543:
520:
844:
42:
2316:
1641:
1605:
1402:
Ozierański K, Balsam P, Kapłon-Cieślicka A, Tymińska A, Kowalik R, Grabowski M, et al. (February 2019). "Comparative
Analysis of Long-Term Outcomes of Torasemide and Furosemide in Heart Failure Patients in Heart Failure Registries of the European Society of Cardiology".
810:, particularly in people with poor thiamine intake, and this depletion may worsen heart failure. It is therefore reasonable to either also give thiamine supplements or to check blood thiamine levels in those being treated with chronic loop diuretics.
787:. Compared with furosemide, torasemide is associated with a lower risk of rehospitalization for heart failure and an improvement in New York Heart Association class of heart failure. In heart failure it may be safer and more effective than
916:), which is the drug naming system coordinated by the USAN Council, which is co-sponsored by the American Medical Association (AMA), the United States Pharmacopeial Convention (USP), and the American Pharmacists Association (APhA).
1180:
Täger T, Fröhlich H, Seiz M, Katus HA, Frankenstein L (March 2019). "READY: relative efficacy of loop diuretics in patients with chronic systolic heart failure-a systematic review and network meta-analysis of randomised trials".
1224:
Miles JA, Hanumanthu BK, Patel K, Chen M, Siegel RM, Kokkinidis DG (June 2019). "Torsemide versus furosemide and intermediate-term outcomes in patients with heart failure: an updated meta-analysis".
924:
In May 2024, the US FDA conditionally approved the first torsemide animal medication for dogs. UpCard-CA1 (torsemide oral solution) was conditionally approved for use with concurrent therapy with
1137:
Abraham B, Megaly M, Sous M, Fransawyalkomos M, Saad M, Fraser R, et al. (January 2020). "Meta-Analysis
Comparing Torsemide Versus Furosemide in Patients With Heart Failure".
173:
673:
81:
629:
615:
1586:
2356:
2270:
1744:
1038:
1011:
756:
1676:
1996:
905:
891:
649:
1456:
Dunn CJ, Fitton A, Brogden RN (January 1995). "Torasemide. An update of its pharmacological properties and therapeutic efficacy".
2094:
2256:
2173:
2142:
2123:
294:
203:
113:
657:
InChI=1S/C16H20N4O3S/c1-11(2)18-16(21)20-24(22,23)15-10-17-8-7-14(15)19-13-6-4-5-12(3)9-13/h4-11H,1-3H3,(H,17,19)(H2,18,20,21)
1090:
869:
720:
Common side effects include headache, increased urination, diarrhea, cough, and dizziness. Other side effects may include
2306:
1713:
419:
2287:
1267:
Wargo KA, Banta WM (November 2009). "A comprehensive review of the loop diuretics: should furosemide be first line?".
865:
499:
854:
132:
1982:
913:
68:
873:
858:
2346:
1817:
1054:
909:
2361:
2128:
1909:
1821:
1622:
368:
2336:
2275:
1669:
941:
143:
538:
2341:
1986:
1905:
1698:
246:
2024:
1740:
791:. Long-term outcomes with torasemide may be better than with furosemide in patients with heart failure.
359:
488:
763:. In 2021, it was the 180th most commonly prescribed medication in the United States, with more than 2
2054:
1541:
269:
1870:
1840:
784:
725:
515:
314:
150:
2216:
1895:
1875:
1662:
1481:
1438:
1335:
1292:
1249:
1206:
1162:
760:
2351:
2210:
2184:
1561:
1522:
1473:
1430:
1384:
1327:
1284:
1241:
1198:
1154:
1034:
1028:
1007:
218:
54:
468:
2086:
1885:
1855:
1553:
1512:
1465:
1420:
1412:
1374:
1366:
1319:
1276:
1233:
1190:
1146:
1062:
555:
228:
428:
408:
323:
2320:
2132:
2090:
1717:
937:
256:
236:
1353:
Buggey J, Mentz RJ, Pitt B, Eisenstein EL, Anstrom KJ, Velazquez EJ, et al. (2015).
