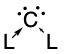192:
of two lone pairs present on the central carbon atom. Further calculations revealed the two highest-occupied molecular orbitals to be primarily localised on the central carbon atom as two lone pairs, like with the hexaphenylcarbodiphosphorane, albeit with slightly more delocalisation of the π-symmetric orbital onto the N-heterocyclic carbene carbon atoms due to their improved π-accepting properties. This is suggestive of a donor-acceptor interaction between the N-heterocyclic carbene ligands and a formally carbon (0) atom with two free lone pairs.
416:
are comparable to those of carbodiphosphoranes and exhibit variability depending on the identity of the N-heterocyclic carbene substituent with a range of values from 155.3 kcal mol (649.8 kJ mol) to 168.4 kcal mol (704.6 kJ mol). This is due to the increased delocalisation of the π-symmetric lone pair over the carbon atoms of the N-heterocyclic carbene substituents which increases the dependence of the second proton affinity on the identity of the substituent.
1857:
517:
389:
349:
321:
247:
191:
at 1.358 Å (compared with 1.308 Å for allene), but with a significantly bent bond angle of 131.8° (compared to 180° for a standard linear allene). X-ray crystallography confirmed the structure with an experimentally-measured C=C bond length of 1.348 Å and a C-C-C bond angle of 131.8° indicative
173:
revealed that the highest-occupied molecular orbitals were both primarily localised on carbon and possessed shapes that were indicative of σ- and π-symmetric lone pairs rather than bonding molecular orbitals. Additional calculations showed σ-bonding orbitals between the central carbon atom and
216:
O) revealed a P-C-C bond angle of 145.5° consistent with the bent structure of other carbon (0) compounds. While both computational and experimental data indicated a linear structure for carbon suboxide, the same models predicted only an energy difference of 1.9 kcal mol (7.9 kJ mol) between linear
415:
is 1101.8 kJ mol, indicating that carbodiphosphoranes can function as strong bases. Carbodicarbenes can act as even stronger bases than carbodiphosphoranes with first proton affinities reaching as high as 294.3 kcal mol (1231 kJ mol). However, the second proton affinities for carbodicarbenes
410:
and accept two protons from an acid. The typical first proton affinity for a carbodiphosphorane ranges from 209.3 kcal mol (875.7 kJ mol) for the weakest base to 287.6 kcal mol (1203 kJ mol) for the strongest base and second proton affinities ranging from 70.6 kcal mol (295 kJ mol) to 188.9
472:
was observed at 2014 cm which is significantly lower than the same carbon-oxygen stretching frequency when rhodium is coordinated to a N-heterocyclic carbene (between 2058 cm and 2036 cm) which is indicative of a strong π-donating effect from the second carbon lone-pair of the carbone.
529:
Carbones can also form complexes with main group elements. The strong σ- and π-donating properties of carbones have made them optimal tools for stabilising reactive main-group-based species. Carbodicarbenes have been employed in the successful synthesis of novel
234:
on a carbon reagent in its +2 or +4 oxidation state. The first successful synthesis of a compound now recognised as a carbodiphosphorane was achieved by
Ramirez et al. in 1961 with this method. By stirring methylidebis-(triphenylphosphonium) bromide with
424:
In addition to being strong Brønsted-Lowry bases, carbones are also nucleophilic and act as strong Lewis bases when coordinating with transition metals and main group elements. Several computational studies found that carbodiphosphoranes bound tightly to
554:
282:
162:
376:
to form a tetraaminoallene which acts as a carbodicarbene. Additionally, a method of facile synthesis of asymmetric carbodicarbenes was developed by Chen et al. in 2015 by using a simple nucleophilic substitution reaction. Reacting an
186:
The structure of carbodicarbenes greatly resembles that of carbodiphosphoranes. Computational data for a N-methyl-substituted carbodicarbene predicted a carbon-carbon bond with a length only marginally longer than a C=C bond in a typical
217:
carbon suboxide and bent carbon suboxide. The ease of bending and relatively large contribution of carbon in the two highest-occupied molecular orbitals imply a certain degree of carbone-like character in spite of the linear geometry.
208:(O=C=C=C=O) have also exhibited carbone-like character where a carbon (0) species participates in a donor-acceptor interaction with carbon monoxide. The crystal structure of triphenylphosphoranylideneketen (Ph
174:
complexed phosphorus atoms but no orbitals localised on phosphorus, indicating the phosphorus atoms were donating their lone pairs into unoccupied valence orbitals on carbon to form a donor-acceptor complex.
