478:
810:
492:
485:
590:
110:
1640:
1491:
1559:
1396:
644:
is very strong at 160 kcal/mol, as the single bond joins two carbons of sp hybridization. Carbon–carbon multiple bonds are generally stronger; the double bond of ethylene and triple bond of acetylene have been determined to have bond dissociation energies of 174 and 230 kcal/mol, respectively. A very
153:
In "structurally complex organic molecules", it is the three-dimensional orientation of the carbon–carbon bonds at quaternary loci which dictates the shape of the molecule. Further, quaternary loci are found in many biologically active small molecules, such as
219:
The directed synthesis of desired three-dimensional structures for tertiary carbons was largely solved during the late 20th century, but the same ability to direct quaternary carbon synthesis did not start to emerge until the first decade of the 21st century.
82:. A double bond is formed with an sp-hybridized orbital and a p-orbital that is not involved in the hybridization. A triple bond is formed with an sp-hybridized orbital and two p-orbitals from each atom. The use of the p-orbitals forms a
928:
Bock, Hans; Borrmann, Horst; Havlas, Zdenek; Oberhammer, Heinz; Ruppert, Klaus; Simon, Arndt (1991). "Tetrakis(dimethylamino)ethene: An
Extremely Electron-Rich Molecule with Unusual Structure both in the Crystal and in the Gas Phase".
65:
orbitals, but single bonds formed between carbon atoms with other hybridizations do occur (e.g. sp to sp). In fact, the carbon atoms in the single bond need not be of the same hybridization. Carbon atoms can also form
844:
Bochkarev, L. N.; Molosnova, N. E.; Zakharov, L. N.; Fukin, G. K.; Yanovsky, A. I.; Struchkov, Y. T. (1995). "1-Diphenylmethylene-4-(triphenylmethyl)cyclohexa-2,5-diene
Benzene Solvate".
102:
of the carbon–carbon bond gives rise to an enormous number of molecular forms, many of which are important structural elements of life, so carbon compounds have their own field of study:
232:
than C-H, O-H, N-H, H-H, H-Cl, C-F, and many double or triple bonds, and comparable in strength to C-O, Si-O, P-O, and S-H bonds, but is commonly considered as strong.
1074:
604:, the bond dissociation energy to form the stabilized triarylmethyl radical is only 8 kcal/mol. Also a consequence of its severe steric congestion, hexakis(3,5-di-
383:
The values given above represent C-C bond dissociation energies that are commonly encountered; occasionally, outliers may deviate drastically from this range.
628:
C ends is 28º although the C=C distance is normal 135 pm. The nearly isostructural tetraisopropylethylene also has a C=C distance of 135 pm, but its C
831:
733:
1067:
1032:
702:
617:
1060:
186:
117:
120:
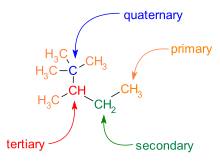
is also common in C−C skeletons. Carbon atoms in a molecule are categorized by the number of carbon neighbors they have:
177:
in which a new carbon–carbon bond is formed. They are important in the production of many human-made chemicals such as
1772:
1629:
1624:
1619:
1614:
1609:
1604:
1599:
1594:
1589:
1584:
1579:
1574:
1564:
1507:
1401:
1322:
1317:
1174:
645:
short triple bond of 115 pm has been observed for the iodonium species , due to the strongly electron-withdrawing
1777:
1665:
1367:
1337:
1327:
1307:
1295:
1263:
1228:
1196:
1164:
1159:
1119:
822:
Yu-Ran Luo and Jin-Pei Cheng "Bond
Dissociation Energies" in CRC Handbook of Chemistry and Physics, 96th Edition.
