1094:. Seed treatment allows for a long lasting immunity to insects damaging the crops. The use of nitenpyram has been shown to be highly effective in protecting crops, as it is generally less toxic for non-target organisms, while killing off crop-destroying insects. While usage is still common, unlike other neonicotinoids, the global market share for nitenpyram seems to decrease based on product sale data from 2003, 2005, 2007 and 2009. The reason for this is not yet fully understood, as other first generation neonicotinoids do not seem to follow the same trend, and nitenpyram is known to be less toxic to non-target organisms as compared to the compounds of the same generation.
341:
228:
876:
493:
45:
892:
844:
1521:
595:
1053:
directly affected by nitenpyram, only after they come out. Administering nitenpyram might have to be repeated or continued until the pest infestation has subsided. The half life of nitenpyram is around eight hours. Thus, 24 hours after treatment roughly 100% of the adult fleas were killed. Between 24 hours and 48 hours the
734:, remains among the most controversial matters on the topic of neonicotinoids. This is primarily due to the lack of concrete systematic work. However, studies have been done on binding phenomena between neonicotinoids and proteins, serving as an indicator to its likely behavior in human physiological conditions.
813:
enzymes in humans could generate some metabolites with greater toxicity than the parent compound, certified to cause tumors in combination with nitrates and induce genetic damage. A precautionary approach to anything understudied would be advised, until the biotransformation is better and its effects
673:
Due to its use as an insecticide and treatment of non-food producing animals, it was not deemed necessary to research the human toxicology during its main use, and, as such, not much is known about the details of nitenpyram's effects on humans. Looking at rat experiments however, the lethal amount of
908:. It has been shown that this type of modification can substantially increase the affinity of nitenpyram to bind to the insect nACh receptor, allowing for more directed and ecologically friendly pest control. Changes to these compounds could also help circumvent the growing resistance in nitenpyram.
1074:
Being one of the first generation neonicotinoids, nitenpyram has seen extensible commercial use since its introduction, including pest control in agriculture. While the development of newer generation nicotinoids has caused a decrease in its use, a
Worldwide Integrated Assessment (WIA) report still
792:, rapidly distributes from the intravascular space to the peripheral tissues and organs, like the kidney, liver and lungs, proceeding biotransformation. Vets and pet owners have reported the effect of nitenpyram on flea-infested pets starting within 30 minutes after administering the neonicotinoid.
760:
Although nitenpyram is an agonist of nicotine for the nicotinic acetylcholine receptor, it has a much lower affinity for the nicotine acetylcholine receptor in mammals. For most insects nitenpyram is a very lethal compound. Nitenpyram will bind irreversibly to the nicotinic acetylcholine receptors,
964:
Ecologic effects of nitenpyram on bee populations is under controversy, as contradicting studies show the presence of nitenpyram in honey bees and their honey, while others do not detect nitenpyram at all. This, however, may be due to the decrease in usage of nitenpyram, as the global market share
798:
Nitenpyram in mice metabolizes into nitenpyram-COOH, nitenpyram-deschloropyridine, desmethyl-nitenpyram, nitenpyram-CN, and nitenpyram-deschloropyridine derivatives. The nitenpyram metabolites have not been through in-depth study. However, these metabolites can undergo oxidation reactions like the
1052:
can be detected and the first fleas dislodge from the pet host. A study showed that six hours after application the infestation of fleas on decreased by 96.7% for dogs and 95.2% for cats. The adult fleas present on the hosts are severely interrupted, hence, egg production is reduced. Eggs are not
1066:
reported side effects are hyperactivity, panting, lethargy, vomiting, fever, decreased appetite, nervousness, diarrhea, difficulty breathing, salivation, incoordination, seizures, pupil dilation, increased heart rate, trembling and nervousness. In other studies no adverse effects were observed.
1065:
One observed side effect is itchiness, suspected to be from the fleas dislodging. In the five hours after the treatment it was observed that cats were grooming themselves more, i.e. scratching, biting, licking, and twitching. This will stop when the fleas have either flagged or have died. Other
1047:
Nitenpyram tablets, brand name
Capstar, are used to treat flea infestations in cats and dogs. After oral administration of the tablet the drug is readily and quickly absorbed into the blood. If a flea bites the animal it will ingest with the blood the nitenpyram. The effect of nitenpyram can be
1020:
The Oxford
University chemical safety data documents an LD50 toxicology test on rats, both male and female, where doses are recorded as 1680 mg and 1575 mg per kg body weight respectively. As such, the overdose limits for humans and animals are quite high, reaching into grams, and the
1029:
In the hope to understand neonicotinoid degradation in various types of water, an interesting find was made. In testing ground water, surface water and finished drinking water, researchers found degradation of nitenpyram was occurring primarily in the drinking water, which was attributed to
903:
Being a first generation neonicotinoid, nitenpyram has been subject to a variety of modifications to its original structure, to either increase the effectiveness or specificity of the compound. One such variation is on the configuration of the reactive group/pharmacophore, from
761:
paralysing those exposed to the compound. Despite lower affinity levels, mammals can still get a nicotine poisoning response from too much neonicotinoids, hence it is of importance to provide the appropriate dose for a flea-infested pet and it's always best to consult a vet.