437:
metal-ligand bonds for certain compounds. Experimentally, a variety of metal-carbodiphosphorane complexes have been synthesised and characterised, including with metals such as tungsten, nickel,
453:
digold complex and provides experimental evidence supporting the structure of carbodiphosphoranes as a carbon (0) compound with two lone pairs on the central carbon atom donating to the gold atoms.
385:
containing a different NHC moiety generates a product which can be readily deprotonated to afford a carbodicarbene with two different carbene substituents with improved functionality.
538:, which can exhibit useful optical properties, as well as a dicationic tricoordinate hydridoboron compound. Carbones have also been used in the first synthesis of stable carbon-
309:
carbodiphosphoranes have also been successfully synthesised through the reaction of bis(diisopropylamino)phosphino diazomethane with bis(dialkylamino)phosphenium triflate in excess
1547:"Reaction of Carbodiphosphorane Ph_3P=C=PPh_3 with Ni(CO)_4. Experimental and Theoretical Study of the Structures and Properties of (CO)_3NiC(PPh_3)_2 and (CO)_2NiC(PPh_3)_2"
1287:"Product Class 2: Carbon Dioxide, Carbonyl Sulfide, Carbon Disulfide, Isocyanates, Isothiocyanates, Carbodiimides, and Their Selenium, Tellurium, and Phosphorus Analogues"
1719:
Hollister, Kimberly; Molino, Andrew; Breiner, Grace; Walley, Jacob; Wentz, Kelsie; Conley, Ashley; Dickie, Diane; Wilson, David; Gilliard Jr., Robert (2022).
728:"C(NHC)_2: Divalent Carbon(0) Compounds with N-Heterocyclic Carbene Ligands-Theoretical Evidence for a Class of Molecules with Promising Chemical Properties"
333:
The first carbodicarbene synthesis was achieved much later than the first carbodiphosphorane synthesis, in 2008 by Dyker et al. The first step was the
407:
1026:
Walley, Jacob; Obi, Akachukwu; Breiner, Grace; Wang, Guocang; Dickie, Diane; Molino, Andrew; Dutton, Jason; Wilson, David; Gilliard Jr., Robert (2019).
178:
data also revealed that the hexaphenylcarbodiphosphorane structure was noticeably bent rather than linear with a P-C-P bond angle of 131.7°.
481:
Transition metal complexes containing a carbone ligand also exhibit a wide range of reactivity. In 2015, Pranckevicius et al. synthesised a
1489:
Chen, Wen-Ching; Shen, Jiun-Shian; Jurca, Titel; Peng, Chun-Jung; Lin, Yen-Hsu; Wang, Yi-Ping; Shih, Wei-Chih; Yap, Glenn; Ong, Tiow-Gan (2015).
854:
Hsu, Yu-Chen; Chen, Jiun-Shian; Lin, Bo-Chao; Chen, Wen-Ching; Chan, Yi-Tsu; Ching, Wei-Min; Yap, Glenn; Hsu, Chao-Ping; Ong, Tiow-Gan (2014).
1382:
509:
coupling reactions while rhodium (I) catalysts coordinated to carbodicarbene pincer ligands have been shown to hydroaminate and hydroarylate
1491:"Expanding the Ligand Framework Diversity of Carbodicarbene and Direct Detection of Boron Activation in the Methylation of Amines with CO_2"
856:"Synthesis and Isolation of an Acyclic Tridentate Bis(pyridine)carbodicarbene and Studies on Its Structural Implications and Reactivities"
289:
Synthetic methods have also been developed for more diverse carbodiphosphoranes. Methylenediphosphines will undergo a reaction with
457:
976:
Walley, Jacob; Breiner, Grace; Wang, Guocang; Dickie, Diane; Molino, Andrew; Dutton, Jason; Wilson, David; Gilliard Jr., Robert (2019).
20:
342:
1310:
243:
solution, the potassium reduced the starting material to form hexaphenylcarbodiphosphorane as a stable, yellow, crystalline solid.
468:
and gold. In the former experiment, when a rhodium carbonyl complex was coordinated to a carbodicarbene, the carbon-oxygen
338:
278:-substituted phosphonium salt can undergo an elimination reaction in the presence of a strong base to form a carbodiphosphorane.
1766:
Chen, Wen-Ching; Lee, Ching-Yu; Lin, Bo-Chao; Hsu, Yu-Chen; Shen, Jiun-Shian; Hsu, Chao-Ping; Yap, Glenn; Ong, Tiow-Gan (2014).
