruso, Anthony; Siegler, Maxime A.; Tovar, John D. (7 May 2010). "Synthesis of
Functionalizable Boron-Containing π-Electron Materials that Incorporate Formally Aromatic Fused Borepin Rings".
639:
Most common example of phenyl-borepin fusion. Halide ion is present to showcase borepin before reactions with Lewis bases. Generic borepin on the left explains labeling of atoms for clarity.
655:
These results explained by
Schulman and Disch have been applied many times over to modify borepin frameworks. Some common examples include increasing the number of rings—making boron-doped
628:
As a result, chemists sought ways to increase the aromatic character of borepins. The tried-and-true method by which chemists stabilize borepins is phenyl-borepin ring fusion (
604:
ability of the carbene carbon. The electron density shared with the boron center back bonds slightly with the carbon atom, leading to the single-electron radical species.
592:
Most recently, in 2022 Gilliard et al. were able to apply similar principles from their cationic borepins to form and characterize the first instance of isolated borepin
1747:
Adachi, Yohei; Arai, Fuka; Yamada, Kohei; Kurihara, Maho; Ohshita, Joji (23 May 2022). "Optical
Properties of Boron-Incorporated Analogues of Tetrathienoanthracene".
1547:
Caruso, Anthony; Tovar, John D. (25 February 2011). "Functionalized
Dibenzoborepins as Components of Small Molecule and Polymeric π-Conjugated Electronic Materials".
400:
to yield the borepin ring system seen above. A method similar to this involving a tin-boron exchange is commonly used in modern synthesis of fused borepin systems.
1620:
Messersmith, Reid E.; Siegler, Maxime A.; Tovar, John D. (1 July 2016). "Aromaticity
Competition in Differentially Fused Borepin-Containing Polycyclic Aromatics".
1509:
Levine, David R.; Siegler, Maxime A.; Tovar, John D. (14 May 2014). "Thiophene-Fused
Borepins As Directly Functionalizable Boron-Containing π-Electron Systems".
724:
On another note, scientists have sought to utilize borepins as potential anion sensors. In the past, tri-coordinate boranes have been used to detect anions like
1301:
Ashe, Arthur J.; Klein, Wolfram; Rousseau, Roger (August 1993). "Evaluation of the aromaticity of borepin: synthesis and properties of 1-substituted borepins".
425:
211:
703:
and greater intensity of fluorescence. Similar results were reported by Caruso, Tovar, and Siegler in 2010 when they ran borepins through electrochemical
347:
of borepin is relatively weak compared to traditional aromatics such as benzene or even cycloheptatriene, which has led to the synthesis of many fused,
671:
The presence of extremely bulky stabilizing groups on the boron heteroatom keep borepins, especially the fused diborepin, from rapidly decomposing.
905:
Wang, Lili; Ma, Juan; Si, Erbing; Duan, Zheng (February 2021). "Recent Advances in Luminescent Annulated Borepins, Silepins, and Phosphepins".
700:
740:
791:
1709:
Adachi, Yohei; Yamada, Kohei; Ohshita, Joji (5 June 2022). "Synthesis and Optical Properties of Anthryl-substituted Tetracyclic Borepins".
763:
Fluorescence is not only limited to outside coordination. Upon insertion of nitrogen into the borepin ring, Li et al. were able to observe
736:. Scientists like Adachi and Ohshita have demonstrated that upon coordination of fluoride (F) fluorescence increases by many magnitudes.
290:
308:
710:
The initial excitement behind these results was the potential for use in electronic materials such as organic light-emitting diodes (
445:
to form a 7-membered tin complex. Finally, they completed a tin-boron exchange reaction to afford the bicyclic borepin on the right.
392:
of o,o’-dibromobibenzyl. Next it was reacted with tributyl borate to yield a fused borinic acid ring. This product was reacted with
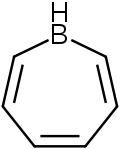
315:, which is a seven-membered ring containing three carbon-carbon double bonds, each of which contributes 2π electrons for a total of
186:
648:
1233:
Caruso, Anthony; Tovar, John D. (17 June 2011). "Conjugated " B -Entacenes": Polycyclic Aromatics Containing Two Borepin Rings".