1134:
1098:
1052:
671:
661:
197:
1710:
1705:
1700:
1695:
1690:
1685:
1680:
1675:
1670:
1655:
1645:
1496:
1471:
1466:
1451:
1436:
1416:
1411:
1362:
1290:
1273:
1223:
1218:
1213:
1208:
1184:
1144:
244:
1660:
1650:
1461:
1446:
1431:
1421:
1406:
1347:
1332:
1312:
1302:
1283:
1278:
1268:
1258:
1201:
1169:
550:
205:
1139:
666:
477:
1569:
1484:
1426:
1389:
1384:
1372:
1352:
1342:
1248:
1243:
1238:
1179:
1154:
979:
970:
Blanksby, Stephen J.; Ellison, G. Barney (April 2003). "Bond
Dissociation Energies of Organic Molecules".
809:
723:
1377:
1189:
1124:
1114:
558:
62:
1456:
1441:
1253:
1233:
772:
984:
1357:
873:"Sizing the role of London dispersion in the dissociation of all-meta tert-butyl hexaphenylethane"
491:
649:
577:
484:
94:
Carbon is one of the few elements that can form long chains of its own atoms, a property called
1038:
1028:
1005:
997:
910:
892:
798:
729:
698:
201:
146:
103:
989:
938:
900:
884:
853:
788:
780:
763:
754:
692:
597:
589:
209:
174:
132:
213:
139:
776:
905:
872:
793:
758:
621:
608:-butylphenyl)ethane has a greatly elongated central bond with a length of 167 pm.
584:. It is this bond that reversibly and readily breaks at room temperature in solution:
193:
178:
125:
1766:
1729:
229:
99:
35:
109:
351:
759:"Review: Catalytic enantioselective synthesis of quaternary carbon stereocentres"
719:
641:
576:
Various extreme cases have been identified where the C-C bond is elongated. In
75:
67:
46:
57:
and is formed between one hybridized orbital from each of the carbon atoms. In
857:
95:
54:
1001:
896:
1042:
581:
410:
155:
1009:
942:
914:
802:
185:. The reverse reaction, where a carbon-carbon bond is broken, is known as
646:
403:
314:
159:
50:
1023:
Streitwieser, Andrew; Heathcock, Clayton H.; Kosower, Edward M. (1992).
784:
17:
888:
372:
334:
286:
182:
83:
1027:(4th ed.). Upper Saddle River, N.J.: Prentice Hall. p. 574.
993:
1084:
466:
461:
456:
396:
262:
79:
71:
58:
53:, one from each of the two atoms. The carbon–carbon single bond is a
39:
640:
On the opposite extreme, the central carbon–carbon single bond of
192:
Some examples of reactions which form carbon–carbon bonds are the
108:
846:
725:
Advanced
Organic Chemistry: Reactions, Mechanisms, and Structure
42:
1056:
694:
Practical
Petroleum Geochemistry for Exploration and Production
871:
Rösel, Sören; Balestrieri, Ciro; Schreiner, Peter R. (2017).
956:
1721:
931:Angewandte Chemie International Edition in English
387:Comparison of bond lengths in simple hydrocarbons
748:
746:
744:
728:(6th ed.), New York: Wiley-Interscience,
1068:
8:
1075:
1061:
1053:
1087:with other elements in the periodic table
983:
904:
792:
385:
234:
683:
580:, one C-C bond is rather long at 159.7
1104:
27:Covalent bond between two carbon atoms
832:CRC Handbook of Chemistry and Physics
7:
1746:Academic research, no widespread use
596:In the even more congested molecule
171:Carbon–carbon bond-forming reactions
25:
1025:Introduction to organic chemistry
228:The carbon-carbon single bond is
1638:
1557:
1489:
1394:
1094:
808:
620:(TDAE) is highly distorted. The
588:
490:
483:
476:
618:tetrakis(dimethylamino)ethylene
547:Structure determination method
691:Dembicki, Harry (2016-10-06).
636:Short, strong C-C triple bonds
612:Twisted, weak C-C double bonds
533:Proportion of C-C single bond
45:. The most common form is the
1:
972:Accounts of Chemical Research
187:carbon-carbon bond activation
572:Long, weak C-C single bonds
142:has three carbon neighbors.
1794:
224:Bond strengths and lengths
149:has four carbon neighbors.