1097:
However, the decrease of use could possibly be explained through the formation of resistance in various insect species. In a study conducted on nine commonly used nicotinoids, nitenpyram was found to have the greatest increase in resistance of the group within
657:
Known under the codename TI 304 during field testing starting in 1989, the compound's first documented commercial use was in 1995 under the name "Bestguard" as an agricultural insecticide. Later, nitenpyram was expanded for use as a flea treatment by the
713:
pharmacophore which is known to be the main reaction site in the binding of the compound to the nACh receptor, though the specificity of the reaction is not yet fully understood for neonicotinoids in general. Due to its polar groups, nitenpyram is quite
1075:
judged it as an ecologically viable treatment in pest control projects such as
Integrated Pest management (IPM). This is due to its lower toxicity and high uptake in plants in relation to soil as opposed to other commercially used neonicotinoids.
1658:
Hong, Xiangsheng; Zhao, Xu; Tian, Xue; Li, Jiasu; Zha, Jinmiao (2018). "Changes of hematological and biochemical parameters revealed genotoxicity and immunotoxicity of neonicotinoids on
Chinese rare minnows ( Gobiocypris rarus )".
501:
473:
1124:. Other non-target organisms, such as earthworms, are also reported to be negatively affected by nitenpyram. Plants themselves do not seem to have a negative response, as they do not possess nicotine nACh receptors.
1579:
Codling, Garry; Naggar, Yahya Al; Giesy, John P.; Robertson, Albert J. (2018-03-01). "Neonicotinoid insecticides in pollen, honey and adult bees in colonies of the
European honey bee (Apis mellifera L.) in Egypt".
826:
compound of this reaction is 2-chloro-5-chloromethylpyridine, which is also used in the preparation of other neonicotinoids such as imidacloprid. The reaction of this compound undergoes three reaction steps.
1535:
Shao, Xusheng; Lu, Haiyan; Bao, Haibo; Xu, Xiaoyong; Liu, Zewen; Li, Zhong (July 2011). "The mode of action of a nitroconjugated neonicotinoid and the effects of target site mutation Y151S on its potency".
1034:
of the compound. Some of these degradation products are thought to have toxic properties in non-target organisms, though the actual toxicities are not known. Nitenpyram is also degraded under the effect of
670:
chemical company. Nitenpyram continues to be used commercially, though data from market surveys indicate a significant decrease in the global usage compared to other insecticides or neonicotinoids.
1931:
Furlan, Lorenzo; Pozzebon, Alberto; Duso, Carlo; Simon-Delso, Noa; Sánchez-Bayo, Francisco; Marchand, Patrice A.; Codato, Filippo; Bijleveld van
Lexmond, Maarten; Bonmatin, Jean-Marc (2018-02-25).
1482:
Schulz-Jander, Daniel A; Casida, John E (2002). "Imidacloprid insecticide metabolism: human cytochrome P450 isozymes differ in selectivity for imidazolidine oxidation versus nitroimine reduction".
784:
The literature on the biotransformation of nitenpyram has been scarce. However, some studies have been conducted. Toxicokinetic studies have shown that human intestinal caco-2 cell line can absorb
685:
Neonicotinoids, in general, have a low degradation rate when used for agricultural purposes, which allows for long-lasting protection of the crops against plant-sucking insects and indirectly the
2054:
Sabatino, Leonardo; Scordino, Monica; Pantò, Valentina; Chiappara, Elena; Traulo, Pasqualino; Gagliano, Giacomo (2013). "Survey of neonicotinoids and fipronil in corn seeds for agriculture".
654:
leading to paralysis and death. Nitenpyram is highly selective towards the variation of the nACHr which insects possess, and has seen extensive use in targeted, insecticide applications.
1021:
compound is seen as safe for daily use for animals. Human consumption is not recommended, though no side effects of indirect exposure (such as eating treated plants) are known to occur.
1995:
Pisa, Lennard; Goulson, Dave; Yang, En-Cheng; Gibbons, David; Sánchez-Bayo, Francisco; Mitchell, Edward; Aebi, Alexandre; van der Sluijs, Jeroen; MacQuarrie, Chris J. K. (2017-11-09).
1631:
Iwasa, Takao; Motoyama, Naoki; Ambrose, John T.; Roe, R.Michael (2004). "Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera".
1190:
Pisa, Lennard; Goulson, Dave; Yang, En-Cheng; Gibbons, David; Sánchez-Bayo, Francisco; Mitchell, Edward; Aebi, Alexandre; Sluijs, Jeroen van der; MacQuarrie, Chris J. K. (2017).
564:
1001:), nitenpyram was shown to not have much genotoxic effects or adversely affect the immune system, either through short or chronic exposure in comparison to the other compounds.
1694:
Yan, Saihong; Wang, Jinhua; Zhu, Lusheng; Chen, Aimei; Wang, Jun (2015). "Toxic effects of nitenpyram on antioxidant enzyme system and DNA in zebrafish (Danio rerio) livers".
