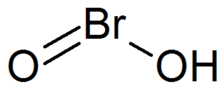33:
207:
42:
463:) resulted in a different reaction mechanism. From numbers of equivalent portions of acid bromine formed from the previous reaction, the ratio between oxygen and bromine was calculated, with the exact value of O:Br (0.149975:0.3745), suggesting the acid compound contains two oxygen atom to one bromine atom. Thus, the chemical structure of the acid compound was deducted as HBrO
357:
24:
665:
916:
for bromous acid was estimated in research studying the decomposition of bromites. The research measured the rate of bromite decomposition as a function of hydrogen and bromite ion concentrations. The experimental data of the log of the initial velocity were plotted against pH. Using this method, the
978:
In comparison to other oxygen-centered oxidants (hypohalites, anions of peroxides) and in line with its low basicity, bromite is a rather weak nucleophile. Rate constants of bromite towards carbocations and acceptor-substituted olefins are by 1–3 orders of magnitude lower than the ones measured with
931:
for bromous acid was measured based on the initial velocity of the reaction between sodium bromites and potassium iodine in a pH range of 2.9–8.0, at 25 °C and ionic strength of 0.06 M. The first order dependence of the initial velocity of this
528:
has a bent structure with ∠(H−O−Br) angles of 106.1°. HOBrO also adopts a non-planar conformation with one isomer structure (2a) adopting a dihedral angle ∠(H−O−Br−O) of 74.2°. Moreover, the planar structures of two other isomers
712:
resulting from the combination of potassium bromate, cerium(IV) sulfate, propanedioic acid and citric acid in dilute sulfuric acid. Bromous acid is an intermediate stage of the reaction between bromate ion
1199:
Field, Richard J.; Koros, Endre; Noyes, Richard M. (1972-12-01). "Oscillations in chemical systems. II. Thorough analysis of temporal oscillation in the bromate-cerium-malonic acid system".
370:
700:
have been crystallized. Upon treatment of these aqueous solutions with salts of Pb, Hg, and Ag, the corresponding heavy metal bromites precipitate as solids.
1349:
256:
400:. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.
709:
1009:
221:
1342:
1050:
Glaser, Rainer; Jost, Mary (2012-08-16). "Disproportionation of bromous acid HOBrO by direct O-transfer and via anhydrides O(BrO)
1261:
Faria, R. B.; Epstein, Irving R.; Kustin, Kenneth (1994-01-01). "Kinetics of
Disproportionation and pKa of Bromous Acid".
2219:
2256:
1335:
185:
377:
2251:
2261:
2043:
1026:
1990:
2122:
1727:
54:
1162:"Spatial and Temporal Control of Information Storage in Cellulose by Chemically Activated Oscillations"
1058:. An ab initio study of the mechanism of a key step of the Belousov–Zhabotinsky oscillating reaction".
408:
In 1905, Richards A. H. proved the existence of bromous acid through a series of experiments involving
108:
1819:
1067:
685:
202:
1897:
1861:
1752:
1664:
1407:
1397:
74:
32:
2155:
2010:
1923:
1879:
1669:
1358:
1142:
936:
on in a pH range of 4.5–8.0. The value of acid dissociation constant measured by this method is
933:
601:
393:
1296:
Mayer, Robert J.; Ofial, Armin R. (2018-02-22). "Nucleophilic
Reactivities of Bleach Reagents".
2246:
2075:
2057:
1937:
1763:
1581:
1576:
1562:
1474:
1469:
1402:
1313:
1278:
1234:
Massagli, A. (1970). "Kinetic investigation of the decomposition of bromite - ScienceDirect".