542:
519:
2281:
2179:
2039:
1850:
1768:
1703:
1379:
1354:
929:
713:. It is a less preferred treatment for high blood pressure. It is taken by mouth or by
706:
2330:
2221:
1918:
1860:
1798:
1736:
1726:
1645:
1609:
1557:
1485:
1469:
1166:
933:
780:
741:
733:
710:
702:
531:
166:
105:
1442:
1339:
1296:
1253:
1210:
2064:
2033:
1963:
1890:
1689:
776:
721:
698:
186:
181:
154:
1542:"Torasemide: An Update of its Pharmacological Properties and Therapeutic Efficacy"
1150:
1082:
348:
1237:
1112:
974:
91:
2240:
2235:
2229:
2206:
2014:
1933:
1923:
1778:
843:
800:
755:
Torasemide was patented in 1974 and came into medical use in 1993. It is on the
729:
714:
99:
1194:
2158:
2153:
2148:
2070:
2049:
1958:
1943:
1938:
1880:
1793:
1783:
1763:
1753:
1517:
1500:
1416:
1370:
925:
823:
788:
591:
399:
161:
1323:
1059:
World Health
Organization model list of essential medicines: 22nd list (2021)
2163:
2044:
2004:
1953:
1948:
1928:
1865:
1788:
1758:
827:
737:
278:
85:
1526:
1434:
1388:
1331:
1288:
1245:
1202:
1158:
33:
17:
1565:
1477:
940:(fluid build-up in lungs) in dogs with congestive heart failure caused by
2225:
2108:
2103:
2009:
1968:
1845:
1835:
1826:
1813:
1773:
1685:
819:
807:
379:
127:
1067:
904:
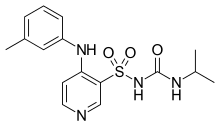
Torasemide is the recommended name of the drug (rINN) according to the (
388:
2202:
749:
334:
41:
1425:
1280:
1310:
Roush GC, Kaur R, Ernst ME (2014). "Diuretics: a review and update".
745:
479:
818:
Compared with other loop diuretics, torasemide has a more prolonged
448:
1654:
614:
605:
459:
261:
1644:
This article incorporates text from this source, which is in the
1608:
This article incorporates text from this source, which is in the
1587:"FDA Conditionally Approves First Torsemide Animal Drug for Dogs"
1355:"A reappraisal of loop diuretic choice in heart failure patients"
2113:
439:
1658:
912:. Torsemide is the official name of the drug according to the (
212:
122:
837:
1006:(76 ed.). Pharmaceutical Press. 2018. pp. 227–228.
806:
Loop diuretics, including torsemide, may decrease total body
504:
1501:"Thiamine Therapy for Heart Failure: a Promise or Fiction?"
757:
680:
908:), which is the drug naming system coordinated by the
2304:
2249:
2195:
2122:
2085:
2023:
1995:
1981:
1904:
1812:
1735:
1712:
1697:
603:
590:
554:
549:
530:
498:
478:
458:
438:
418:
398:
378:
367:
358:
333:
313:
285:
268:
255:
245:
235:
227:
202:
197:
172:
160:
142:
112:
98:
80:
63:
53:
48:
347:
981:. American Society of Health-System Pharmacists
322:
1670:
934:angiotensin-converting enzyme (ACE) inhibitor
744:. It works by decreasing the reabsorption of
8:
1499:Kattoor AJ, Goel A, Mehta JL (August 2018).
131:
32:
872:. Unsourced material may be challenged and
1992:
1709:
1677:
1663:
1655:
969:
967:
965:
963:
961:
959:
957:
541:
518:
407:
40:
1516:
1424:
1378:
1066:
892:Learn how and when to remove this message
697:, is a diuretic medication used to treat
637:CC(C)NC(=O)NS(=O)(=O)c1cnccc1Nc2cc(C)ccc2
427:
1004:British national formulary : BNF 76
2311:
998:
996:
975:"Torsemide Monograph for Professionals"
953:
654:
634:
514:
387:
299:
104:
1033:. John Wiley & Sons. p. 458.