1366:
Ding, Fei; Peng, Wei (2015). "Biological assessment of neonicotinoids imidacloprid and its major metabolites for potentially human health using globular proteins as a model".
768:, have not been through in-depth toxicological investigations. Similarly genotoxicity effects remain ambiguous. 6-chloronicotinic acid, according to a research group, is non-
608:
133:
726:
Though neonicotinoids are the largest group of insecticides used in today's agricultural world and prevalent in veterinary treatments, toxicity in general, e.g.,
941:
is a common earthworm, which is partly responsible for the natural aeration of soil, including agricultural soil. In a 14-day exposure period, the
Toxicity in
380:
1300:
Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L. P.; Bonmatin, J. M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D. W.; Giorio, C. (2015-01-01).
1269:
741:-related chemical (neonicotinoid), has an effect on the nicotinic acetylcholine receptors and, for this reason, is considered similar to nicotine (
1864:
Dobson, P.; Tinembart, O.; Fisch, R. D.; Junquera, P. (2000-12-16). "Efficacy of nitenpyram as a systemic flea adulticide in dogs and cats".
1163:
1451:
949:
activity and damage to the epidermal cells and gut cells. This, however, was significantly less toxic than similar insecticides such as
886:
is added and reacts with the intermediate, replacing the pharmacophore chloride group, obtaining nitenpyram as the final end product.
1519:
CN patent 102816112], 曾挺, 陈华, 陈共华, 潘光飞, 浙江禾本科技有限公司, "Method for preparing pesticide nitenpyram", published 2012-09-13
355:
1408:
Casida, John E. (2018-01-07). "Neonicotinoids and Other Insect
Nicotinic Receptor Competitive Modulators: Progress and Prospects".
968:
Nitenpyram is also commonly used in the elimination of and protection from mosquitoes. Specifically, the toxicity of nitenpyram on
643:
1933:"An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 3: alternatives to systemic insecticides"
543:
1997:"An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems"
1192:"An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems"
750:
1116:
Due to its use on pollen carrying plants, nitenpyram has been linked to a decrease in population of pollinators such as
663:
529:
298:
319:
1729:
Noestheden, Matthew; Roberts, Simon; Hao, Chunyan (2016-07-15). "Nitenpyram degradation in finished drinking water".
492:
928:
746:
615:
1244:
630:
is a chemical frequently used as an insecticide in agriculture and veterinary medicine. The compound is an insect
235:
753:
nervous systems, present on the muscle cells where the cells from the nervous systems and the muscle cells form
2131:
2126:
905:
2146:
1102:, a common agricultural pest, between 2011 and 2012. A substantial increase of resistance was also found in
1054:
1009:
697:
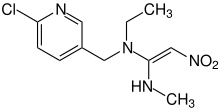
Nitenpyram ( (E)-N-(6-Chloro-3-pyridylmethyl)- N-ethyl-N'-methyl-2-nitrovinylidenediamine) is an open-chain
223:
970:
789:
765:
666:
approval for non-food producing animals in
October 2000. The current producer of nitenpyram itself is the
639:
185:
44:
2141:
1039:, suggesting that exposure to the sun will also degrade the compound into various degradation products.
875:
859:
851:
823:
702:
515:
485:
891:
843:
1738:
667:
57:
1302:"Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites"
539:
364:
InChI=1S/C11H15ClN4O2/c1-3-15(11(13-2)8-16(17)18)7-9-4-5-10(12)14-6-9/h4-6,8,13H,3,7H2,1-2H3/b11-8+
336:
99:
1826:
Wismer, Tina; Means, Charlotte (March 2012). "Toxicology of newer insecticides in small animals".
1458:
2136:
2087:
1613:
1169:
145:
1245:"ChemSpider | Data Source Details | Oxford University Chemical Safety Data (No longer updated)"
2079:
2071:
2036:
2018:
1972:
1954:
1881:
1873:
1843:
1808:
1762:
1754:
1711:
1676:
1605:
1597:
1561:
1553:
1499:
1433:
1425:
1383:
1339:
1321:
1223:
1159:
1099:
993:
807:
group. 6-chloronicotinic acid can make hydrogen bonds with the hydrogen atom of amino groups.
731:
1012:(ROS) were severely affected, causing oxidative DNA damage increasing with chronic exposure.
205:
2063:
2026:
2008:
1962:
1944:
1835:
1798:
1746:
1703:
1668:
1640:
1589:
1545:
1491:
1417:
1375:
1329:
1313:
1213:
1203:
1151:
757:. Variations in nicotinic-acetylcholine-receptor-binding affinity persists between species.