1216:
1181:
1134:
1091:
1083:
1005:
561:
553:
1161:
2208:
1912:
1833:
1709:
1626:
1509:
1434:
1429:
1418:
1305:
1270:
1243:
1208:
1173:
1126:
1075:
637:
613:
605:
577:
549:
471:
417:
331:
327:
279:
148:
41:
84:
2104:
2005:
1847:
1591:
1586:
1499:
1109:
Souza, Gabriel L. C. de; Brown, Alex (2016-07-01). "The ground and excited states of HBrO
1071:
206:
128:
2166:
2086:
2015:
1679:
1459:
673:
456:
421:
409:
348:
339:
304:
1247:
455:
Richards discovered that the effect of adding excess liquid bromine in a concentrated
2240:
2180:
2029:
1948:
1738:
1650:
1636:
1616:
1548:
1514:
1479:
1369:
1146:
2194:
2140:
1976:
1962:
1805:
1791:
1689:
1519:
1489:
1383:
808:
173:
1699:
1596:
1449:
1309:
629:
425:
335:
1777:
1606:
1130:
294:
139:
1282:
1220:
1138:
1087:
812:
23:
1317:
1185:
1177:
1095:
1684:
1274:
1212:
160:
1079:
474:(HBrO) arises by the reaction of bromine and silver nitrate solution:
1327:
347:
Except where otherwise noted, data are given for materials in their
664:
628:
A rearrangement reaction, which results from the syn-proportion of
540:
Another study identified three isomers: HOOBr, HOBrO, and HBr(O)O.
119:
107:
97:
1331:
321:
748:
Other relevant reactions in such oscillating reactions are:
190:
416:) and bromine. The reaction of excess cold aqueous to form
608:(HBrO) results in the formation of both bromous acid (HBrO
1027:"Journal of the Society of Chemical Industry. v.25 1906"
365:
996:
994:
992:
537:) are transition state for fast enantiomerization.
887:The acid dissociation constant of bromous acid,
556:(HClO) can be used to produce bromous acid (HBrO
172:
83:
1000:Egon Wiberg, Arnold Frederick Holleman (2001)
1343:
8:
1160:Vassalini, Irene; Alessandri, Ivano (2015).
1350:
1336:
1328:
906:, was determined using different methods.
205:
147:
15:
2222:
2211:
2201:
2197:
2187:
2183:
2173:
2169:
2158:
2147:
2143:
2133:
2129:
2125:
2115:
2111:
2107:
2097:
2093:
2089:
2078:
2068:
2064:
2060:
2050:
2046:
2036:
2032:
2022:
2018:
1997:
1993:
1983:
1979:
1969:
1965:
1955:
1951:
1940:
1930:
1926:
1915:
1904:
1900:
1890:
1886:
1882:
1872:
1868:
1864:
1854:
1850:
1840:
1836:
1826:
1822:
1812:
1808:
1798:
1794:
1784:
1780:
1770:
1766:
1755:
1745:
1741:
1730:
1720:
1716:
1712:
1702:
1692:
1672:
1657:
1653:
1643:
1639:
1629:
1619:
1609:
1599:
1569:
1565:
1555:
1551:
1541:
1537:
1533:
1526:
1522:
1502:
1492:
1482:
1462:
1452:
1442:
1421:
1410:
1390:
1386:
1376:
1372:
1201:Journal of the American Chemical Society
663:
988:
261:
226:
201:
1166:ACS Applied Materials & Interfaces
233:Key: DKSMCEUSSQTGBK-UHFFFAOYSA-N
127:
7:
1021:
1019:
1017:
1060:The Journal of Physical Chemistry A
243:Key: DKSMCEUSSQTGBK-UHFFFAOYAC
163:
668:The bromite ion in sodium bromite.
580:(HBrO) can form bromous acid (HBrO
14:
1263:The Journal of Physical Chemistry
924:value for bromous acid was 6.25.
708:Bromous acid is a product of the
355:
40:
31:
22:
351:(at 25 °C , 100 kPa).
1119:Theoretical Chemistry Accounts
1117:(HOOOBr and HOOBrO) isomers".
640:(HBr) gives bromous acid (HBrO
230:InChI=1S/BrHO2/c2-1-3/h(H,2,3)
1:
1248:10.1016/S0020-1693(00)93357-7
710:Belousov–Zhabotinsky reaction
704:Belousov–Zhabotinsky reaction
548:A oxidation reaction between
490:O → HBrO + AgBr + HNO
240:InChI=1/BrHO2/c2-1-3/h(H,2,3)
927:Using another method, the pK
1310:10.1021/acs.orglett.8b00645
934:disproportionation reaction
602:disproportionation reaction
299:112.911 g/mol
2278:
1365:
1131:10.1007/s00214-016-1931-8
345:
313:
272:
252:
217:
67:
53:
48:
39:
30:
21:
396:with the formula of HBrO
1236:Inorganica Chimica Acta
470:According to Richards,
1178:10.1021/acsami.5b11857
669:
512:+ 2 AgBr + 2 HNO
667:
576:A redox reaction of
448:O → HBrO + AgBr + HNO
60:hydroxidooxidobromine
740:+ 2 Br → HBrO
725:) and bromine (Br):
1275:10.1021/j100055a051
1213:10.1021/ja00780a001
1172:(51): 28708–28713.