532:
31:
1061:. Geneva: World Health Organization.
487:
467:
90:
72:
7:
1093:from the original on 15 January 2024
870:adding citations to reliable sources
185:
1113:"Torsemide - Drug Usage Statistics"
447:
338:
799:No evidence of torasemide-induced
25:
1627:U.S. Food and Drug Administration
1591:U.S. Food and Drug Administration
803:has been demonstrated in humans.
2314:
1639:
1603:
1558:10.2165/00003495-199549010-00009
1470:10.2165/00003495-199549010-00009
1405:Cardiovascular Drugs and Therapy
842:
822:effect than equipotent doses of
783:. It is sometimes used to treat
572:
566:
2257:Hydrochlorothiazide/triamterene
2124:Vasopressin receptor inhibitors
1027:Fischer J, Ganellin CR (2006).
662:Key:NGBFQHCMQULJNZ-UHFFFAOYSA-N
1312:J. Cardiovasc. Pharmacol. Ther
584:
578:
560:
1:
2357:Carbonic anhydrase inhibitors
1226:J Cardiovasc Med (Hagerstown)
1151:10.1016/j.amjcard.2019.09.039
1030:Analogue-based Drug Discovery
1629:(Press release). 14 May 2024
1593:(Press release). 10 May 2024
1540:Christopher J. Dunn (1995).
1238:10.2459/JCM.0000000000000794
736:. Use is not recommended in
1623:"FDA Roundup: May 14, 2024"
2378:
1195:10.1007/s10741-019-09771-8
1071:. WHO/MHP/HPS/EML/2021.02.
550:Chemical and physical data
305:--4-pyridine-3-sulfonamide
2265:
1518:10.1007/s10557-018-6808-8
1417:10.1007/s10557-018-6843-5
1371:10.1016/j.ahj.2014.12.009
1055:World Health Organization
910:World Health Organization
826:and relatively decreased
670:
645:
625:
290:
39:
1324:10.1177/1074248413497257
2025:Aldosterone antagonists
942:myxomatous mitral valve
767:million prescriptions.
759:. It is available as a
251:Highly bound (>99%).
936:for the management of
67:Torsemide, Torsemide (
59:Demadex, Tortas, Wator
1505:Cardiovasc Drugs Ther
1083:"The Top 300 of 2021"
715:injection into a vein
2250:Combination products
2055:Potassium canrenoate
866:improve this section
775:It is used to treat
2217:mercurial diuretics
1871:Hydrochlorothiazide
1841:Bendroflumethiazide
785:high blood pressure
726:low blood potassium
36:
27:Diuretic medication
2292:Never to phase III
1896:Trichlormethiazide
1876:Hydroflumethiazide
761:generic medication
728:. Torasemide is a
2302:
2301:
2185:Lithium carbonate
2087:Osmotic diuretics
2081:
2080:
1983:Potassium-sparing
1977:
1976:
1281:10.1345/aph.1M177
1040:978-3-527-60749-5
1013:978-0-85711-338-2
902:
901:
894:
688:
687:
616:Interactive image
500:CompTox Dashboard
216:
125:
75:
16:(Redirected from
2369:
2347:4-Aminopyridines
2319:
2318:
2317:
2310:
1993:
1886:Methyclothiazide
1856:Cyclopenthiazide
1710:
1679:
1672:
1665:
1656:
1649:
1643:
1642:
1638:
1636:
1634:
1619:
1613:
1607:
1606:
1602:
1600:
1598:
1583:
1577:
1576:
1574:
1572:
1537:
1531:
1530:
1520:
1496:
1490:
1489:
1453:
1447:
1446:
1428:
1399:
1393:
1392:
1382:
1350:
1344:
1343:
1307:
1301:
1300:
1269:Ann Pharmacother
1264:
1258:
1257:
1221:
1215:
1214:
1177:
1171:
1170:
1134:
1128:
1127:
1125:
1123:
1109:
1103:
1102:
1100:
1098:
1079:
1073:
1072:
1070:
1051:
1045:
1044:
1024:
1018:
1017:
1000:
991:
990:
988:
986:
971:
944:disease (MMVD).