698:
647:
403:
109:
307:
927:. Nitenpyram specifically was found to have the lowest toxicity, making it useful in IPM (
855:
810:
804:
686:
575:
2109:
1048:
observed half an hour after the administration. At this time a high concentration in the
945:
of nitenpyram on e.fetida was found to be 4.34 mg/kg soil, showing an inhibition of
340:
227:
1787:"Efficacy and longevity of nitenpyram against adult cat fleas (Siphonaptera: Pulicidae)"
1742:
165:
2031:
1996:
1967:
1932:
1421:
1334:
1301:
1218:
1191:
1104:
1091:
997:) as a general fish model. Of the neonicotinoids tested (imidacloprid, nitenpyram, and
936:
586:
1495:
934:
In 2015, researchers conducted a study on the toxicity of nitenpyram on the earthworm
788:
at a very high rate of efficiency. The compound completely absorbs (>92%) from the
2120:
1379:
867:
706:
635:
458:
216:
1517:
1173:
1057:
is highly decreased and after 72 hours no effect could be shown anymore in studies.
551:
287:
2091:
1617:
1049:
958:
950:
923:
785:
769:
727:
679:
570:
535:
2067:
1707:
1672:
1644:
991:
In a study a 60-day chronic toxicity test was conducted on Chinese rare minnows (
1785:
Rust, MK; Waggoner, MM; Hinkle, NC; Stansfield, D; Barnett, S (September 2003).
1087:
1036:
998:
954:
883:
31:
1839:
1803:
1786:
1549:
1148:
Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor | SpringerLink
1004:
In a similar study, nitenpyram was shown to have adverse effects on the DNA of
2013:
1949:
1593:
1317:
1208:
1155:
1031:
831:
715:
710:
631:
430:
196:
2075:
2022:
1958:
1877:
1758:
1601:
1557:
1429:
1325:
1121:
1117:
1005:
961:, making nitenpyram a viable substitute for many other neonicotinoids used.
946:
2083:
2040:
1976:
1899:
1885:
1847:
1812:
1766:
1715:
1680:
1609:
1565:
1503:
1437:
1387:
1343:
1227:
1108:
or the cotton aphid, as compared to other compounds such as imidacloprid.
921:
In a 2015 study, neonicotinoids toxicity was tested on the egg parasitoid
773:
738:
659:
795:
Nitenpyram has been reported to metabolize into 6-chloronicotinic acid.
754:
742:
448:
274:
236:
17:
1086:, and can be applicated in various ways. Commonly used techniques are
1750:
1079:
675:
651:
176:
585:
Except where otherwise noted, data are given for materials in their
521:
262:
1083:
800:
156:
132:
122:
975:
942:
253:
709:, the reactive group of the molecule. Nitenpyram possesses a
547:
324:
30:"Capstar" redirects here. For the broadcasting company, see
1078:
Nitenpyram has been used on many commercial crops, such as
2001:
Environmental Science and Pollution Research International
1937:
Environmental Science and Pollution Research International
1828:
Veterinary Clinics of North America: Small Animal Practice
662:
company under the trade name "Capstar", with a subsequent
866:-ethyl-2-chloro-5-pyridylmethyl amine with an additional
745:). Nicotinic acetylcholine receptors are involved in the
830:
First step, 2-chloro-5-chloromethylpyridine reacts with
822:
Nitenpyram is synthesized in a multistage reaction. The
701:
neonicotinoid. Nitenpyram consists of a chloronicotinyl
682:
will die with only micro or nanograms of the substance.
2056:
Food Additives & Contaminants. Part B, Surveillance
603:
1368:
Journal of Photochemistry and Photobiology B: Biology
705:
common to all first generation neonicotinoids and a
674:
nitenpyram is quite high (on the order of grams) in
978:of the compound was found to be 0.493 ug/ml.
764:Nitenpyram itself and its metabolites, apart from
646:(nACHr) causing a stop of the flow of ions in the
638:which works by blocking neural signaling of the
286:
974:or the southern house mosquito was tested. The
108:
834:on its phase boundary acquiring the molecule
8:
1780:
1778:
1776:
1306:Environmental Science and Pollution Research
1196:Environmental Science and Pollution Research
642:. It does so by binding irreversibly to the
2113:in the Pesticide Properties DataBase (PPDB)
718:, with an extremely high water solubility.
574:
890:
874:
842:
339:
226:
204:
36:
2030:
2012:
1966:
1948:
1802:
1731:Rapid Communications in Mass Spectrometry
1538:Insect Biochemistry and Molecular Biology
1333:
1217:
1207:
306:
1133:
838:-ethyl-2-chloro-5-pyridylmethyl amine.
385:
360:
335:
1990:
1988:
1986:
1696:Ecotoxicology and Environmental Safety
1185:
1183:
1008:. Enzymes inhibiting the formation of
772:and is not considered a developmental
217:
1926:
1924:
1859:
1857:
1295:
1293:
1291:
1289:
463:82 °C (180 °F; 355 K)
367:Key: CFRPSFYHXJZSBI-DHZHZOJOSA-N
184:
164:
7:
1403:
1401:
1399:
1397:
1361:
1359:
1357:
1355:
1353:
1239:
1237:
1141:
1139:
1137:
814:are better studied and understood.
388:ClC1=CC=C(C=N1)CN(\C(=C\(=O))\NC)CC
277:
261:
1422:10.1146/annurev-ento-020117-043042
1146:Yamamoto, I.; Casida, J.E (1999).