1072:2012JPCA..116.8352G
1002:Inorganic Chemistry
652:+ HBr → 3 HBrO
620:2 HBrO → HBrO
604:of two equivalents
58:hydroxy-λ-bromanone
18:
2257:Hydrogen compounds
1359:Hydrogen compounds
909:The value of the p
670:
584:) as its product:
568:HBrO + HClO → HBrO
394:inorganic compound
378:Infobox references
314:Related compounds
16:
2234:
2233:
1304:(10): 2816–2820.
1207:(25): 8649–8664.
1080:10.1021/jp301329g
1066:(32): 8352–8365.
562:hydrochloric acid
554:hypochlorous acid
524:The molecule HBrO
386:Chemical compound
384:
383:
186:CompTox Dashboard
109:Interactive image
2269:
2252:Halogen oxoacids
2226:
2215:
2204:
2190:
2176:
2162:
2151:
2136:
2118:
2100:
2082:
2071:
2053:
2039:
2025:
2001:
1986:
1972:
1958:
1944:
1933:
1919:
1908:
1893:
1875:
1857:
1843:
1829:
1815:
1801:
1787:
1773:
1759:
1748:
1734:
1723:
1705:
1695:
1675:
1660:
1646:
1632:
1622:
1612:
1602:
1572:
1558:
1544:
1529:
1505:
1495:
1485:
1465:
1455:
1445:
1425:
1414:
1393:
1379:
1352:
1345:
1338:
1329:
1322:
1321:
1293:
1287:
1286:
1269:(4): 1363–1367.
1258:
1252:
1251:
1231:
1225:
1224:
1196:
1190:
1189:
1157:
1151:
1150:
1106:
1100:
1099:
1047:
1041:
1040:
1038:
1037:
1023:
1012:
998:
969:
967:
954:
952:
948:
904:
902:
901:
866:
865:
864:
854:
853:
852:
842:
841:
840:
830:
829:
828:
807:Bromites reduce
802:
801:
800:
779:
778:
777:
767:
766:
765:
739:
738:
737:
724:
723:
722:
638:hydrobromic acid
614:hydrobromic acid
606:hypobromous acid
578:hypobromous acid
550:hypobromous acid
472:hypobromous acid
418:hypobromous acid
368:
362:
359:
358:
332:hypobromous acid
328:Hydrobromic acid
280:Chemical formula
210:
209:
194:
192:
176:
165:
151:
131:
111:
87:
44:
35:
26:
19:
2277:
2276:
2272:
2271:
2270:
2268:
2267:
2266:
2262:Oxidizing acids
2237:
2236:
2235:
2230:
2224:
2220:
2213:
2209:
2203:
2199:
2195:
2189:
2185:
2181:
2175:
2171:
2167:
2160:
2156:
2149:
2145:
2141:
2135:
2131:
2127:
2123:
2117:
2113:
2109:
2105:
2099:
2095:
2091:
2087:
2080:
2076:
2070:
2066:
2062:
2058:
2052:
2048:
2044:
2038:
2034:
2030:
2024:
2020:
2016:
1999:
1995:
1991:
1985:
1981:
1977:
1971:
1967:
1963:
1957:
1953:
1949:
1942:
1938:
1932:
1928:
1924:
1917:
1913:
1906:
1902:
1898:
1892:
1888:
1884:
1880:
1874:
1870:
1866:
1862:
1856:
1852:
1848:
1842:
1838:
1834:
1828:
1824:
1820:
1814:
1810:
1806:
1800:
1796:
1792:
1786:
1782:
1778:
1772:
1768:
1764:
1757:
1753:
1747:
1743:
1739:
1732:
1728:
1722:
1718:
1714:
1710:
1704:
1700:
1694:
1690:
1674:
1670:
1659:
1655:
1651:
1645:
1641:
1637:
1631:
1627:
1621:
1617:
1611:
1607:
1601:
1597:
1571:
1567:
1563:
1557:
1553:
1549:
1543:
1539:
1535:
1531:
1528:
1524:
1520:
1504:
1500:
1494:
1490:
1484:
1480:
1464:
1460:
1454:
1450:
1444:
1440:
1423:
1419:
1412:
1408:
1392:
1388:
1384:
1378:
1374:
1370:
1361:
1356:
1326:
1325:
1298:Organic Letters
1295:
1294:
1290:
1260:
1259:
1255:
1233:
1232:
1228:
1198:
1197:
1193:
1159:
1158:
1154:
1116:
1112:
1108:
1107:
1103:
1057:
1053:
1049:
1048:
1044:
1035:
1033:
1025:
1024:
1015:
999:
990:
985:
976:
965:
963:
961:
950:
946:
944:
942:
930:
923:
915:
905:
899:
898:
896:
893:
885:
882:
870:
863:
860:
859:
858:
856:
851:
848:
847:
846:
844:
839:
836:
835:
834:
832:
827:
824:
823:
822:
820:
799:
796:
795:
794:
792:
790:
783:
776:
773:
772:
771:
769:
764:
761:
760:
759:
757:
755:
743:
736:
733:
732:
731:
729:
721:
718:
717:
716:
714:
706:
697:
693:
689:
681:
677:
662:
655:
651:
643:
635:
623:
611:
595:
591:
583:
571:
559:
546:
527:
522:
515:
511:
507:
503:
499:
493:
489:
485:
481:
466:
462:
451:
447:
443:
439:
431:
415:
406:
399:
387:
380:
375:
374:
373: ?)
364:
360:
356:
352:
324:
288:
282:
268:
265:
260:
259:
248:
245:
244:
241:
235:
234:
231:
225:
224:
213:
195:
188:
179:
166:
154:
134:
114:
101:
90:
77:
63:
61:
59:
12:
11:
5:
2275:
2273:
2265:
2264:
2259:
2254:
2249:
2239:
2238:
2232:
2231:
2229:
2228:
2217:
2206:
2192:
2178:
2164:
2153:
2138:
2120:
2102:
2084:
2073:
2055:
2041:
2027:
2013:
2008:
2003:
1988:
1974:
1960:
1946:
1935:
1921:
1910:
1895:
1877:
1859:
1845:
1831:
1817:
1803:
1789:
1775:
1761:
1750:
1736:
1725:
1707:
1697:
1687:
1682:
1677:
1667:
1662:
1648:
1634:
1624:
1614:
1604:
1594:
1589:
1584:
1579:
1574:
1560:
1546:
1517:
1512:
1507:
1497:
1487:
1477:
1472:
1467:
1457:
1447:
1437:
1432:
1427:
1416:
1405:
1400:
1395:
1381:
1366:
1363:
1362:
1357:
1355:
1354:
1347:
1340:
1332:
1324:
1323:
1288:
1253:
1226:
1191:
1152:
1114:
1110:
1101:
1055:
1051:
1042:
1013:
987:
986:
984:
981:
975:
972:
959:
940:
928:
921:
913:
895:
891:
884:
880:
874:
873:
872:
868:
861:
849:
843:+ OH → 2
837:
825:
805:
804:
797:
788:
785:
781:
774:
762:
753:
746:
745:
741:
734:
719:
705:
702:
695:
691:
687:
679:
675:
661:
658:
657:
656:
653:
649:
641:
633:
626:
625:
621:
609:
598:
597:
593:
592:O − 2e → HBrO
589:
581:
574:
573:
569:
557:
545:
542:
525:
521:
518:
517:
516:
513:
509:
505:
501:
500:+ HBrO + Br
497:
494:
491:
487:
483:
479:
464:
460:
457:silver nitrate
453:
452:
449:
445:
441:
437:
429:
422:silver bromide
413:
410:silver nitrate
405:
402:
397:
385:
382:
381:
376:
354:
353:
349:standard state
346:
343:
342:
340:perbromic acid
325:
319:
316:
315:
311:
310:
307:
305:Conjugate base
301:
300:
297:
291:
290:
286:
283:
278:
275:
274:
270:
269:
267:
266:
263:
255:
254:
253:
250:
249:
247:
246:
242:
239:
238:
236:
232:
229:
228:
220:
219:
218:
215:
214:
212:
211:
203:DTXSID80276549
198:
196:
184:
181:
180:
178:
177:
169:
167:
159:
156:
155:
153:
152:
144:
142:
136:
135:
133:
132:
124:
122:
116:
115:
113:
112:
104:
102:
95:
92:
91:
89:
88:
80:
78:
73:
70:
69:
65:
64:
57:
51:
50:
46:
45:
37:
36:
28:
27:
13:
10:
9:
6:
4:
3:
2:
2274:
2263:
2260:
2258:
2255:
2253:
2250:
2248:
2245:
2244:
2242:
2227:
2218:
2216:
2207:
2205:
2193:
2191:
2179:
2177:
2165:
2163:
2154:
2152:
2139:
2137:
2121:
2119:
2103:
2101:
2085:
2083:
2074:
2072:
2056:
2054:
2042:
2040:
2028:
2026:
2014:
2012:
2009:
2007:
2004:
2002:
1989:
1987:
1975:
1973:
1961:
1959:
1947:
1945:
1936:
1934:
1922:
1920:
1911:
1909:
1896:
1894:
1878:
1876:
1860:
1858:
1846:
1844:
1832:
1830:
1818:
1816:
1804:
1802:
1790:
1788:
1776:
1774:
1762:
1760:
1751:
1749:
1737:
1735:
1726:
1724:
1708:
1706:
1698:
1696:
1688:
1686:
1683:
1681:
1678:
1676:
1668:
1666:
1663:
1661:
1649:
1647:
1635:
1633:
1625:
1623:
1615:
1613:
1605:
1603:
1595:
1593:
1590:
1588:
1585:
1583:
1580:
1578:
1575:
1573:
1561:
1559:
1547:
1545:
1518:
1516:
1513:
1511:
1508:
1506:
1498:
1496:
1488:
1486:
1478:
1476:
1473:
1471:
1468:
1466:
1458:
1456:
1448:
1446:
1438:
1436:
1433:
1431:
1428:
1426:
1417:
1415:
1406:
1404:
1401:
1399:
1396:
1394:
1382:
1380:
1368:
1367:
1364:
1360:
1353:
1348:
1346:
1341:
1339:
1334:
1333:
1330:
1319:
1315:
1311:
1307:
1303:
1299:
1292:
1289:
1284:
1280:
1276:
1272:
1268:
1264:
1257:
1254:
1249:
1245:
1241:
1237:
1230:
1227:
1222:
1218:
1214:
1210:
1206:
1202:
1195:
1192:
1187:
1183:
1179:
1175:
1171:
1167:
1163:
1156:
1153:
1148:
1144:
1140:
1136:
1132:
1128:
1124:
1120:
1105:
1102:
1097:
1093:
1089:
1085:
1081:
1077:
1073:
1069:
1065:
1061:
1046:
1043:
1032:
1028:
1022:
1020:
1018:
1014:
1011:
1010:0-12-352651-5
1007:
1003:
997:
995:
993:
989:
982:
980:
979:hypobromite.