897:
890:
886:
883:
877:
846:
838:
766:
693:, also known as
684:
683:
676:
618:
598:
586:
580:
574:
568:
562:
545:
534:
523:
522:
508:
506:
491:
471:
451:
431:
411:
391:
371:
351:
341:
340:
326:
273:
214:
211:
189:
135:
124:
121:
108:
94:
74:
71:
44:
37:
35:
21:
2377:
2376:
2372:
2371:
2370:
2368:
2367:
2366:
2362:Dog medications
2327:
2326:
2325:
2315:
2313:
2305:
2303:
2298:
2297:
2282:Clinical trials
2261:
2245:
2191:
2126:
2118:
2077:
2019:
1973:
1900:
1827:Calcium-sparing
1825:
1808:
1731:
1701:
1693:
1683:
1653:
1652:
1640:
1632:
1630:
1621:
1620:
1616:
1604:
1596:
1594:
1585:
1584:
1580:
1570:
1568:
1539:
1538:
1534:
1498:
1497:
1493:
1455:
1454:
1450:
1401:
1400:
1396:
1352:
1351:
1347:
1309:
1308:
1304:
1275:(11): 1836–47.
1266:
1265:
1261:
1223:
1222:
1218:
1179:
1178:
1174:
1136:
1135:
1131:
1121:
1119:
1111:
1110:
1106:
1096:
1094:
1081:
1080:
1076:
1053:
1052:
1048:
1041:
1026:
1025:
1021:
1014:
1002:
1001:
994:
984:
982:
973:
972:
955:
950:
938:pulmonary edema
922:
920:Veterinary uses
898:
887:
881:
878:
863:
847:
836:
816:
797:
795:Adverse effects
773:
764:
679:
677:
674:(what is this?)
671:
666:
663:
658:
653:
652:
641:
638:
633:
632:
621:
596:
583:
577:
571:
565:
526:
502:
494:
474:
454:
434:
414:
394:
374:
354:
337:
329:
309:
306:
298:
297:
271:
247:Protein binding
237:Bioavailability
229:Pharmacokinetic
223:
193:
145:
138:
28:
23:
22:
15:
12:
11:
5:
2375:
2373:
2365:
2364:
2359:
2354:
2349:
2344:
2339:
2337:Loop diuretics
2329:
2328:
2324:
2323:
2300:
2299:
2296:
2295:
2294:
2293:
2290:
2279:
2273:
2267:
2266:
2263:
2262:
2260:
2259:
2253:
2251:
2247:
2246:
2244:
2243:
2238:
2233:
2213:
2199:
2197:
2193:
2192:
2190:
2189:
2188:
2187:
2182:
2180:Demeclocycline
2169:
2168:
2167:
2166:
2161:
2156:
2151:
2138:
2136:
2120:
2119:
2117:
2116:
2111:
2106:
2100:
2098:
2083:
2082:
2079:
2078:
2076:
2075:
2074:
2073:
2060:
2059:
2058:
2057:
2052:
2047:
2042:
2040:Spironolactone
2029:
2027:
2021:
2020:
2018:
2017:
2012:
2007:
2001:
1999:
1990:
1979:
1978:
1975:
1974:
1972:
1971:
1966:
1961:
1956:
1951:
1946:
1941:
1936:
1931:
1926:
1921:
1915:
1913:
1906:Thiazide-likes
1902:
1901:
1899:
1898:
1893:
1888:
1883:
1878:
1873:
1868:
1863:
1858:
1853:
1851:Chlorothiazide
1848:
1843:
1838:
1832:
1830:
1810:
1809:
1807:
1806:
1801:
1796:
1791:
1786:
1781:
1776:
1771:
1769:Etacrynic acid
1766:
1761:
1756:
1750:
1748:
1733:
1732:
1730:
1729:
1723:
1721:
1707:
1704:etacrynic acid
1695:
1694:
1684:
1682:
1681:
1674:
1667:
1659:
1651:
1650:
1614:
1578:
1552:(1): 121–142.
