211:
190:
161:
134:, the rate of the reaction is the fastest because the reaction has the smallest energy of activation (ΔG). The ethoxy and cyano groups are able to delocalize the radical ion in the transition state, thus stabilizing the radical center. The rate enhancement is due to the captodative effect. When R = H, the reaction has the largest energy of activation because the radical center is not stabilized by the captodative effect. The hydrogen atom is not able to delocalize the radical ion. Thus, the reaction is slow relative to the R = OCH
232:, where radicals are the chain carriers in the propagation of the process, accounted for 40 billion of the 110 billion pounds of polymers produced in the United States in 2001. Captodative olefins have a specific advantage of being responsive to solvent effects without the effect of destabilizing the radical. They have also shown to undergo their radical transformation spontaneously which allows them to be useful in polymerization mechanism elucidation and better understood through
108:
95:. Delocalizing the radical ion stabilizes the transition state structure. As a result, the energy of activation decreases, enhancing the rate of the overall reaction. According to the captodative effect, the rate of a reaction is the greatest when both the EDG and EWG are able to delocalize the radical ion in the transition state structure.
248:
204:
reactions in cases where nucleophilic olefins react inefficiently, attributed to the transition state being close to a biradical and thus stabilized. These studies have revealed a direct dependence on Δω, difference in electrophilicity, and the polar nature of the reaction. They have been used
154:
because the radical ion is not delocalized over the methyl group . Thus, the captodative does not influence the reaction rate if the radical ion is not delocalized onto both the EWG and EDG substituents. Each of these cases is illustrated below:
256:
579:"1-Acetyvinyl Acrylates: New Captodative Olefins Bearing and Internal Probe for the Evaluation of the Relative Reactivity of Captodative against Electron-Deficient Double Bond in Diels-Alders and Friedel-Crafts Reaction"
43:
and an electron-donating substituent. The name originates as the electron-withdrawing group (EWG) is sometimes called the "captor" group, whilst the electron-donating group (EDG) is the "dative" substituent.
146:, the rate of the reaction is faster relative to when R = H because methyl groups have more electron donating capability. However, the reaction rate is slower relative to when R = OCH
298:
in specific bifunctional polymers( see figure above). However no clear correlation has been developed at this time, since not all combinations of substituents and solubilities have been investigated.
240:
through the known radical mechanisms. The polymers obtained from captodatively substituted starting materials exhibit "desirable" properties such as optical activity, differences in polarity,
688:
Wood, M.; Bissiriou, S.; Lowe, C.; Windeatt, K. M. (2013). "Synthetic Use of the
Primary Kinetic Isotope Effect in Hydrogen Atom Transfer 2: Generation of Captodatively Stabilised Radicals".
17:
48:
with this substituent pattern are sometime described as captodative. Radical reactions play an integral role in several chemical reactions and are also important to the field of
221:, allowing atypical reactions to occur with isotope-labeled molecules and demonstrating that the mechanisms and transition states of these reactions have been influenced.
210:
189:
91:
Certain substituents are better at stabilizing radical centers than others. This is influenced by the substituent's ability to delocalize the radical ion in the
641:
Domingo, L.; Chamorro, E.; Pérez, P. (2008). "Understanding the
Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study".
67:. These stabilization mechanisms lead to an enhanced rate for free-radical reactions. In the figure at right, the radical is delocalized between the captor
196:
Similar effects have been discussed for other cycloadditions such as , , and for captodative ethylenes. Effects have also been reported in cases like
583:
63:
and other radical centers from reacting with the center. The substituents thermodynamically stabilize the center by delocalizing the radical ion via
233:
16:
486:
Ito, Osamu; Arito, Y.; Matsuda, M. (1988). "Captodative
Effects on Rate of Addition Reactions of Arylthiyl Radical to Disubstituted Olefins".
282:(maximum rate of weight change temperatures). Meaning although they will start to melt quicker, they will take longer to fully change phases.
98:
Ito and co-workers observed the rate of addition reactions of aryl thiol radical to disubstituted olefins. The olefins contained an EWG
236:. Furthermore, captodative ethanes are initiators with unique properties giving higher molecular weight distribution and forming block
690:
734:
558:
366:
337:
102:
group and varying EDGs and the effect of varying EDGs on the rate of the addition reactions was observed. The process studied was:
612:
Stella, L.; Boucher, J.-L. (1982). "Capto-dative
Substituent Effects. 12 - New Ketene Equivalents for Diels-Alder Cycloadditions".
465:
285:
Polymers with large captodative stabilizations starting materials can quickly “unzip” to their starting monomer upon heating.
511:
Creary, X.; Mehrisheikh-Mohammadi, M. E. (1985). "Captodative Rate
Enhancement in the Methylenecyclopropane Rearrangement".
184:
is "sluggish". Intramolecular cyclizations have also been reported to be enhanced by captodative effects, as shown below:
395:
546:
291:
polymers, with two different functional groups at every monomer unit, are commonly formed from the captodative monomers.