906:cis (E) to trans (Z) configuration
850:Synthesis can then proceed with a
78:′-methyl-2-nitroethene-1,1-diamine
25:
1380:10.1016/j.jphotobiol.2015.03.010
644:nicotinic acetylcholine receptor
593:
491:
43:
1900:"CAPSTAR Novartis (nitenpyram)"
589:(at 25 °C , 100 kPa).
1452:"Rx_Info_Sheets/rx_nitenpyram"
965:has been steadily decreasing.
854:(step 2), adding the solvents
443:Pale yellow crystalline solid
1:
1791:Journal of Medical Entomology
1496:10.1016/s0378-4274(02)00068-1
2068:10.1080/19393210.2012.717969
1708:10.1016/j.ecoenv.2015.06.030
1673:10.1016/j.envpol.2017.12.036
1645:10.1016/j.cropro.2003.08.018
862:will yield the intermediate
1410:Annual Review of Entomology
689:these insects might carry.
2163:
1840:10.1016/j.cvsm.2011.12.004
1804:10.1603/0022-2585-40.5.678
1550:10.1016/j.ibmb.2011.04.005
929:integrated pest management
634:belonging to the class of
435:270.72 g/mol
29:
2014:10.1007/s11356-017-0341-3
1950:10.1007/s11356-017-1052-5
1594:10.1007/s10646-017-1876-2
1318:10.1007/s11356-014-3470-y
1209:10.1007/s11356-017-0341-3
1156:10.1007/978-4-431-67933-2
1070:Agricultural applications
737:Nitenpyram, a synthetic,
583:
557:
472:
467:
396:
376:
351:
92:
84:
56:
51:
42:
1274:pubchem.ncbi.nlm.nih.gov
530:Precautionary statements
1661:Environmental Pollution
1043:Veterinary applications
1010:reactive oxygen species
971:Culex quinquefasciatus
790:gastrointestinal tract
766:6-chloronicotinic acid
640:central nervous system
1866:The Veterinary Record
860:trichloronitromethane
852:condensation reaction
648:postsynaptic membrane
678:in general, whereas
58:Preferred IUPAC name
2007:(10): 11749–11797.
1943:(10): 11798–11820.
1907:datasheets.scbt.com
1743:2016RCMS...30.1653N
1202:(10): 11749–11797.
868:nitroethylene group
722:Mechanism of action
146:Beilstein Reference
39:
1484:Toxicology Letters
1249:www.chemspider.com
1100:brown planthoppers
703:heterocyclic group
616:Infobox references
37:
1737:(13): 1653–1661.
1165:978-4-431-68011-6
994:Gobiocypris rarus
882:In the last step
732:biotransformation
624:Chemical compound
622:
621:
516:Hazard statements
320:CompTox Dashboard
134:Interactive image
16:(Redirected from
2154:
2096:
2095:
2051:
2045:
2044:
2034:
2016:
1992:
1981:
1980:
1970:
1952:
1928:
1919:
1918:
1916:
1914:
1904:
1896:
1890:
1889:
1861:
1852:
1851:
1823:
1817:
1816:
1806:
1782:
1771:
1770:
1751:10.1002/rcm.7581
1726:
1720:
1719:
1691:
1685:
1684:
1655:
1649:
1648:
1628:
1622:
1621:
1576:
1570:
1569:
1532:
1526:
1525:
1524:
1520:
1514:
1508:
1507:
1479:
1473:
1472:
1470:
1469:
1463:
1457:. Archived from
1456:
1448:
1442:
1441:
1405:
1392:
1391:
1363:
1348:
1347:
1337:
1297:
1284:
1283:
1281:
1280:
1265:
1259:
1258:
1256:
1255:
1241:
1232:
1231:
1221:
1211:
1187:
1178:
1177:
1143:
1120:, wild bees and
894:
878:
846:
606:
600:
597:
596:
578:
553:
549:
545:
541:
537:
523:
495:
404:Chemical formula
344:
343:
328:
326:
310:
290:
279:
265:
238:
230:
219:
208:
188:
168:
136:
112:
47:
40:
21:
2162:
2161:
2157:
2156:
2155:
2153:
2152:
2151:
2132:Cat medications
2127:Dog medications
2117:
2116:
2105:
2100:
2099:
2053:
2052:
2048:
1994:
1993:
1984:
1930:
1929:
1922:
1912:
1910:
1902:
1898:
1897:
1893:
1872:(25): 709–713.
1863:
1862:
1855:
1825:
1824:
1820:
1784:
1783:
1774:
1728:
1727:
1723:
1693:
1692:
1688:
1657:
1656:
1652:
1633:Crop Protection
1630:
1629:
1625:
1578:
1577:
1573:
1534:
1533:
1529:
1522:
1516:
1515:
1511:
1481:
1480:
1476:
1467:
1465:
1461:
1454:
1450:
1449:
1445:
1407:
1406:
1395:
1365:
1364:
1351:
1299:
1298:
1287:
1278:
1276:
1267:
1266:
1262:
1253:
1251:
1243:
1242:
1235:
1189:
1188:
1181:
1166:
1145:
1144:
1135:
1130:
1114:
1072:
1063:
1045:
1027:
1018:
989:
987:Aquatic animals
984:
919:
914:
901:
856:dichloromethane
820:
811:Cytochrome P450
782:
751:parasympathetic
724:
695:
625:
618:
613:
612:
611: ?)