656:
416:
Eisch and Galle isolated the first non-fused borepin in 1975. The heptaphenyl borabicycloheptadiene on the left went through a
1331:
Ashe, Arthur J.; Drone, Frederick J. (March 1987). "1-Methyl-4,5-cyclopentenoborepin: a neutral boron analog of tropylium".
524:
HOMO/LUMO electronic density mapping. Carbon atoms are in grey while the boron atom is pink/red in the center of the image.
1793:
351:
borepin systems over the years. Simple and complex borepins have been extensively studied more recently due to their high
277:
644:
then the resulting compound is less stable than dibenzoborepins by around 34 kcal/mol, quite a large energy difference.
472:
of borepin, shown below. This bicyclic, boron-containing heterocycle can be interconverted to its borepin isomer using
1788:
748:
566:
421:
150:
1455:
Schulman, Jerome M.; Disch, Raymond L. (1 July 2000). "Borepin and Its Analogues: Planar and Nonplanar Compounds".
651:
Example of a dibenzoborepin. Note how the phenyls are no longer positioned in conjunction with the borepin π-bonds.
600:
where there is multiple bonding between a boron-carbon center. The generation of the radical comes from the strong
1482:
Subramanian, Govindan; Schleyer, Paul von Ragué; Jiao, Haijun (1 May 1997). "Aromaticity of Annelated Borepins".
506:
612:
570:
533:. The borepin-hydride complex has not been able to be isolated due to its instability, whereas the boron-doped
945:
Schickedanz, Kai; Radtke, Julian; Bolte, Michael; Lerner, Hans-Wolfram; Wagner, Matthias (22 February 2017).
707:
and by Messersmith, Siegler, and Tovar in 2016 when testing the effects of variable aromaticity of borepins.
517:
is centered around the boron atom. An example of the HOMO/LUMO distribution can be seen in the figure below.
562:
1655:
De Simone, Bruna Clara; Mazzone, Gloria; Marino, Tiziana; Russo, Nino; Toscano, Marirosa (31 August 2018).
497:
center and additional aromatic π-systems that can donate electron density into the empty boron p-orbital.
717:
Another potential of redox chemistry is the use of boron-containing polycyclic aromatic hydrocarbons as
450:
389:
340:
300:
39:
694:
that by expanding the π-system (i.e. adding more fused phenyl rings) they could dramatically shift the
718:
581:
557:
Using the concept of zwitterions, Gilliard et al. was recently able to synthesize and characterize a
417:
167:
93:
1078:
Adachi, Yohei; Ohshita, Joji (26 March 2018). "Synthesis and Properties of Benzodithienoborepins".
815:
Schulman, Jerome M.; Disch, Raymond L. (March 1989). "Thermochemistry of borabenzene and borepin".
593:
473:
465:
397:
59:
632:). The addition of two fused phenyl rings increases the 6π borepin system to a 14π fused system.
1764:
1726:
1384:
1185:
1134:
922:
874:
597:
558:
412:
First synthetic procedure for a borepin lacking additional π-conjugation from fused phenyl rings.
393:
385:
304:
324:
1686:
1637:
1602:
1564:
1526:
1434:
1376:
1250:
1177:
1126:
1020:
968:
348:
328:
113:
320:
1756:
1718:
1676:
1668:
1629:
1594:
1556:
1518:
1491:
1464:
1424:
1416:
1368:
1340:
1310:
1278:
1242:
1212:
1169:
1118:
1087:
1057:
1010:
958:
914:
864:
824:
695:
336:
332:
312:
234:
780:
776:
764:
757:
573:
of NHCs and CAACs, boron has only two covalent bonds, giving it a formal positive charge.
442:
171:
851:
Ashe, A. J.; Drone, F. J.; Kausch, C. M.; Kroker, J.; Al-Taweel, S. M. (1 January 1990).
69:
1681:
1656:
1429:
1404:
1203:
van Tamelen, E.E.; Brieger, G.; Untch, K.G. (14 March 1960). "Synthesis of a borepin".
751:(TBAF). Increase of intensity correlates positively between compounds as TBAF is added.