973:
971:
958:
939:
935:
925:
920:
912:
907:
890:
879:
875:
818:
817:
816:
814:
810:
809:permanganates
786:
768:+ H → 2
751:
750:
749:
728:
727:
726:
711:
703:
701:
699:
683:
666:
659:
647:
646:
645:
639:
631:
619:
618:
617:
615:
607:
603:
587:
586:
585:
579:
567:
566:
565:
563:
555:
551:
543:
541:
538:
536:
532:
519:
495:
477:
476:
475:
473:
468:
458:
435:
434:
433:
427:
423:
419:
411:
403:
401:
395:
391:
379:
372:
367:
350:
344:
341:
337:
333:
329:
326:
323:
318:
317:
312:
308:
306:
303:
302:
298:
296:
293:
292:
284:
281:
277:
276:
271:
262:
258:
251:
237:
227:
223:
216:
208:
204:
200:
199:
197:
187:
183:
182:
175:
171:
170:
168:
162:
158:
157:
150:
146:
145:
143:
141:
138:
137:
130:
126:
125:
123:
121:
118:
117:
110:
106:
105:
103:
99:
94:
93:
86:
82:
81:
79:
76:
72:
71:
66:
56:
52:
47:
43:
38:
34:
29:
25:
20:
17:Bromous acid
2221:H[Co(CO)
1439:
1301:
1297:
1291:
1266:
1262:
1256:
1239:
1235:
1229:
1204:
1200:
1194:
1169:
1165:
1155:
1122:
1118:
1104:
1063:
1059:
1045:
1034:. Retrieved
1030:
1001:
977:
956:
937:
926:
918:
910:
908:
888:
886:
877:
806:
747:
707:
671:
627:
599:
575:
547:
539:
534:
530:
523:
508:O → HBrO
469:
454:
407:
390:Bromous acid
389:
388:
68:Identifiers
62:bromous acid
1242:: 593–596.
1054:and BrO-BrO
1004:, Elsevier
917:estimated p
883:measurement
787:2 HBrO
648:2 HBrO
630:bromic acid
552:(HBrO) and
496:2 AgNO
426:nitric acid
424:(AgBr) and
336:bromic acid
273:Properties
129:CHEBI:29247
55:IUPAC names
2241:Categories
1125:(7): 178.
1036:2017-04-28
1031:HathiTrust
983:References
974:Reactivity
813:manganates
803:+ HOBr + H
672:The salts
295:Molar mass
140:ChemSpider
96:3D model (
85:37691-27-3
75:CAS Number
1903:[PtCl
1283:0022-3654
1221:0002-7863
1139:1432-881X
1088:1520-5215
953:10 M
544:Synthesis
520:Isomerism
404:Discovery
2247:Bromites
1996:[SiF
1420:H[BF
1318:29741385
1186:26654462
1147:99067360
1113:and HBrO
1096:22871057
588:HBrO + H
420:(HBrO),
309:Bromite
1068:Bibcode
903:
897:
819:2
616:(HBr):
564:(HCl).
533:and 2c-
482:+ AgNO
392:is the
371:what is
369: (
289:
161:PubChem
1316:
1281:
1219:
1184:
1145:
1137:
1094:
1086:
1008:
815:(VI):
744:+ HBrO
686:Ba(BrO
636:) and
612:) and
560:) and
440:+ AgNO
366:verify
363:
322:anions
320:Other
257:SMILES
174:165616
149:145144
49:Names
1671:NaHCO
1143:S2CID
955:and p
674:NaBrO
660:Salts
632:(HBrO
624:+ HBr
572:+ HCl
535:trans
459:(AgNO
412:(AgNO
222:InChI
120:ChEBI
98:JSmol
2011:HNCS
2006:HSCN
1680:HNCO
1628:HMnO
1515:HCNO
1501:HClO
1491:HClO
1481:HClO
1475:HClO
1461:HBrO
1451:HBrO
1441:HBrO
1435:HBrO
1398:HArF
1314:PMID
1279:ISSN
1217:ISSN
1182:PMID
1135:ISSN
1092:PMID
1084:ISSN
1006:ISBN
968:0.05
964:3.43
949:0.9)
945:(3.7
752:HBrO
684:and
600:The
596:+ 2H
529:(2b-
504:+ H
486:+ H
432:):
428:(HNO
285:HBrO
2200:TiO
2186:TeO
2172:TeO
1982:SiO
1968:SeO
1954:SeO
1744:NSO
1729:HNO
1701:HNO
1691:HNO
1685:HNO
1665:HNC
1656:MoO
1642:MnO
1618:HIO
1608:HIO
1598:HIO
1592:HIO
1582:HFO
1525:CrO
1510:HCN
1470:HCl
1430:HBr
1409:HSO
1403:HAt
1389:AsO
1375:AsO
1306:doi
1271:doi
1244:doi
1209:doi
1174:doi
1127:doi
1123:135
1076:doi
1064:116
867:+ H
857:BrO
845:MnO
833:BrO
821:MnO
811:to
793:BrO
780:+ H
770:BrO
758:BrO
730:BrO
715:BrO
678:·3H
644:):
531:cis
444:+ H
191:EPA
164:CID
2243::
2214:Po
2161:Te
2146:SO
2142:CF
2049:SO
2035:SO
2021:SO
1943:Se
1891:10
1853:PO
1839:PO
1825:PO
1587:HI
1577:HF
1568:CS
1554:CO
1536:Cr
1312:.