1635:
1554:
1106:
1102:
1092:
858:10.1107/S0108270194009005
135:has two carbon neighbors.
49:: a bond composed of two
499:Hybridisation of carbon
245:Bond dissociation energy
206:cross-coupling reactions
128:has one carbon neighbor.
98:. This coupled with the
697:. Elsevier. p. 7.
555:microwave spectroscopy
1741:Many uses in chemistry
1736:Core organic chemistry
943:10.1002/anie.199116781
551:microwave spectroscopy
114:
113:2,2,3-trimethylpentane
61:, the orbitals are sp-
559:infrared spectroscopy
112:
672:Carbon–nitrogen bond
662:Carbon–hydrogen bond
198:Diels–Alder reaction
90:Chains and branching
78:in compounds called
70:in compounds called
785:10.1038/nature14007
777:2014Natur.516..181Q
753:Quasdorf, Kyle W.;
718:Smith, Michael B.;
602:-butylphenyl)ethane
388:
889:10.1039/c6sc02727j
667:Carbon–oxygen bond
386:
115:
32:carbon–carbon bond
1773:Organic chemistry
1760:
1759:
1716:
1715:
994:10.1021/ar020230d
937:(12): 1678–1681.
771:(7530): 181–191.
755:Overman, Larry E.
735:978-0-471-72091-1
616:The structure of
564:
563:
381:
380:
202:Grignard reaction
175:organic reactions
147:quaternary carbon
104:organic chemistry
16:(Redirected from
1785:
1778:Chemical bonding
1752:
1747:
1742:
1737:
1642:
1641:
1561:
1560:
1493:
1492:
1398:
1397:
1095:
1077:
1070:
1063:
1054:
1047:
1046:
1020:
1014:
1013:
987:
967:
961:
960:
953:
947:
946:
925:
919:
918:
908:
877:Chemical Science
868:
862:
861:
841:
835:
829:
823:
820:
814:
813:
812:
806:
796:
750:
739:
738:
715:
709:
708:
688:
632:core is planar.
592:
513:C-C bond length
494:
487:
480:
389:
235:
210:Michael reaction
133:secondary carbon
21:
1793:
1792:
1788:
1787:
1786:
1784:
1783:
1782:
1763:
1762:
1761:
1756:
1755:
1750:
1745:
1740:
1735:
1717:
1639:
1558:
1490:
1395:
1088:
1081:
1051:
1050:
1035:
1022:
1021:
1017:
985:10.1.1.616.3043
969:
968:
964:
955:
954:
950:
927:
926:
922:
870:
869:
865:
843:
842:
838:
830:
826:
821:
817:
807:
752:
751:
742:
736:
717:
716:
712:
705:
690:
689:
685:
680:
658:
638:
631:
627:
614:
598:hexakis(3,5-di-
578:Gomberg's dimer
574:
569:
448:
444:
438:
434:
428:
424:
368:
364:
347:
331:
327:
311:
307:
303:
299:
283:
279:
275:
259:
255:
226:
214:Wittig reaction
179:pharmaceuticals
168:
140:tertiary carbon
92:
28:
23:
22:
15:
12:
11:
5:
1791:
1789:
1781:
1780:
1775:
1765:
1764:
1758:
1757:
1754:
1753:
1748:
1743:
1738:
1733:
1730:Chemical bonds
1726:
1725:
1723:
1719:
1718:
1714:
1713:
1708:
1703:
1698:
1693:
1688:
1683:
1678:
1673:
1668:
1663:
1658:
1653:
1648:
1643:
1636:
1633:
1632:
1627:
1622:
1617:
1612:
1607:
1602:
1597:
1592:
1587:
1582:
1577:
1572:
1567:
1562:
1555:
1552:
1551:
1547:
1546:
1543:
1540:
1537:
1534:
1531:
1528:
1525:
1522:
1519:
1516:
1513:
1510:
1505:
1502:
1499:
1494:
1487:
1482:
1478:
1477:
1474:
1469:
1464:
1459:
1454:
1449:
1444:
1439:
1434:
1429:
1424:
1419:
1414:
1409:
1404:
1399:
1392:
1387:
1381:
1380:
1375:
1370:
1365:
1360:
1355:
1350:
1345:
1340:
1335:
1330:
1325:
1320:
1315:
1310:
1305:
1300:
1298:
1293:
1287:
1286:
1281:
1276:
1271:
1266:
1261:
1256:
1251:
1246:
1241:
1236:
1231:
1226:
1221:
1216:
1211:
1206:
1204:
1199:
1193:
1192:
1187:
1182:
1177:
1172:
1167:
1162:
1157:
1151:
1150:
1147:
1142:
1137:
1132:
1127:
1122:
1117:
1111:
1110:
1107:
1105:
1103:
1101:
1093:
1090:
1089:
1082:
1080:
1079:
1072:
1065:
1057:
1049:
1048:
1034:978-0139738500
1033:
1015:
978:(4): 255–263.