601:
596:. These radicals were also capable of being reduced to the first instance of a borepin
548:
Early examples of Lewis acid-base adducts formed from the functionalization of borepins
534:
483:
477:
469:
271:
1216:
739:
513:
of borepin lies mostly with the carbon moieties of the seven-membered ring, while the
420:, leading to the intermediate in the middle. This intermediate subsequently underwent
1782:
1768:
1730:
1189:
1138:
926:
530:
138:
1388:
878:
790:
784:
690:
408:
352:
1760:
1091:
380:
Reaction scheme of o,o’-dibromobibenzyl to make the first reported borepin system.
1173:
747:-substituted tetracyclic borepins upon titration with increasing equivalents of
520:
344:
289:
424:
ring opening to yield heptaphenylborepin on the right. The isolated borepin is
355:
and potential applications in technologies like organic light-emitting diodes (
744:
629:
585:
538:
376:
360:
259:
104:
1672:
487:
Borepin valence isomer interconversion via photochemical reaction conditions.
1633:
869:
852:
667:
1690:
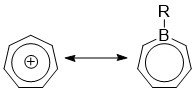
1641:
1606:
1598:
1568:
1530:
1438:
1420:
1380:
1372:
1254:
1181:
1130:
1122:
1024:
1015:
999:"Benzo- and Napthoborepins: Blue-Emitting Boron Analogues of Higher Acenes"
998:
972:
918:
607:
1657:"On the Electrochromic Properties of Borepins: A Computational Prediction"
635:
576:
963:
946:
725:
1344:
1314:
1282:
1061:
828:
544:
432:
772:
733:
729:
647:
316:
125:
26:
1722:
1560:
1522:
1495:
1468:
1246:
396:(NBS) to yield a bromo-substituted product. Finally, they performed a
997:
Mercier, Lauren G.; Piers, Warren E.; Parvez, Masood (29 July 2009).
768:
660:
270:
Except where otherwise noted, data are given for materials in their
580:
First reported instance of NHC/CAAC stabilized borepinium cations.
311:
with a tricoordinate boron in it. Simple borepins are analogues of
789:
738:
704:
666:
646:
634:
606:
575:
543:
519:
482:
431:
407:
375:
288:
92:
82:
711:
514:
510:
384:
The first synthesis of a stable borepin was reported in 1960 by
356:
339:, aromatizing the borepin while also allowing it to act as a
659:(PAHs), adding additional R groups to the framework such as
155:
615:
the borepin ring adopts to coordinate to the CAAC ligand.
327:, boron has a vacant p-orbital that can interact with the
787:(MeCN), rather drastic changes in color were observed.
537:
on the right side satisfies boron's octet, forming a
505:
Borepins are of interest due to their Lewis acidity.
588:
and does not participate in bonding to the borepin.
1405:"Isolation of Stable Borepin Radicals and Anions"
794:Solvatochromic property of N-doped diazoborepins.
436:Synthetic route to minimally substituted borepin.
388:, Brieger, and Untch. The synthesis began with a
137:
68:
947:"Facile Route to Quadruply Annulated Borepins"
319:. Unlike other seven-membered systems such as
8:
307:chemistry. They consist of a seven-membered
611:CAAC stabilized borepin radical. Note the
331:of the cycloheptatriene. This leads to an
170:
112:
18:
1680:
1428:
1014:
962:
868:
853:"Borepins and group 15 element heteroles"
767:effects. Upon addition of the borepin to
1511:Journal of the American Chemical Society
1333:Journal of the American Chemical Society
1050:Journal of the American Chemical Society
951:Journal of the American Chemical Society
1587:Angewandte Chemie International Edition
1409:Angewandte Chemie International Edition
1003:Angewandte Chemie International Edition
804:
509:(DFT) calculations have shown that the
216:
191:
166:
1742:
1740:
1704:
1702:
1700:
1580:
1578:
1542:
1540:
1450:
1448:
1326:
1324:
1296:
1294:
1292:
1228:
1226:
553:Borepin cations, anions, and radicals
418:suprafacial sigmatropic rearrangement
198:Key: LYCCRAPWFYEFFC-UHFFFAOYSA-N
195:InChI=1S/C6H7B/c1-2-4-6-7-5-3-1/h1-7H
7:
1154:
1152:
1150:
1148:
1103:
1101:
1073:
1071:
1042:
1040:
1038:
1036:
1034:
992:
990:
988:
986:
984:
982:
940:
938:
936:
900:
898:
896:
894:
892:
890:
888:
846:
844:
842:
840:
838:
810:
808:
743:Figure shows fluorescence output of
128:
14:
1622:The Journal of Organic Chemistry
1549:The Journal of Organic Chemistry
657:polycyclic-aromatic hydrocarbons
299:are a class of boron-containing
246:
25:
16:Aromatic, boron-containing rings
372:First reported synthetic method
274:(at 25 °C , 100 kPa).