1878:
547:
262:
of carbon (IV) or carbon (II) starting materials. Reacting a carbon (IV) or carbon (II) diphosphine salt with a strong
1807:"Carbodicarbene Bismaalkene Cations: Unravelling the Complexities of Carbene versus Carbone in Heavy Pnictogen Chemistry"
170:
1805:
Walley, Jacob; Warring, Levi; Wang, Guocang; Dickie, Diane; Pan, Sudip; Frenking, Gernot; Gilliard Jr., Robert (2020).
158:
species that places a positive charge on both phosphorus atoms and an overall charge of -2 on the central carbon atom.
150:
between an overall neutral species in which double bonds exists between the central carbon atom and the two complexed
1867:
1286:
814:"Cyclic Bent Allene Hydrido-Carbonyl Complexes of Ruthenium: Highly Active Catalysts for Hydrogenation of Olefins"
770:"Divalent Carbon(0) Chemistry, Part 2: Protonation and Complexes with Main Group and Transition Metal Lewis Acids"
1907:
365:
314:
78:
1331:"An unusual reaction of hexafluoroacetone with methylenediphosphines. Facile synthesis of carbodiphosphoranes"
769:
627:
546:
chemistry with the synthesis of a five-membered beryllacycle through C-H activation as well as beryllacycle
1680:
Inés, Blanca; Patil, Mahendra; Carreras, Javier; Goddard, Richard; Thiel, Walter; Alcarazo, Manuel (2011).
1651:"Synthesis and Ligand Properties of Stable Five-Membered-Ring Allenes Containing Only Second-Row Elements"
494:
114:
892:"Intermolecular Hydroamination of 1,3-Dienes Catalyzed by Bis(phosphine)carbodicarbene-Rhodium Complexes"
469:
147:
90:
978:"s-Block carbodicarbene chemistry: C(sp^3)-H activation and cyclization mediated by a beryllium center"
501:(II) catalysts with bis(pyridine)carbodicarbene ligands have been shown to be successful catalysts for
1252:
1191:
542:
species with π-bonding character. Carbodicarbenes have also seen significant utility in the field of
274:
can deprotonate the centre carbon atom to form the desired carbodiphosphorane. Alternatively, a
259:
977:
412:
1330:
406:
The presence of two lone pairs on the central carbon atom make it possible for carbones to act as
1748:
1055:
1005:
601:
485:(II) catalyst coordinated to two different carbodicarbene ligands that was able to catalytically
143:
106:
1362:
464:
Carbodicarbenes have also been shown to form complexes with different transition metals such as
1580:
1836:
1787:
1740:
1701:
1510:
1466:
1422:
1378:
1306:
1172:
1047:
997:
955:
911:
833:
789:
747:
693:
647:
382:
361:
290:
126:
122:
82:
1681:
1650:
1490:
1446:
1402:
1152:
936:"Lewis acid Activation of Carbodicarbene Catalysts for Rh-Catalyzed Hydroarylation of Dienes"
855:
727:
673:
1826:
1818:
1779:
1732:
1693:
1662:
1628:
1592:
1558:
1502:
1458:
1414:
1370:
1338:
1298:
1264:
1230:
1199:
1164:
1126:
1095:
1039:
989:
947:
903:
867:
825:
781:
739:
685:
672:
Tonner, Ralf; Öxler, Florian; Neumüller, Bernhard; Petz, Wolfgang; Frenking, Gernot (2006).
639:
591:
263:
110:
50:
1153:"Synthesis of an Extremely Bent Acyclic Allene (A "Carbodicarbene"): A Strong Donor Ligand"
535:
502:
434:
306:
205:
175:
98:
46:
1027:
520:
Sample transition-metal catalysed reactions where the catalyst contains a carbone ligand
1831:
1806:
1028:"Cyclic(alkyl)(amino) Carbene-Promoted Ring Expansion of a Carbodicarbene Beryllacycle"
373:
267:
94:
1901:
1752:
1059:
506:
486:
369:
341:
and the second step was the deprotonation of the carbon (II) species using potassium
255:
42:
1009:
605:
254:
Alternative methods to synthesise alkyl-substituted carbodiphosphoranes involve the
1251:
Gruber, Marco; Bauer, Walter; Maid, Harald; Schöll, Kilian; Tykwinski, Rik (2017).
1218:
1114:
1083:
490:
310:
271:
1043:
113:
complexes. Carbone-coordinated elements also exhibit a variety of different
1720:
1445:
Fürstner, Alois; Alcarazo, Manuel; Goddard, Richard; Lehmann, Christian (2008).
1401:
Marrot, Sebastien; Kato, Tsuyoshi; Gornitzka, Heinz; Baceiredo, Antoine (2006).