602:
598:
594:
590:
567:
532:
518:
504:
488:
424:
420:
416:
412:
406:
392:
389:
384:
383:
372:
369:
368:
365:
359:
358:
347:
329:
322:
313:
293:
280:
268:
248:
211:
191:
171:
148:
139:
126:
115:
102:
88:
80:
79:
35:
28:
23:
22:
15:
12:
11:
5:
2160:
2158:
2150:
2149:
2147:Neonicotinoids
2144:
2139:
2134:
2129:
2119:
2118:
2115:
2114:
2104:
2103:External links
2101:
2098:
2097:
2046:
1982:
1920:
1909:. 2 April 2014
1891:
1853:
1834:(2): 335–347.
1818:
1772:
1721:
1686:
1650:
1639:(5): 371–378.
1623:
1588:(2): 122–131.
1571:
1544:(7): 440–445.
1527:
1509:
1474:
1443:
1416:(1): 125–144.
1393:
1349:
1285:
1260:
1233:
1179:
1164:
1132:
1131:
1129:
1126:
1113:
1110:
1105:Aphis gossypii
1092:seed treatment
1071:
1068:
1062:
1059:
1044:
1041:
1026:
1023:
1017:
1014:
988:
985:
983:
980:
918:
915:
913:
910:
900:
897:
896:
895:
880:
879:
848:
847:
819:
816:
781:
778:
723:
720:
694:
691:
687:plant diseases
636:neonicotinoids
623:
620:
619:
614:
592:
591:
587:standard state
584:
581:
580:
568:
563:
560:
559:
555:
554:
533:
528:
525:
524:
519:
514:
511:
510:
505:
500:
497:
496:
489:
484:
481:
480:
470:
469:
465:
464:
461:
455:
454:
451:
445:
444:
441:
437:
436:
433:
427:
426:
422:
418:
414:
410:
407:
402:
399:
398:
394:
393:
391:
390:
387:
379:
378:
377:
374:
373:
371:
370:
366:
363:
362:
354:
353:
352:
349:
348:
346:
345:
332:
330:
318:
315:
314:
312:
311:
303:
301:
295:
294:
292:
291:
283:
281:
273:
270:
269:
267:
266:
258:
256:
250:
249:
247:
246:
242:
240:
232:
231:
221:
213:
212:
210:
209:
201:
199:
193:
192:
190:
189:
181:
179:
173:
172:
170:
169:
161:
159:
153:
152:
149:
144:
141:
140:
138:
137:
129:
127:
120:
117:
116:
114:
113:
105:
103:
98:
95:
94:
90:
89:
86:
82:
81:
61:
60:
54:
53:
49:
48:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2159:
2148:
2145:
2143:
2140:
2138:
2135:
2133:
2130:
2128:
2125:
2124:
2122:
2112:
2111:
2107:
2106:
2102:
2093:
2089:
2085:
2081:
2077:
2073:
2069:
2065:
2061:
2057:
2050:
2047:
2042:
2038:
2033:
2028:
2024:
2020:
2015:
2010:
2006:
2002:
1998:
1991:
1989:
1987:
1983:
1978:
1974:
1969:
1964:
1960:
1956:
1951:
1946:
1942:
1938:
1934:
1927:
1925:
1921:
1908:
1901:
1895:
1892:
1887:
1883:
1879:
1875:
1871:
1867:
1860:
1858:
1854:
1849:
1845:
1841:
1837:
1833:
1829:
1822:
1819:
1814:
1810:
1805:
1800:
1797:(5): 678–81.
1796:
1792:
1788:
1781:
1779:
1777:
1773:
1768:
1764:
1760:
1756:
1752:
1748:
1744:
1740:
1736:
1732:
1725:
1722:
1717:
1713:
1709:
1705:
1701:
1697:
1690:
1687:
1682:
1678:
1674:
1670:
1666:
1662:
1654:
1651:
1646:
1642:
1638:
1634:
1627:
1624:
1619:
1615:
1611:
1607:
1603:
1599:
1595:
1591:
1587:
1583:
1582:Ecotoxicology
1575:
1572:
1567:
1563:
1559:
1555:
1551:
1547:
1543:
1539:
1531:
1528:
1518:
1513:
1510:
1505:
1501:
1497:
1493:
1489:
1485:
1478:
1475:
1464:on 2015-02-26
1460:
1453:
1447:
1444:
1439:
1435:
1431:
1427:
1423:
1419:
1415:
1411:
1404:
1402:
1400:
1398:
1394:
1389:
1385:
1381:
1377:
1373:
1369:
1362:
1360:
1358:
1356:
1354:
1350:
1345:
1341:
1336:
1331:
1327:
1323:
1319:
1315:
1311:
1307:
1303:
1296:
1294:
1292:
1290:
1286:
1275:
1271:
1264:
1261:
1250:
1246:
1240:
1238:
1234:
1229:
1225:
1220:
1215:
1210:
1205:
1201:
1197:
1193:
1186:
1184:
1180:
1175:
1171:
1167:
1161:
1157:
1153:
1149:
1142:
1140:
1138:
1134:
1127:
1125:
1123:
1119:
1111:
1109:
1107:
1106:
1101:
1095:
1093:
1089:
1085:
1081:
1076:
1069:
1067:
1060:
1058:
1056:
1051:
1042:
1040:
1038:
1033:
1024:
1022:
1015:
1013:
1011:
1007:
1002:
1000:
996:
995:
986:
981:
979:
977:
973:
972:
966:
962:
960:
956:
952:
948:
944:
940:
938:
932:
931:) treatment.