1361:Chemistry – A European Journal
1111:Chemistry – A European Journal
240:
1:
1761:10.1021/acs.organomet.2c00106
1217:10.1016/S0040-4039(01)82703-9
1092:10.1021/acs.organomet.7b00844
464:As a final note, in 2018 the
252:
685:Fluorescence/phosphorescence
567:cyclic(alkyl)(amino)carbenes
541:between boron and nitrogen.
449:presence of aromaticity and
1174:10.1021/acs.orglett.1c02528
453:within the borepin system.
1810:
857:Pure and Applied Chemistry
749:tetrabutylammoniumfluoride
507:Density functional theory
268:
264:89.93 g/mol
227:
207:
182:
52:
38:
33:
24:
1673:10.1021/acsomega.8b01288
680:Photophysical properties
1634:10.1021/acs.joc.6b00927
870:10.1351/pac199062030513
563:N-heterocyclic carbenes
501:Lewis acid-base adducts
1599:10.1002/anie.201000411
1421:10.1002/anie.202202516
1373:10.1002/chem.201803509
1123:10.1002/chem.201903348
1016:10.1002/anie.200902803
919:10.1055/s-0040-1705946
795:
752:
672:
652:
640:
620:Framework manipulation
616:
589:
549:
525:
488:
437:
426:kinetically stabilized
413:
404:Synthetic developments
381:
293:
793:
742:
689:The first reports of
670:
650:
638:
610:
584:(SbF6-) is used as a
582:Antimony hexafluoride
579:
547:
523:
486:
435:
411:
379:
292:
1794:Seven-membered rings
964:10.1021/jacs.7b00268
569:(CAACS). Due to the
561:borepin state using
468:group synthesized a
335:akin to that of the
1367:(57): 15387–15391.
1345:10.1021/ja00240a058
1315:10.1021/om00032a051
1283:10.1021/om00158a035
1205:Tetrahedron Letters
1117:(54): 12512–12516.
1062:10.1021/ja00848a070
829:10.1021/om00105a024
398:dehydrohalogenation
333:isoelectronic state
21:
1789:Boron heterocycles
1415:(23): e202202516.
796:
753:
673:
653:
641:
617:
590:
550:
526:
489:
438:
414:
394:n-bromosuccinimide
382:
361:photovoltaic cells
294:
278:Infobox references
19:
1755:(10): 1225–1231.
1723:10.1246/cl.220139
1711:Chemistry Letters
1628:(13): 5595–5605.
1593:(25): 4213–4217.
1561:10.1021/jo2001726
1523:10.1021/ja502644e
1517:(19): 7132–7139.
1496:10.1021/om970008q
1490:(11): 2362–2369.
1469:10.1021/om0002733
1463:(15): 2932–2936.
1247:10.1021/ol2010159
1241:(12): 3106–3109.
1168:(18): 7123–7128.
1056:(15): 4436–4437.
1009:(33): 6108–6111.
613:boat conformation
329:π and π* orbitals
286:Chemical compound
284:
283:
151:CompTox Dashboard
94:Interactive image
1801:
1773:
1772:
1744:
1735:
1734:
1706:
1695:
1694:
1684:
1667:(8): 9556–9563.
1652:
1646:
1645:
1617:
1611:
1610:
1582:
1573:
1572:
1555:(7): 2227–2239.
1544:
1535:
1534:
1506:
1500:
1499:
1479:
1473:
1472:
1452:
1443:
1442:
1432:
1399:
1393:
1392:
1355:
1349:
1348:
1339:(6): 1879–1880.