1374:
935:
813:
553:
516:
456:
356:
Similar non-cyclic carbodicarbenes have also been successfully synthesised from
294:
102:
1115:"Structure and triboluminescence of polymorphs of hexaphenylcarbodiphosphorane"
388:
348:
320:
281:
246:
161:
19:
1532:
1268:
360:
salts through the condensation of two equivalents of the starting material in
334:
155:
151:
62:
1767:
1616:
1596:
1579:
Schmidbaur, Hubert; Zybill, Christian; Müller, Gerhard; Krüger, Carl (1983).
1546:
1447:"Coordination Chemistry of Ene-1,1-diamines and a Prototype "Carbodicarbene""
1302:
891:
596:
579:
433:
with metal-ligand bond dissociation energies that were greater than those of
934:
Roberts, Courtney; Matías, Desirée; Goldfogel, Matthew; Meek, Simon (2015).
543:
498:
482:
236:
230:
One strategy for the synthesis of carbodiphosphoranes involves the use of a
118:
70:
54:
1856:
1840:
1822:
1791:
1744:
1705:
1697:
1666:
1514:
1506:
1470:
1462:
1426:
1418:
1253:"Synthetic and NMR studies on hexaphenylcarbodiphosphorane (Ph_3P=C=PPh_3)"
1176:
1168:
1051:
1001:
959:
915:
871:
837:
793:
785:
751:
743:
697:
689:
651:
643:
1649:
Lavallo, Vincent; Dyker, C. Adam; Donnadieu, Bruno; Bertrand, Guy (2008).
1151:
Dyker, C. Adam; Lavallo, Vincent; Donnadieu, Bruno; Bertrand, Guy (2008).
1736:
1203:
951:
829:
426:
31:
1234:
1130:
1099:
1403:"Cyclic Carbodiphosphoranes: Strongly Nucleophilic sigma-Donor Ligands"
993:
539:
465:
450:
357:
275:
240:
188:
86:
74:
1783:
1632:
1562:
1545:
Petz, Wolfgang; Weller, Frank; Uddin, Jamal; Frenking, Gernot (1999).
907:
411:
kcal mol (790.4 kJ mol). For comparison, the proton affinity of
1682:"Synthesis, Structure, and Reactivity of a Dihydrido Borenium Cation"
1342:
442:
438:
430:
378:
302:
298:
66:
35:
1369:. Topics in Organometallic Chemistry. Vol. 30. pp. 49–92.
1196:
Journal of the
Chemical Society A: Inorganic, Physical, Theoretical
77:(carbodicarbenes), that stabilises the central carbon atom through
552:
531:
515:
510:
455:
449:. The gold complex is of particular note as it is the first
387:
347:
319:
280:
245:
231:
160:
18:
1721:"Air-Stable Thermoluminescent Carbodicarbene-Borafluorenium Ions"
1617:"New Ylide-, Alkynyl-, and Mixed Alkynyl/Ylide-Gold(I) Complexes"
1285:
Braverman, S.; Cherkinsky, M.; Birsa, M.L. (2005). Knight (ed.).
1113:
Hardy, Gordon; Zink, Jeffrey; Kaska, W.C.; Baldwin, J.C. (1978).
345:
to yield the desired N-heterocyclic-carbene-substituted carbone.
460:
Gem-Digold complex synthesised from hexaphenylcarbodiphosphorane
446:
38:
1768:"The Elusive Three-Coordinate Dicationic Hydrido Boron Complex"
1531:. National Institute of Standards and Technology. May 2022.
1082:
Ramirez, Fausto; Desai, N.B.; Hansen, B.; McKelvie, N. (1961).
1850:
557:
Examples of main group complexes stabilised by carbodicarbenes
890:
Goldfogel, Matthew; Roberts, Courtney; Meek, Simon (2014).
812:
Pranckevicius, Conor; Fan, Louie; Stephan, Douglas (2015).
674:"Carbodiphosphoranes: The Chemistry of Divalent Carbon(0)"
381:
substituted with a N-heterocyclic carbene scaffold with a
1581:"Money Metal Complexes with Hexaphenylcarbodiphosphorane"
1529:
Computational
Chemistry Comparison and Benchmark Database
1367:
Transition Metal
Complexes of Neutral eta1-Carbon Ligands
1219:"Bis(trimethylphosphoranylidene)methane, (CH3)3PCP(CH3)3"
146:
of carbodiphosphoranes, the structure was described as a
105:
which allows them to function as ligands in a variety of
628:"Divalent Carbon(0) Chemistry, Part 1: Parent Compounds"
165:
Initial proposed carbodiphosphorane resonance structures
1874:
1881:
to it so that it can be listed with similar articles.
1615:
Vincente, José; Singhal, Anshu; Jones, Peter (2002).