1302:20
1300:.
1277:.
1267:98
1265:.
1238:.
1215:.
1205:94
1203:.
1180:.
1168:.
1164:.
1141:.
1133:.
1121:.
1090:.
1082:.
1074:.
1062:.
1029:.
1016:^
991:^
970:.
962:=
943:=
894:=
855:+
831:+
791:→
756:+
694:·H
478:Br
467:.
436:Br
338:;
334:;
330:;
2225:]
2223:4
2212:2
2210:H
2202:4
2198:4
2196:H
2188:6
2184:6
2182:H
2174:3
2170:2
2168:H
2159:2
2157:H
2150:H
2148:3
2144:3
2134:8
2132:O
2130:2
2128:S
2126:2
2124:H
2116:7
2114:O
2112:2
2110:S
2108:2
2106:H
2098:6
2096:O
2094:2
2092:S
2090:2
2088:H
2081:O
2079:3
2077:H
2069:3
2067:O
2065:2
2063:S
2061:2
2059:H
2051:5
2047:2
2045:H
2037:4
2033:2
2031:H
2023:3
2019:2
2017:H
2000:]
1998:6
1994:2
1992:H
1984:4
1980:4
1978:H
1970:4
1966:2
1964:H
1956:3
1952:2
1950:H
1941:2
1939:H
1931:2
1929:S
1927:2
1925:H
1918:S
1916:2
1914:H
1907:]
1905:6
1901:2
1899:H
1889:O
1887:3
1885:P
1883:5
1881:H
1873:7
1871:O
1869:2
1867:P
1865:4
1863:H
1855:4
1851:3
1849:H
1841:3
1837:3
1835:H
1827:2
1823:3
1821:H
1813:5
1811:O
1809:2
1807:H
1799:4
1797:O
1795:2
1793:H
1785:3
1783:O
1781:2
1779:H
1771:2
1769:O
1767:2
1765:H
1758:O
1756:2
1754:H
1746:3
1742:3
1740:H
1733:S
1731:5
1721:2
1719:O
1717:2
1715:N
1713:2
1711:H
1703:3
1693:2
1673:3
1658:4
1654:2
1652:H
1644:4
1640:2
1638:H
1630:4
1620:4
1610:3
1600:2
1570:3
1566:2
1564:H
1556:3
1552:2
1550:H
1542:7
1540:O
1538:2
1534:2
1532:H
1530:/
1527:4
1523:2
1521:H
1503:4
1493:3
1483:2
1463:4
1453:3
1443:2
1424:]
1422:4
1413:F
1411:3
1391:4
1387:3
1385:H
1377:3
1373:3
1371:H
1351:e
1344:t
1337:v
1320:.
1308::
1285:.
1273::
1250:.
1246::
1240:4
1223:.
1211::
1188:.
1176::
1170:7
1149:.
1129::
1115:3
1111:2
1098:.
1078::
1070::
1056:2
1052:2
1039:.
966:±
960:a
957:K
951:×
947:±
941:a
938:K
929:a
922:a
919:K
914:a
911:K
900:/
892:a
889:K
881:a
878:K
876:p
871:O
869:2
862:3
850:4
838:2
826:4
798:3
789:2
784:O
782:2
775:2
763:3
754:2
742:2
735:3
720:3
713:(
698:O
696:2
692:2
690:)
688:2
682:O
680:2
676:2
654:2
650:3
642:2
634:3
622:2
610:2
594:2
590:2
582:2
570:2
558:2
526:2
514:3
510:2
506:2
502:2
498:3
492:3
488:2
484:3
480:2
465:2
461:3
450:3
446:2
442:3
438:2
430:3
414:3
398:2
361:Y
287:2
264:O
193:)
189:(
100:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.