1328:
1319:
1318:
1309:(8): 3225–3231.
1298:
1287:
1286:
1277:(8): 2342–2349.
1265:
1259:
1258:
1230:
1221:
1220:
1200:
1194:
1193:
1156:
1143:
1142:
1105:
1096:
1095:
1075:
1066:
1065:
1044:
1029:
1028:
1018:
994:
977:
976:
966:
957:(7): 2842–2851.
942:
931:
930:
902:
883:
882:
872:
848:
833:
832:
812:
337:tropylium cation
313:cycloheptatriene
309:unsaturated ring
254:
248:
242:
235:Chemical formula
175:
174:
159:
157:
141:
130:
116:
96:
72:
29:
22:
1809:
1808:
1804:
1803:
1802:
1800:
1799:
1798:
1779:
1778:
1777:
1776:
1749:Organometallics
1746:
1745:
1738:
1708:
1707:
1698:
1654:
1653:
1649:
1619:
1618:
1614:
1584:
1583:
1576:
1546:
1545:
1538:
1508:
1507:
1503:
1484:Organometallics
1481:
1480:
1476:
1457:Organometallics
1454:
1453:
1446:
1401:
1400:
1396:
1357:
1356:
1352:
1330:
1329:
1322:
1303:Organometallics
1300:
1299:
1290:
1271:Organometallics
1267:
1266:
1262:
1235:Organic Letters
1232:
1231:
1224:
1202:
1201:
1197:
1162:Organic Letters
1158:
1157:
1146:
1107:
1106:
1099:
1080:Organometallics
1077:
1076:
1069:
1046:
1045:
1032:
996:
995:
980:
944:
943:
934:
904:
903:
886:
850:
849:
836:
817:Organometallics
814:
813:
806:
801:
781:dichloromethane
777:tetrahydrofuran
758:phosphorescence
705:redox reactions
687:
682:
622:
571:dative donation
555:
503:
494:
462:
406:
374:
369:
287:
280:
275:
251:
245:
237:
223:
220:
215:
214:
203:
200:
199:
196:
190:
189:
178:
168:DTXSID001045782
160:
153:
144:
131:
119:
99:
86:
75:
62:
48:
17:
12:
11:
5:
1807:
1805:
1797:
1796:
1791:
1781:
1780:
1775:
1774:
1736:
1717:(6): 654–657.
1696:
1647:
1612:
1574:
1536:
1501:
1474:
1444:
1394:
1350:
1320:
1288:
1260:
1222:
1195:
1144:
1097:
1086:(6): 869–881.
1067:
1030:
978:
932:
913:(4): 623–635.
884:
863:(3): 513–517.
834:
823:(3): 733–737.
803:
802:
800:
797:
765:solvatochromic
719:semiconductors
686:
683:
681:
678:
621:
618:
554:
551:
502:
499:
493:
490:
470:valence isomer
461:
458:
405:
402:
373:
370:
368:
365:
285:
282:
281:
276:
272:standard state
269:
266:
265:
262:
256:
255:
249:
243:
238:
233:
230:
229:
225:
224:
222:
221:
218:
210:
209:
208:
205:
204:
202:
201:
197:
194:
193:
185:
184:
183:
180:
179:
177:
176:
163:
161:
149:
146:
145:
143:
142:
134:
132:
124:
121:
120:
118:
117:
109:
107:
101:
100:
98:
97:
89:
87:
80:
77:
76:
74:
73:
65:
63:
58:
55:
54:
50:
49:
42:
36:
35:
31:
30:
15:
13:
10:
9:
6:
4:
3:
2:
1806:
1795:
1792:
1790:
1787:
1786:
1784:
1770:
1766:
1762:
1758:
1754:
1750:
1743:
1741:
1737:
1732:
1728:
1724:
1720:
1716:
1712:
1705:
1703:
1701:
1697:
1692:
1688:
1683:
1678:
1674:
1670:
1666:
1662:
1658:
1651:
1648:
1643:
1639:
1635:
1631:
1627:
1623:
1616:
1613:
1608:
1604:
1600:
1596:
1592:
1588:
1581:
1579:
1575:
1570:
1566:
1562:
1558:
1554:
1550:
1543:
1541:
1537:
1532:
1528:
1524:
1520:
1516:
1512:
1505:
1502:
1497:
1493:
1489:
1485:
1478:
1475:
1470:
1466:
1462:
1458:
1451:
1449:
1445:
1440:
1436:
1431:
1426:
1422:
1418:
1414:
1410:
1406:
1398:
1395:
1390:
1386:
1382:
1378:
1374:
1370:
1366:
1362:
1354:
1351:
1346:
1342:
1338:
1334:
1327:
1325:
1321:
1316:
1312:
1308:
1304:
1297:
1295:
1293:
1289:
1284:
1280:
1276:
1272:
1264:
1261:
1256:
1252:
1248:
1244:
1240:
1236:
1229:
1227:
1223:
1218:
1214:
1211:(29): 14–15.