1532:
1511:(4): 313–317.
1491:
1448:
1394:
1345:
1302:
1259:
1232:(6): 379–388.
1216:
1189:(4): 461–472.
1183:Heart Fail Rev
1172:
1139:Am. J. Cardiol
1129:
1104:
1074:
1046:
1039:
1019:
1012:
992:
952:
951:
949:
946:
930:spironolactone
921:
918:
900:
899:
850:
848:
841:
835:
832:
815:
812:
796:
793:
777:fluid overload
772:
769:
707:kidney disease
699:fluid overload
686:
685:
668:
667:
665:
664:
661:
659:
656:
648:
647:
646:
643:
642:
640:
639:
636:
628:
627:
626:
623:
622:
620:
619:
611:
609:
601:
600:
594:
588:
587:
581:
575:
569:
563:
558:
552:
551:
547:
546:
536:
528:
527:
525:
524:
511:
509:
496:
495:
493:
492:
484:
482:
476:
475:
473:
472:
464:
462:
456:
455:
453:
452:
444:
442:
436:
435:
433:
432:
424:
422:
416:
415:
413:
412:
404:
402:
396:
395:
393:
392:
384:
382:
376:
375:
373:
372:
364:
362:
356:
355:
353:
352:
344:
342:
331:
330:
328:
327:
319:
317:
311:
310:
308:
307:
301:
293:
292:
291:
288:
287:
283:
282:
275:
266:
265:
259:
253:
252:
249:
243:
242:
239:
233:
232:
225:
224:
222:
221:
208:
206:
200:
199:
195:
194:
192:
191:
178:
176:
170:
169:
164:
158:
157:
148:
146:administration
140:
139:
137:
136:
118:
116:
110:
109:
102:
96:
95:
88:
78:
77:
65:
61:
60:
57:
51:
50:
46:
45:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2374:
2363:
2360:
2358:
2355:
2353:
2350:
2348:
2345:
2343:
2342:Sulfonylureas
2340:
2338:
2335:
2334:
2332:
2322:
2312:
2308:
2291:
2289:
2286:
2285:
2283:
2280:
2277:
2274:
2272:
2269:
2268:
2264:
2258:
2255:
2254:
2252:
2248:
2242:
2239:
2237:
2234:
2231:
2227:
2223:
2222:Chlormerodrin
2219:
2218:
2214:
2212:
2208:
2204:
2201:
2200:
2198:
2194:
2186:
2183:
2181:
2178:
2177:
2176:
2175:
2171:
2170:
2165:
2162:
2160:
2157:
2155:
2152:
2150:
2147:
2146:
2145:
2144:
2140:
2139:
2137:
2134:
2130:
2125:
2121:
2115:
2112:
2110:
2107:
2105:
2102:
2101:
2099:
2096:
2092:
2088:
2084:
2072:
2069:
2068:
2067:
2066:
2062:
2061:
2056:
2053:
2051:
2048:
2046:
2043:
2041:
2038:
2037:
2036:
2035:
2034:Spirolactones
2031:
2030:
2028:
2026:
2022:
2016:
2013:
2011:
2008:
2006:
2003:
2002:
2000:
1998:
1994:
1991:
1988:
1984:
1980:
1970:
1967:
1965:
1962:
1960:
1957:
1955:
1952:
1950:
1947:
1945:
1942:
1940:
1937:
1935:
1932:
1930:
1927:
1925:
1922:
1920:
1919:Chlortalidone
1917:
1916:
1914:
1911:
1907:
1903:
1897:
1894:
1892:
1889:
1887:
1884:
1882:
1879:
1877:
1874:
1872:
1869:
1867:
1864:
1862:
1861:Cyclothiazide
1859:
1857:
1854:
1852:
1849:
1847:
1844:
1842:
1839:
1837:
1834:
1833:
1831:
1828:
1823:
1819:
1815:
1811:
1805:
1802:
1800:
1799:Tienilic acid
1797:
1795:
1792:
1790:
1787:
1785:
1782:
1780:
1777:
1775:
1772:
1770:
1767:
1765:
1762:
1760:
1757:
1755:
1752:
1751:
1749:
1746:
1742:
1738:
1734:
1728:
1727:Acetazolamide
1725:
1724:
1722:
1719:
1715:
1714:CA inhibitors
1711:
1708:
1705:
1700:
1696:
1691:
1687:
1680:
1675:
1673:
1668:
1666:
1661:
1660:
1657:
1647:
1646:public domain
1628:
1624:
1618:
1615:
1611:
1610:public domain
1592:
1588:
1582:
1579:
1567:
1563:
1559:
1555:
1551:
1547:
1543:
1536:
1533:
1528:
1524:
1519:
1514:
1510:
1506:
1502:
1495:
1492:
1487:
1483:
1479:
1475:
1471:
1467:
1464:(1): 121–42.
1463:
1459:
1452:
1449:
1444:
1440:
1436:
1432:
1427:
1422:
1418:
1414:
1410:
1406:
1398:
1395:
1390:
1386:
1381:
1376:
1372:
1368:
1365:(3): 323–33.
1364:
1360:
1356:
1349:
1346:
1341:
1337:
1333:
1329:
1325:
1321:
1317:
1313:
1306:
1303:
1298:
1294:
1290:
1286:
1282:
1278:
1274:
1270:
1263:
1260:
1255:
1251:
1247:
1243:
1239:
1235:
1231:
1227:
1220:
1217:
1212:
1208:
1204:
1200:
1196:
1192:
1188:
1184:
1176:
1173:
1168:
1164:
1160:
1156:
1152:
1148:
1144:
1140:
1133:
1130:
1118:
1114:
1108:
1105:
1092:
1088:
1084:
1078:
1075:
1069:
1064:
1060:
1056:
1050:
1047:
1042:
1036:
1032:
1031:
1023:
1020:
1015:
1009:
1005:
999:
997:
993:
980:
976:
970:
968:
966:
964:
962:
960:
958:
954:
947:
945:
943:
939:
935:
931:
927:
919:
917:
915:
911:
907:
896:
893:
885:
882:February 2024
875:
871:
867:
861:
860:
856:
851:This section
849:
845:
840:
839:
833:
831:
829:
825:
821:
813:
811:
809:
804:
802:
794:
792:
790:
786:
782:
781:heart failure
778:
770:
768:
762:
758:
753:
751:
747:
743:
742:breastfeeding
739:
735:
734:loop diuretic
731:
727:
723:
718:
716:
712:
711:liver disease
708:
704:
703:heart failure
700:
696:
692:
682:
675:
669:
660:
655:
651:
644:
635:
631:
624:
617:
613:
612:
610:
607:
602:
595:
593:
589:
559:
557:
553:
548:
544:
540:
537:
535:
533:ECHA InfoCard
529:
521:
517:
516:DTXSID2023690
513:
512:
510:
501:
497:
490:
486:
485:
483:
481:
477:
470:
466:
465:
463:
461:
457:
450:
446:
445:
443:
441:
437:
430:
426:
425:
423:
421:
417:
410:
406:
405:
403:
401:
397:
390:
386:
385:
383:
381:
377:
370:
366:
365:
363:
361:
357:
350:
346:
345:
343:
336:
332:
325:
321:
320:
318:
316:
312:
304:
300:
296:
289:
284:
280:
276:
274:
267:
263:
260:
258:
254:
250:
248:
244:
240:
238:
234:
230:
226:
220:
210:
209:
207:
205:
201:
196:
188:
183:
180:
179:
177:
175:
171:
168:
167:Loop diuretic
165:
163:
159:
156:
152:
149:
147:
141:
134:
129:
120:
119:
117:
115:
111:
107:
103:
101:
97:
93:
89:
87:
83:
79:
70:
66:
62:
58:
56:
52:
49:Clinical data
47:
43:
38:
30:
19:
2215:
2172:
2141:
2065:Nonsteroidal
2063:
2032:
1997:ESC blockers
1964:Quinethazone
1891:Polythiazide
1803:
1699:Sulfonamides
1631:. Retrieved
1626:
1617:
1595:. Retrieved
1590:
1581:
1569:. Retrieved
1549:
1545:
1535:
1508:
1504:
1494:
1461:
1457:
1451:
1411:(1): 77–86.