643:
513:
327:
180:
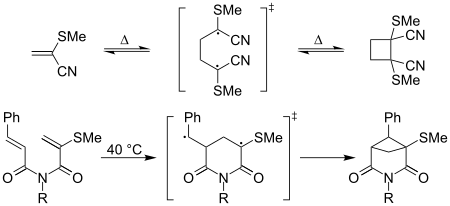
of 2-methylthioacrylonitrile occurs readily at room temperature; formation of the equivalent cyclobutane derivative of
756:
429:
356:
278:(initial decomposition) of these polymers are relatively low compared to their analogues, but have relatively higher T
229:
160:
55:
When EDGs and EWGs are near the radical center, the stability of the radical center increases. The substituents can
114:
The rate of the addition reaction was accelerated by the following EDGs in increasing order: H < CH
247:
264:
Polymers with polar substituents are known to have interesting applications including within electrical and
92:
761:
218:
64:
241:
295:
176:
reactions involving captodative radical intermediates – for example, the thermal head-to-head
614:
488:
33:
205:
because of their highly reactive, stereoselective, regioselective nature within these reactions.
29:
20:
Resonance contributors of the 2-(dimethylamino)propanenitrile free radical, adapted from Anslyn
730:
707:
670:
554:
461:
362:
333:
265:
177:
56:
699:
660:
652:
623:
592:
522:
493:
438:
404:
577:
Herrera, R.; Jimenez-Vazquez, H. A.; Delgado, F.; Soderberg, B. C. G.; Tamariz, J. (2005).
49:
201:
627:
442:
393:
Viehe, H. G.; Janousek, Z.; Merényi, R.; Stella, L. (1985). "The
Captodative Effect".
750:
181:
173:
597:
578:
288:
37:
217:
Captodative olefins in reactions also show interfering effects with the typical
197:
40:
107:
237:
711:
674:
255:
497:
303:
Captodative polymer is highly functional in chelates with certain metals.
60:
665:
526:
408:
703:
99:
68:
656:
45:
427:
Tanaka, H. (2003). "Captodative
Modification in Polymer Science".
254:
246:
72:
15:
172:
The term "captodative ethylenes" has been used in the context of
547:"Captodative Substituent Effects in Cycloaddition Reactions"
332:(Dodr. ed.). Sausalito, CA: University Science Books.
481:
479:
477:
294:
Dative groups substantially alter the solubility through
251:
Substituents on the monomer can affect solvent affinities
388:
386:
384:
382:
380:
378:
321:
319:
317:
489:
Journal of the
Chemical Society, Perkin Transactions 2
549:. In Viehe, H. G.; Janousek, Z.; Merényi, R. (eds.).
460:. San Diego, CA: Academic Press. pp. 131–172.
259:How a captodative monomer can form a polar polymer
572:
570:
729:(4th ed.). New York: Wiley-Interscience.
8:
664:
596:
584:Journal of the Brazilian Chemical Society
271:These polymers are typically transparent.
551:Substituent Effects in Radical Chemistry
540:
538:
536:
422:
420:
418:
361:. V&S Publishers. 2012. p. 51.
326:Anslyn, E. V.; Dougherty, D. A. (2006).
83:), thus stabilizing the radical center.
59:stabilize radical centers by preventing
313:
458:Advances in Physical Organic Chemistry
351:
349:
244:, thermal and mechanical stabilities.
7:
87:Substituent effect on reaction rates
456:Sustmann, R.; Korth, H.-G. (1990).
691:Organic and Biomolecular Chemistry
14:
329:Modern Physical Organic Chemistry
209:
188:
159:
106:
71:(-CN), and the dative secondary
598:10.1590/S0103-50532005000300021
358:Concise Dictionary of Chemistry
553:. Springer. pp. 361–370.
36:by a synergistic effect of an
1:
628:10.1016/S0040-4039(00)86992-0
443:10.1016/S0079-6700(03)00013-3
396:Accounts of Chemical Research
727:Principles of Polymerization
644:Journal of Organic Chemistry
514:Journal of Organic Chemistry
430:Progress in Polymer Science
230:Free-radical polymerization
225:Polymer science application
142:case. When R = CH
778:
93:transition state structure
126:. When R = OCH
260:
252:
219:kinetic isotope effect
21:
258:
250:
19:
498:10.1039/P29880000869
38:electron-withdrawing
615:Tetrahedron Letters
545:Stella, L. (1986).
527:10.1021/jo00364a009
409:10.1021/ar00113a004
118: < OCH
757:Chemical reactions
725:Odian, G. (2004).
704:10.1039/C3OB40275D
261:
253:
26:captodative effect
22:
657:10.1021/jo800572a
651:(12): 4615–4624.
521:(14): 2664–2668.