1210:
1206:
1199:
1196:
1191:
1187:
1183:
1179:
1175:
1171:
1167:
1163:
1155:
1153:
1151:
1149:
1145:
1140:
1136:
1132:
1128:
1124:
1120:
1116:
1112:
1104:
1102:
1098:
1093:
1089:
1085:
1081:
1074:
1072:
1068:
1063:
1059:
1055:
1051:
1043:
1041:
1039:
1037:
1035:
1031:
1026:
1022:
1017:
1012:
1008:
1004:
1000:
993:
991:
989:
987:
985:
983:
979:
974:
970:
965:
960:
956:
952:
948:
941:
939:
937:
933:
928:
924:
920:
916:
912:
908:
901:
899:
897:
895:
893:
891:
889:
885:
880:
876:
871:
866:
862:
858:
854:
847:
845:
843:
841:
839:
835:
830:
826:
822:
818:
811:
809:
805:
798:
792:
788:
786:
782:
778:
774:
770:
766:
761:
760:in solution.
759:
750:
746:
741:
737:
735:
731:
727:
722:
720:
715:
713:
708:
706:
702:
697:
692:
684:
679:
677:
669:
665:
662:
658:
649:
645:
637:
633:
631:
626:
619:
614:
609:
605:
603:
599:
595:
587:
583:
578:
574:
572:
568:
564:
560:
552:
546:
542:
540:
536:
532:
531:leaving group
522:
518:
516:
512:
508:
500:
498:
491:
485:
481:
479:
478:photochemical
475:
471:
467:
460:Isomerization
459:
457:
454:
452:
451:ring currents
446:
444:
434:
430:
427:
423:
419:
410:
403:
401:
399:
395:
391:
387:
378:
371:
366:
364:
362:
358:
354:
350:
346:
342:
338:
334:
330:
326:
322:
318:
314:
310:
306:
302:
298:
291:
279:
273:
267:
263:
261:
258:
257:
239:
236:
232:
231:
226:
217:
213:
206:
192:
188:
181:
173:
169:
165:
164:
162:
152:
148:
147:
140:
136:
135:
133:
127:
123:
122:
115:
111:
110:
108:
106:
103:
102:
95:
91:
90:
88:
84:
79:
78:
71:
67:
66:
64:
61:
57:
56:
51:
46:
41:
37:
32:
28:
23:
1752:
1748:
1714:
1710:
1664:
1660:
1650:
1625:
1621:
1615:
1590:
1586:
1552:
1548:
1514:
1510:
1504:
1487:
1483:
1477:
1460:
1456:
1412:
1408:
1397:
1364:
1360:
1353:
1336:
1332:
1306:
1302:
1274:
1270:
1263:
1238:
1234:
1208:
1204:
1198:
1165:
1161:
1114:
1110:
1083:
1079:
1053:
1049:
1006:
1002:
954:
950:
910:
906:
860:
856:
820:
816:
785:acetonitrile
762:
754:
723:
716:
709:
691:fluorescence
688:
674:
654:
642:
627:
623:
591:
556:
527:
504:
495:
466:Braunschweig
463:
455:
447:
443:ring closure
439:
415:
383:
353:fluorescence
349:π-conjugated
317:6π electrons
301:heterocycles
296:
295:
219:B1C=CC=CC=C1
53:Identifiers
44:
732:, and even
701:absorptions
625:p-orbital.
602:π-accepting
565:(NHCs) and
480:reactions.