1408:
1404:
1397:
1362:
1358:
1348:
1315:
1311:
1305:
1272:
1268:
1262:
1229:
1225:
1219:
1186:
1182:
1175:
1145:(1): 92–99.
1142:
1138:
1132:
1120:. Retrieved
1116:
1107:
1095:. Retrieved
1086:
1077:
1068:10665/345533
1058:
1049:
1029:
1022:
1003:
983:. Retrieved
978:
923:
903:
888:
879:
864:Please help
852:
817:
805:
798:
774:
771:Medical uses
754:
722:hearing loss
719:
694:
690:
689:
678:
672:
302:
270:Elimination
204:Legal status
198:Legal status
155:intraveneous
114:License data
29:
2278:from market
2241:Cicletanine
2236:Theobromine
2230:Meralluride
2207:Isopropanol
2015:Triamterene
1934:Clorexolone
1924:Clofenamide
1908:(primarily
1779:Indacrinone
1359:Am. Heart J
1318:(1): 5–13.
801:ototoxicity
730:sulfonamide
599: g·mol
539:100.164.924
286:Identifiers
281:: 7-8 hours
277:3.5 hours;
100:MedlinePlus
64:Other names
55:Trade names
18:C16H20N4O3S
2331:Categories
2159:Satavaptan
2154:Mozavaptan
2149:Conivaptan
2071:Finerenone
2050:Eplerenone
1959:Metolazone
1944:Indapamide
1939:Fenquizone
1881:Mebutizide
1804:Torasemide
1794:Piretanide
1784:Muzolimine
1764:Bumetanide
1754:Furosemide
1426:2183/22704
1122:14 January
1097:14 January
948:References
926:pimobendan
824:furosemide
789:furosemide
691:Torasemide
604:3D model (
592:Molar mass
489:ChEMBL1148
469:CHEBI:9637
429:W31X2H97FB
400:ChemSpider
360:IUPHAR/BPS
324:56211-40-6
315:CAS Number
295:IUPAC name
257:Metabolism
162:Drug class
34:Torasemide
2288:Phase III
2276:Withdrawn
2164:Tolvaptan
2045:Canrenone
2005:Amiloride
1954:Meticrane
1949:Mefruside
1929:Clopamide
1866:Epitizide
1814:Thiazides
1789:Ozolinone
1759:Azosemide
1686:Diuretics
1486:261404564
1167:207937875
979:Drugs.com
932:, and an
853:does not
828:potassium
814:Chemistry
738:pregnancy
695:torsemide
279:Cirrhosis
272:half-life
144:Routes of
133:Torsemide
92:Monograph
86:Drugs.com
2352:Anilines
2321:Medicine
2226:Mersalyl
2109:Glycerol
2104:Mannitol
2010:Benzamil
1969:Xipamide
1846:Butizide
1836:Altizide
1774:Etozolin
1527:30022355
1443:58014640
1435:30649675
1389:25728721
1340:21204143
1332:24243991
1297:43339236
1289:19843838
1254:96436158
1246:30950982
1211:77394851
1203:30874955
1159:31699358
1117:ClinCalc
1091:Archived
1087:ClinCalc
1057:(2021).