266:optical materials
168:Uses in synthesis
769:
741:
740:
722:
716:
715:
685:
679:
678:
668:
638:
632:
631:
609:
603:
602:
600:
574:
565:
564:
542:
531:
530:
508:
502:
501:
483:
472:
471:
453:
447:
446:
437:(7): 1171–1203.
424:
413:
412:
390:
373:
372:
353:
344:
343:
323:
296:Hydrogen bonding
242:solvent affinity
213:
192:
163:
110:
777:
776:
772:
771:
770:
768:
767:
766:
747:
746:
745:
744:
737:
724:
723:
719:
698:(16): 2712–23.
687:
686:
682:
640:
639:
635:
611:
610:
606:
591:(3A): 456–466.
576:
575:
568:
561:
544:
543:
534:
510:
509:
505:
485:
484:
475:
468:
455:
454:
450:
426:
425:
416:
392:
391:
376:
369:
355:
354:
347:
340:
325:
324:
315:
310:
281:
277:
227:
170:
153:
149:
145:
141:
137:
133:
129:
125:
121:
117:
89:
82:
78:
50:polymer science
12:
11:
5:
775:
773:
765:
764:
759:
749:
748:
743:
742:
735:
717:
680:
633:
622:(9): 953–956.
604:
566:
559:
532:
503:
492:(6): 869–873.
473:
466:
448:
414:
403:(5): 148–154.
374:
367:
345:
338:
312:
311:
309:
306:
305:
304:
301:
300:
299:
286:
283:
279:
275:
272:
269:
226:
223:
215:
214:
202:Friedel-Crafts
194:
193:
169:
166:
165:
164:
151:
147:
143:
139:
135:
131:
127:
123:
119:
115:
112:
111:
88:
85:
80:
76:
13:
10:
9:
6:
4:
3:
2:
774:
763:
762:Free radicals
760:
758:
755:
754:
752:
738:
736:9780471274001
732:
728:
721:
718:
713:
709:
705:
701:
697:
693:
692:
684:
681:
676:
672:
667:
662:
658:
654:
650:
646:
645:
637:
634:
629:
625:
621:
617:
616:
608:
605:
599:
594:
590:
586:
585:
580:
573:
571:
567:
562:
560:9789027723406
556:
552:
548:
541:
539:
537:
533:
528:
524:
520:
516:
515:
507:
504:
499:
495:
491:
490:
482:
480:
478:
474:
469:
463:
459:
452:
449:
444:
440:
436:
432:
431:
423:
421:
419:
415:
410:
406:
402:
398:
397:
389:
387:
385:
383:
381:
379:
375:
370:
368:9789381588628
364:
360:
359:
352:
350:
346:
341:
339:9781891389313
335:
331:
330:
322:
320:
318:
314:
307:
302:
297:
293:
292:
290:
287:
284:
273:
270:
267:
263:
262:
257:
249:
245:
243:
239:
235:
231:
224:
222:
220:
212:
208:
207:
206:
203:
199:
191:
187:
186:
185:
183:
182:acrylonitrile
179:
175:
174:cycloaddition
167:
162:
158:
157:
156:
109:
105:
104:
103:
101:
96:
94:
86:
84:
74:
70:
66:
62:
58:
53:
51:
47:
42:
39:
35:
31:
30:stabilization
27:
18:
726:
720:
695:
689:
683:
666:10533/139635
648:
642:
636:
619:
613:
607:
588:
582:
550:
518:
512:
506:
487:
457:
451:
434:
428:
400:
394:
357:
328:
289:Bifunctional
228:
216:
195:
178:dimerization
171:
113:
97:
90:
54:
25:
23:
234:NMR Studies
198:Diels-Alder
57:kinetically
41:substituent
751:Categories
467:0120335263
308:References
238:copolymers
65:resonance
61:molecules
712:23479029
675:18484771
34:radicals
100:nitrile
69:nitrile
46:Olefins
28:is the
733:
710:
673:
557:
464:
365:
336:
75:(-N(CH
274:The T
73:amine
731:ISBN
708:PMID
671:PMID
555:ISBN
462:ISBN
363:ISBN
334:ISBN
200:and
24:The
700:doi
661:hdl
653:doi
624:doi
593:doi
523:doi
494:doi
439:doi
405:doi
32:of
753::
706:.
696:11
694:.
669:.
659:.
649:73
647:.
620:22
618:.
589:16
587:.
581:.
569:^
535:^
519:51
517:.
476:^
435:28
433:.
417:^
401:18
399:.
377:^
348:^
316:^
280:dm
276:di
150:CH
138:CH
130:CH
122:CH
52:.
739:.
714:.
702::
677:.
663::
655::
630:.
626::
601:.
595::
563:.
529:.
525::
500:.
496::
470:.
445:.
441::
411:.
407::
371:.
342:.
268:.
152:3
148:2
144:3
140:3
136:2
132:3
128:2
124:3
120:2
116:3
81:2
79:)
77:3
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.