962:
957:"NIST Webbook"
948:
920:
883:(1): 405–410.
863:
852:(3): 489–491.
836:
834:, 88th edition
824:
815:
740:
734:
710:
703:
682:
681:
679:
676:
675:
674:
669:
664:
657:
654:
637:
634:
629:
625:
622:dihedral angle
613:
610:
594:
593:
573:
570:
568:
565:
562:
561:
556:
553:
548:
544:
543:
540:
537:
534:
530:
529:
524:
519:
514:
510:
509:
506:
503:
500:
496:
495:
488:
481:
474:
470:
469:
464:
459:
454:
450:
449:
446:
442:
439:
436:
432:
429:
426:
422:
419:
415:
414:
407:
400:
393:
379:
378:
375:
370:
366:
362:
358:
357:
354:
349:
345:
341:
340:
337:
332:
329:
325:
321:
320:
317:
312:
309:
305:
301:
297:
293:
292:
289:
284:
281:
277:
273:
269:
268:
265:
260:
257:
253:
249:
248:
242:
239:
225:
222:
194:aldol reaction
167:
164:
151:
150:
143:
136:
129:
126:primary carbon
91:
88:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1790:
1779:
1776:
1774:
1771:
1770:
1768:
1749:
1744:
1739:
1734:
1731:
1728:
1727:
1724:
1720:
1712:
1709:
1707:
1704:
1702:
1699:
1697:
1694:
1692:
1689:
1687:
1684:
1682:
1679:
1677:
1674:
1672:
1669:
1667:
1664:
1662:
1659:
1657:
1654:
1652:
1649:
1647:
1644:
1637:
1634:
1631:
1628:
1626:
1623:
1621:
1618:
1616:
1613:
1611:
1608:
1606:
1603:
1601:
1598:
1596:
1593:
1591:
1588:
1586:
1583:
1581:
1578:
1576:
1573:
1571:
1568:
1566:
1563:
1556:
1553:
1549:
1548:
1544:
1541:
1538:
1535:
1532:
1529:
1526:
1523:
1520:
1517:
1514:
1511:
1509:
1506:
1503:
1500:
1498:
1495:
1488:
1486:
1483:
1480:
1479:
1475:
1473:
1470:
1468:
1465:
1463:
1460:
1458:
1455:
1453:
1450:
1448:
1445:
1443:
1440:
1438:
1435:
1433:
1430:
1428:
1425:
1423:
1420:
1418:
1415:
1413:
1410:
1408:
1405:
1403:
1400:
1393:
1391:
1388:
1386:
1383:
1382:
1379:
1376:
1374:
1371:
1369:
1366:
1364:
1361:
1359:
1356:
1354:
1351:
1349:
1346:
1344:
1341:
1339:
1336:
1334:
1331:
1329:
1326:
1324:
1321:
1319:
1316:
1314:
1311:
1309:
1306:
1304:
1301:
1299:
1297:
1294:
1292:
1289:
1288:
1285:
1282:
1280:
1277:
1275:
1272:
1270:
1267:
1265:
1262:
1260:
1257:
1255:
1252:
1250:
1247:
1245:
1242:
1240:
1237:
1235:
1232:
1230:
1227:
1225:
1222:
1220:
1217:
1215:
1212:
1210:
1207:
1205:
1203:
1200:
1198:
1195:
1194:
1191:
1188:
1186:
1183:
1181:
1178:
1176:
1173:
1171:
1168:
1166:
1163:
1161:
1158:
1156:
1153:
1152:
1148:
1146:
1143:
1141:
1138:
1136:
1133:
1131:
1128:
1126:
1123:
1121:
1118:
1116:
1113:
1112:
1108:
1100:
1097:
1096:
1091:
1086:
1083:Compounds of
1078:
1073:
1071:
1066:
1064:
1059:
1058:
1055:
1044:
1040:
1036:
1030:
1026:
1019:
1016:
1011:
1007:
1003:
999:
995:
991:
986:
981:
977:
973:
966:
963:
958:
952:
949:
944:
940:
936:
932:
924:
921:
916:
912:
907:
902:
898:
894:
890:
886:
882:
878:
874:
867:
864:
859:
855:
851:
847:
840:
837:
833:
828:
825:
819:
816:
811:
804:
800:
795:
790:
786:
782:
778:
774:
770:
766:
765:
760:
756:
749:
747:
745:
741:
737:
731:
727:
726:
721:
714:
711:
706:
704:9780128033517
700:
696:
695:
687:
684:
677:
673:
670:
668:
665:
663:
660:
659:
655:
653:
651:
648:
643:
635:
633:
624:for the two N
623:
619:
611:
609:
607:
603:
601:
591:
587:
586:
585:
583:
579:
571:
567:Extreme cases
566:
560:
557:
554:
552:
549:
546:
545:
541:
538:
535:
532:
531:
528:
525:
523:
520:
518:
515:
512:
511:
507:
504:
501:
498:
497:
493:
489:
486:
482:
479:
475:
472:
471:
468:
465:
463:
460:
458:
455:
452:
451:
440:
430:
420:
417:
416:
413:
412:
408:
406:
405:
401:
399:
398:
394:
391:
390:
384:
376:
374:
371:
360:
359:
355:
353:
350:
343:
342:
338:
336:
333:
323:
322:
318:
316:
313:
295:
294:
290:
288:
285:
271:
270:
266:
264:
261:
251:
250:
246:
243:
240:
237:
236:
233:
231:
223:
221:
217:
215:
211:
207:
203:
199:
195:
190:
188:
184:
180:
176:
172:
165:
163:
161:
157:
148:
144:
141:
137:
134:
130:
127:
123:
122:
121:
119:
111:
107:
105:
101:
97:
89:
87:
85:
81:
77:
73:
69:
64:
60:
56:
52:
48:
44:
41:
37:
36:covalent bond
33:
19:
1751:Bond unknown
1129:
1024:
1018:
975:
971:
965:
951:
934:
930:
923:
880:
876:
866:
849:
845:
839:
827:
818:
768:
762:
724:
720:March, Jerry
713:
693:
686:
639:
615:
605:
599:
595:
575:
526:
521:
516:
409:
402:
395:
382:
352:acetonitrile
227:
218:
191:
170:
169:
152:
116:
93:
76:triple bonds
68:double bonds
38:between two
31:
29:
642:diacetylene
247:(kcal/mol)
47:single bond
1767:Categories
678:References
582:picometers
473:Structure
96:catenation
63:hybridized
55:sigma bond
1732:to carbon
1002:0001-4842
980:CiteSeerX
897:2041-6520
767:(paper).
411:Acetylene
392:Molecule
241:Molecule
238:C–C bond
166:Synthesis
156:cortisone
118:Branching
51:electrons
1043:52836313
1010:12693923
915:28451185
803:25503231
757:(2014).