985:18 March
820:diuretic
808:thiamine
681:(verify)
380:DrugBank
174:ATC code
151:By mouth
128:DailyMed
2203:Ethanol
2143:Vaptans
1741:Na-K-Cl
1571:8 March
1566:7705212
1478:7705212
1380:4346710
874:removed
859:sources
779:due to
750:kidneys
748:by the
701:due to
556:Formula
389:DB00214
335:PubChem
190:)
184: (
182:C03CA04
130::
106:a601212
2307:Portal
2271:WHO-EM
2174:Others
1633:15 May
1597:15 May
1564:
1525:
1484:
1476:
1441:
1433:
1387:
1377:
1338:
1330:
1295:
1287:
1252:
1244:
1209:
1201:
1165:
1157:
1037:
1010:
830:loss.
765:
746:sodium
709:, and
630:SMILES
597:348.42
480:ChEMBL
449:D00382
241:80-90%
219:℞-only
217:
126:
2196:Other
1818:Na-Cl
1702:(and
1546:Drugs
1482:S2CID
1458:Drugs
1439:S2CID
1336:S2CID
1293:S2CID
1250:S2CID
1207:S2CID
1163:S2CID
834:Names
650:InChI
606:JSmol
460:ChEBI
409:38123
349:41781
264:(80%)
262:Liver
2211:2M2B
2131:and
2114:Urea
1985:(at
1737:Loop
1716:(at
1635:2024
1599:2024
1573:2024
1562:PMID
1523:PMID
1474:PMID
1431:PMID
1385:PMID
1328:PMID
1285:PMID
1242:PMID
1199:PMID
1155:PMID
1124:2024
1099:2024
1035:ISBN
1008:ISBN
987:2019
914:USAN
857:any
855:cite
732:and
724:and
440:KEGG
420:UNII
369:7312
231:data
82:AHFS
69:USAN
2129:DCT
1910:DCT
1822:DCT
1820:at
1743:at
1690:C03
1554:doi
1513:doi
1466:doi
1421:hdl
1413:doi
1375:PMC
1367:doi
1363:169
1320:doi
1277:doi
1234:doi
1191:doi
1147:doi
1143:125
1063:hdl
906:INN
868:by
740:or
505:EPA
339:CID
187:WHO
2333::
2284::
2228:,
2224:,
2209:,
2205:,
2133:CD
2095:DL
2093:,
2091:PT
1987:CD
1745:AL
1718:PT
1625:.
1589:.
1560:.
1550:49
1548:.
1544:.
1521:.
1509:32
1507:.
1503:.
1480:.
1472:.
1462:49
1460:.
1437:.
1429:.
1419:.
1409:33
1407:.
1383:.
1373:.
1361:.
1357:.
1334:.
1326:.
1316:19
1314:.
1291:.
1283:.
1273:43
1271:.
1248:.
1240:.
1230:20
1228:.
1205:.
1197:.
1187:24
1185:.
1161:.
1153:.
1141:.
1115:.
1089:.
1085:.
995:^
977:.
956:^
928:,
752:.
717:.
705:,
570:20
564:16
213:US
153:,
123:US
73:US
2309::
2232:)
2220:(
2135:)
2127:(
2097:)
2089:(
1989:)
1912:)
1829:)
1824:,
1816:(
1747:)
1739:(
1720:)
1706:)
1692:)
1688:(
1678:e
1671:t
1664:v
1648:.
1637:.
1612:.
1601:.
1575:.
1556::
1529:.
1515::
1488:.
1468::
1445:.
1423::
1415::
1391:.
1369::
1342:.
1322::
1299:.
1279::
1256:.
1236::
1213:.
1193::
1169:.
1149::
1126:.
1101:.
1065::
1043:.
1016:.
989:.
895:)
889:(
884:)
880:(
876:.
862:.
608:)
585:S
582:3
579:O
576:4
573:N
567:H
561:C
507:)
503:(
303:N
215::
84:/
76:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.