930:
926:
925:
917:Invertebrates
916:
911:
909:
907:
898:
893:
889:
888:
887:
885:
877:
873:
872:
871:
869:
865:
861:
857:
853:
845:
841:
840:
839:
837:
833:
828:
825:
817:
815:
812:
808:
806:
803:group into a
802:
796:
793:
791:
787:
779:
777:
775:
771:
767:
762:
758:
756:
752:
748:
744:
740:
735:
733:
729:
721:
719:
717:
712:
708:
707:pharmacophore
704:
700:
699:chloropyridyl
692:
690:
688:
683:
681:
680:invertebrates
677:
671:
669:
665:
661:
655:
653:
649:
645:
641:
637:
633:
629:
617:
610:
605:
588:
582:
577:
572:
569:
566:
562:
561:
558:Pharmacology
556:
534:
531:
527:
526:
520:
517:
513:
512:
509:
506:
503:
499:
498:
494:
490:
487:
483:
482:
478:
476:
471:
466:
462:
460:
459:Melting point
457:
456:
452:
450:
447:
446:
442:
439:
438:
434:
432:
429:
428:
408:
405:
401:
400:
395:
386:
382:
375:
361:
357:
350:
342:
338:
337:DTXSID8041080
334:
333:
331:
321:
317:
316:
309:
305:
304:
302:
300:
297:
296:
289:
285:
284:
282:
276:
272:
271:
264:
260:
259:
257:
255:
252:
251:
244:
243:
241:
239:
234:
233:
229:
225:
222:
220:
218:ECHA InfoCard
215:
214:
207:
203:
202:
200:
198:
195:
194:
187:
183:
182:
180:
178:
175:
174:
167:
163:
162:
160:
158:
155:
154:
150:
147:
143:
142:
135:
131:
130:
128:
124:
119:
118:
111:
107:
106:
104:
101:
97:
96:
91:
83:
77:
73:
69:
65:
59:
55:
50:
46:
41:
33:
19:
2142:Nitroethenes
2108:
2062:(1): 11–16.
2059:
2055:
2049:
2004:
2000:
1940:
1936:
1911:. Retrieved
1906:
1894:
1869:
1865:
1831:
1827:
1821:
1794:
1790:
1734:
1730:
1724:
1699:
1695:
1689:
1664:
1660:
1653:
1636:
1632:
1626:
1585:
1581:
1574:
1541:
1537:
1530:
1512:
1490:(1): 65–70.
1487:
1483:
1477:
1466:. Retrieved
1459:the original
1446:
1413:
1409:
1371:
1367:
1309:
1305:
1277:. Retrieved
1273:
1270:"Nitenpyram"
1263:
1252:. Retrieved
1248:
1199:
1195:
1147:
1115:
1112:Side effects
1103:
1096:
1077:
1073:
1064:
1061:Side effects
1046:
1028:
1019:
1003:
992:
990:
969:
967:
963:
959:clothianidin
951:imidacloprid
935:
933:
924:trichogramma
922:
920:
902:
881:
863:
849:
835:
829:
821:
809:
797:
794:
786:imidacloprid
783:
770:carcinogenic
763:
759:
736:
728:genotoxicity
725:
696:
684:
672:
656:
627:
626:
507:
474:
186:ChEMBL259728
93:Identifiers
85:Other names
75:
71:
67:
63:
1667:: 862–871.
1312:(1): 5–34.
1122:butterflies
1025:Degradation
999:dinotefuran
982:Vertebrates
955:thiacloprid
899:Derivatives
884:methylamine
747:sympathetic
716:hydrophilic
565:ATCvet code
502:Signal word
453:1.4 (g/mL)
440:Appearance
397:Properties
224:100.162.838
166:CHEBI:39170
110:150824-47-8
38:Nitenpyram
32:iHeartMedia
27:Insecticide
2121:Categories
2110:Nitenpyram
1468:2018-03-21
1279:2018-03-21
1254:2018-03-21
1128:References
1118:honey bees
1032:hydrolysis
939:. E.fetida
912:Toxicology
832:ethylamine
805:carboxylic
780:Metabolism
711:nitroamine
632:neurotoxin
628:Nitenpyram
486:Pictograms
431:Molar mass
308:3A837VZ81Y
197:ChemSpider
121:3D model (
100:CAS Number
2137:Pyridines
2076:1939-3229
2023:1614-7499
1959:1614-7499
1878:0042-4900
1759:1097-0231
1702:: 54–60.