722:(2007),
656:See also
647:iodonium
527:120.3 pm
522:133.9 pm
517:153.5 pm
418:Formula
404:Ethylene
315:biphenyl
212:and the
183:plastics
160:morphine
100:strength
18:C-C bond
1550:
906:5365070
794:4697831
773:Bibcode
373:ethanol
335:acetone
328:C(O)−CH
287:toluene
84:pi bond
80:alkynes
72:alkenes
1722:Legend
1085:carbon
1041:
1031:
1008:
1000:
982:
913:
903:
895:
801:
791:
764:Nature
732:
701:
650:moiety
467:alkyne
462:alkene
457:alkane
453:Class
397:Ethane
263:ethane
230:weaker
208:, the
59:ethane
40:carbon
536:100%
43:atoms
34:is a
1039:OCLC
1029:ISBN
1006:PMID
998:ISSN
911:PMID
893:ISSN
799:PMID
730:ISBN
699:ISBN
606:tert
600:tert
542:78%
539:87%
356:136
348:−CN
319:114
291:102
181:and
173:are
158:and
1696:CEs
1691:CCf
1686:CBk
1681:CCm
1676:CAm
1671:CPu
1666:CNp
1656:CPa
1651:CTh
1630:CYb
1625:CTm
1620:CEr
1615:CHo
1610:CDy
1605:CTb
1600:CGd
1595:CEu
1590:CSm
1585:CPm
1580:CNd
1575:CPr
1570:CCe
1565:CLa
1545:Og
1542:Ts
1539:Lv
1536:Mc
1533:Fl
1530:Nh
1527:Cn
1524:Rg
1521:Ds
1518:Mt
1515:Hs
1512:Bh
1508:CSg
1504:Db
1501:Rf
1485:CRa
1481:Fr
1476:Rn
1472:CAt
1467:CPo
1462:CBi
1457:CPb
1452:CTl
1447:CHg
1442:CAu
1437:CPt
1432:CIr
1427:COs
1422:CRe
1412:CTa
1407:CHf
1402:CLu
1390:CBa
1385:CCs
1378:CXe
1368:CTe
1363:CSb
1358:CSn
1353:CIn
1348:CCd
1343:CAg
1338:CPd
1333:CRh
1328:CRu
1323:CTc
1318:CMo
1313:CNb
1308:CZr
1296:CSr
1291:CRb
1284:CKr
1279:CBr
1274:CSe
1269:CAs
1264:CGe
1259:CGa
1254:CZn
1249:CCu
1244:CNi
1239:CCo
1234:CFe
1229:CMn
1224:CCr
1214:CTi
1209:CSc
1202:CCa
1190:CAr
1185:CCl
1170:CSi
1165:CAl
1160:CMg
1155:CNa
1149:Ne
1120:CBe
1115:CLi
1109:He
990:doi
939:doi
901:PMC
885:doi
854:doi
789:PMC
781:doi
769:516
508:sp
505:sp
502:sp
377:88
369:OH
365:−CH
339:84
280:−CH
267:90
256:−CH
74:or
1769::
1711:No
1706:Md
1701:Fm
1661:CU
1646:Ac
1497:Lr
1417:CW
1373:CI
1303:CY
1219:CV
1197:CK
1180:CS
1175:CP
1145:CF
1140:CO
1135:CN
1130:CC
1125:CB
1099:CH
1037:.
1004:.
996:.
988:.
976:36
974:.
935:30
933:.
909:.
899:.
891:.
879:.
875:.
850:51
848:.
797:.
787:.
779:.
761:.
743:^
652:.
361:CH
344:CH
324:CH
304:−C
252:CH
216:.
204:,
200:,
196:,
189:.
162:.
145:A
138:A
131:A
124:A
106:.
86:.
30:A
1076:e
1069:t
1062:v
1045:.
1012:.
992::
959:.
945:.
941::
917:.
887::
881:8
860:.
856::
805:.
783::
775::
707:.
630:6
626:2
447:2
445:H
443:2
441:C
437:4
435:H
433:2
431:C
427:6
425:H
423:2
421:C
367:2
363:3
346:3
330:3
326:3
310:5
308:H
306:6
302:5
300:H
298:6
296:C
282:3
278:5
276:H
274:6
272:C
258:3
254:3
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.