422:disrotatory
386:van Tamelen
345:aromaticity
325:phosphepins
228:Properties
1783:Categories
799:References
783:(DCM) and
745:anthracene
696:wavelength
630:annulation
586:counterion
539:zwitterion
535:spirocycle
492:Reactivity
474:pericyclic
390:lithiation
341:Lewis acid
305:main group
260:Molar mass
105:ChemSpider
81:3D model (
60:CAS Number
40:IUPAC name
1769:248735151
1731:248313365
1661:ACS Omega
1190:237339643
1139:198170504
927:228982156
907:Synthesis
367:Synthesis
47:-borepine
1691:31459087
1642:27224845
1607:20455227
1569:21351778
1531:24738628
1439:35289046
1389:51953498
1381:30095190
1255:21604774
1182:34449226
1131:31334883
1025:19598197
973:28125773
879:96223530
726:fluoride
594:radicals
559:cationic
321:silepins
303:used in
297:Borepins
139:14970100
114:26666607
70:291-62-3
20:Borepin
1682:6645310
1430:9324096
779:(THF),
773:toluene
769:hexanes
734:ammonia
730:cyanide
661:alkynes
126:PubChem
1767:
1729:
1689:
1679:
1640:
1605:
1567:
1529:
1437:
1427:
1387:
1379:
1253:
1188:
1180:
1137:
1129:
1023:
971:
925:
877:
359:) and
343:. The
212:SMILES
34:Names
1765:S2CID
1727:S2CID
1385:S2CID
1186:S2CID
1135:S2CID
923:S2CID
875:S2CID
712:OLEDs
598:anion
357:OLEDs
187:InChI
83:JSmol
1687:PMID
1638:PMID
1603:PMID
1565:PMID
1527:PMID
1435:PMID
1377:PMID
1251:PMID
1178:PMID
1127:PMID
1021:PMID
969:PMID
515:LUMO
511:HOMO
323:and
1757:doi
1719:doi
1677:PMC
1669:doi
1630:doi
1595:doi
1557:doi
1519:doi
1515:136
1492:doi
1465:doi
1425:PMC
1417:doi
1369:doi
1341:doi
1337:109
1311:doi
1279:doi
1243:doi
1213:doi
1170:doi
1119:doi
1088:doi
1058:doi
1011:doi
959:doi
955:139
915:doi
865:doi
825:doi
156:EPA
129:CID
1785::
1763:.
1753:41
1751:.
1739:^
1725:.
1715:51
1713:.
1699:^
1685:.
1675:.
1663:.
1659:.
1636:.
1626:81
1624:.
1601:.
1591:49
1589:.
1577:^
1563:.
1553:76
1551:.
1539:^
1525:.
1513:.
1488:16
1486:.
1461:19
1459:.
1447:^
1433:.
1423:.
1413:61
1411:.
1407:.
1383:.
1375:.
1365:24
1363:.
1335:.
1323:^
1307:12
1305:.
1291:^
1273:.
1249:.
1239:13
1237:.
1225:^
1207:.
1184:.
1176:.
1166:23
1164:.
1147:^
1133:.
1125:.
1115:25
1113:.
1100:^
1084:37
1082:.
1070:^
1054:97
1052:.
1033:^
1019:.
1007:48
1005:.
1001:.
981:^
967:.
953:.
949:.
935:^
921:.
911:53
909:.
887:^
873:.
861:62
859:.
855:.
837:^
819:.
807:^
775:,
771:,
728:,
476:,
363:.
1771:.
1759::
1733:.
1721::
1693:.
1671::
1665:3
1644:.
1632::
1609:.
1597::
1571:.
1559::
1533:.
1521::
1498:.
1494::
1471:.
1467::
1441:.
1419::
1391:.
1371::
1347:.
1343::
1317:.
1313::
1285:.
1281::
1275:9
1257:.
1245::
1219:.
1215::
1209:1
1192:.
1172::
1141:.
1121::
1094:.
1090::
1064:.
1060::
1027:.
1013::
975:.
961::
929:.
917::
881:.
867::
831:.
827::
821:8
253:B
250:7
247:H
244:6
241:C
158:)
154:(
85:)
45:H
43:1
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.