1602:0963-9292
1558:1879-0240
1430:0066-4170
1374:: 24–36.
1326:0944-1344
1268:Pubchem.
1006:Zebrafish
947:cellulase
824:precursor
818:Synthesis
693:Structure
544:P301+P312
477:labelling
245:601-735-5
237:EC Number
2084:24786619
2041:29124633
1977:29478160
1886:11140929
1848:22381183
1813:14596282
1767:27321854
1716:26202306
1681:29253827
1610:29143171
1566:21549193
1504:12084621
1438:29324040
1388:25837412
1344:25233913
1228:29124633
1174:34374399
1055:efficacy
1037:UV light
937:E.fetida
774:toxicant
755:synapses
743:agonists
739:nicotine
668:Sumitomo
660:Novartis
579:)
571:QP53BX02
468:Hazards
151:8489488
2092:6769499
2032:7921077
1968:7921064
1913:12 June
1739:Bibcode
1618:3917697
1335:4284386
1219:7921077
1088:dusting
1016:Mammals
676:mammals
652:neurons
609:what is
607: (
573: (
508:Warning
449:Density
425:
288:3034287
275:PubChem
206:2298774
87:Capstar
74:-ethyl-
18:Capstar
2090:
2082:
2074:
2039:
2029:
2021:
1975:
1965:
1957:
1884:
1876:
1846:
1811:
1765:
1757:
1714:
1679:
1616:
1608:
1600:
1564:
1556:
1523:
1502:
1436:
1428:
1386:
1342:
1332:
1324:
1226:
1216:
1172:
1162:
1080:cotton
1050:plasma
604:verify
601:
381:SMILES
263:C18511
177:ChEMBL
52:Names
2088:S2CID
1903:(PDF)
1614:S2CID
1462:(PDF)
1455:(PDF)
1170:S2CID
801:cyano
356:InChI
157:ChEBI
123:JSmol
2080:PMID
2072:ISSN
2037:PMID
2019:ISSN
1973:PMID
1955:ISSN
1915:2019
1882:PMID
1874:ISSN
1844:PMID
1809:PMID
1763:PMID
1755:ISSN
1712:PMID
1677:PMID
1606:PMID
1598:ISSN
1562:PMID
1554:ISSN
1500:PMID
1434:PMID
1426:ISSN
1384:PMID
1340:PMID
1322:ISSN
1224:PMID
1160:ISBN
1090:and
1084:corn
1082:and
976:LC50
957:and
943:LC50
858:and
749:and
730:and
552:P501
548:P330
540:P270
536:P264
522:H302
299:UNII
254:KEGG
2064:doi
2027:PMC
2009:doi
1963:PMC
1945:doi
1870:147
1836:doi
1799:doi
1747:doi
1704:doi
1700:122
1669:doi
1665:233
1641:doi
1590:doi
1546:doi
1492:doi
1488:132
1418:doi
1376:doi
1372:147
1330:PMC
1314:doi
1214:PMC
1204:doi
1152:doi
664:FDA
650:of
576:WHO
475:GHS
417:ClN
325:EPA
278:CID
2123::
2086:.
2078:.
2070:.
2058:.
2035:.
2025:.
2017:.
2005:28
2003:.
1999:.
1985:^
1971:.
1961:.
1953:.
1941:28
1939:.
1935:.
1923:^
1905:.
1880:.
1868:.
1856:^
1842:.
1832:42
1830:.
1807:.
1795:40
1793:.
1789:.
1775:^
1761:.
1753:.
1745:.
1735:30
1733:.
1710:.
1698:.
1675:.
1663:.
1637:23
1635:.
1612:.
1604:.
1596:.
1586:27
1584:.
1560:.
1552:.
1542:41
1540:.
1498:.
1486:.
1432:.
1424:.
1414:63
1412:.
1396:^
1382:.
1370:.
1352:^
1338:.
1328:.
1320:.
1310:22
1308:.
1304:.
1288:^
1272:.
1247:.
1236:^
1222:.
1212:.
1200:28
1198:.
1194:.
1182:^
1168:.
1158:.
1150:.
1136:^
953:,
870:.
776:.
550:,
546:,
542:,
538:,
479::
415:15
411:11
70:--
66:)-
2094:.
2066::
2060:6
2043:.
2011::
1979:.
1947::
1917:.
1888:.
1850:.
1838::
1815:.
1801::
1769:.
1749::
1741::
1718:.
1706::
1683:.
1671::
1647:.
1643::
1620:.
1592::
1568:.
1548::
1506:.
1494::
1471:.
1440:.
1420::
1390:.
1378::
1346:.
1316::
1282:.
1257:.
1230:.
1206::
1176:.
1154::
864:N
836:N
599:N
423:2
421:O
419:4
413:H
409:C
327:)
323:(
125:)
76:N
72:N
68:N
64:E
62:(
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.