305:-substituted carbodiphosphoranes respectively.
57:. These carbon-based compounds are of the formula CL
477:Reactivity in transition metal complexes
285:Alternative syntheses of alkyl-carbodiphosphoranes
1084:"Hexaphenylcarbodiphosphorane, (C6H5)3Pcp(C6H5)3"
337:of bis(N-methylbenzimidazol-2-yl)methane using
1192:"Structure of triphenylphosphoranylideneketen"
324:Synthesis of more diverse carbodiphosphoranes
8:
1533:https://cccbdb.nist.gov/palistx.asp#webbook
1217:Gasser, Oswald; Schmidbaur, Hubert (1975).
97:substituents. Carbones possess high
1830:
1363:"Carbodiphosphoranes and Related Ligands"
1361:Petz, Wolfgang; Frenking, Gernot (2009).
595:
1772:Journal of the American Chemical Society
1725:Journal of the American Chemical Society
1223:Journal of the American Chemical Society
1119:Journal of the American Chemical Society
1088:Journal of the American Chemical Society
940:Journal of the American Chemical Society
896:Journal of the American Chemical Society
818:Journal of the American Chemical Society
1811:Angewandte Chemie International Edition
1686:Angewandte Chemie International Edition
1495:Angewandte Chemie International Edition
1451:Angewandte Chemie International Edition
1407:Angewandte Chemie International Edition
1157:Angewandte Chemie International Edition
768:Tonner, Ralf; Frenking, Gernot (2008).
732:Angewandte Chemie International Edition
726:Tonner, Ralf; Frenking, Gernot (2007).
678:Angewandte Chemie International Edition
626:Tonner, Ralf; Frenking, Gernot (2008).
578:Frenking, Gernot; Tonner, Ralf (2009).
567:
200:Phosphaketene ylides (general formula R
1644:
1642:
1610:
1608:
1606:
1574:
1572:
1484:
1482:
1480:
1440:
1438:
1436:
1396:
1394:
1356:
1354:
1352:
1324:
1322:
1280:
1278:
1246:
1244:
1146:
1144:
1142:
1140:
1077:
1075:
1073:
1071:
1069:
1021:
1019:
971:
969:
81:. Carbones possess high-energy
7:
1190:Daley, J.J.; Wheatley, P.J. (1966).
929:
927:
925:
885:
883:
881:
849:
847:
807:
805:
803:
763:
761:
721:
719:
717:
715:
713:
711:
709:
707:
667:
665:
663:
661:
621:
619:
617:
615:
573:
571:
392:Alternative carbodicarbene syntheses
1866:needs additional or more specific
525:Reactivity in main group complexes
250:First carbodiphosphorane synthesis
169:However, computational studies on
14:
1527:Experimental Proton Affinities.
1855:
343:bis(trimethylsilyl)amide (KHMDS)
313:followed by deprotonation with
580:"Divalent carbon(0) compounds"
534:-containing compounds such as
352:First carbodicarbene synthesis
89:-symmetry, making them strong
1:
1044:10.1021/acs.inorgchem.9b01643
774:Chemistry: A European Journal
632:Chemistry: A European Journal
196:Other carbene structures
171:hexaphenylcarbodiphosphorane
1375:10.1007/978-3-642-04722-0_3
73:(carbodiphosphoranes) or a
1924:
584:Pure and Applied Chemistry
372:, then deprotonation with
75:N-heterocyclic carbene/NHC
1329:Shevchenko, Igor (1998).
1269:10.1016/j.ica.2017.04.018
366:nucleophilic substitution
1597:10.1002/ange.19830950930
1303:10.1055/sos-SD-018-00070
597:10.1351/PAC-CON-08-11-03
493:and similar activity to
1335:Chemical Communications
1257:Inorganica Chimica Acta
982:Chemical Communications
489:olefins with excellent
362:dimethylacetamide (DMA)
49:of zero where all four
1823:10.1002/anie.202014398
1698:10.1002/anie.201103197
1667:10.1002/ange.200801176
1507:10.1002/anie.201507921
1463:10.1002/anie.200705798
1419:10.1002/anie.200504396
1169:10.1002/anie.200705620
872:10.1002/ange.201406481
786:10.1002/chem.200701392
744:10.1002/anie.200701632
690:10.1002/anie.200602552
644:10.1002/chem.200701390
558:
521:
461:
393:
353:
325:
286:
251:
166:
61:where L is a strongly
24:
556:
519:
459:
391:
351:
323:
284:
249:
239:metal suspended in a
164:
133:Structure and bonding
22:
1737:10.1021/jacs.1c11861
1291:Science of Synthesis
1204:10.1039/J19660001703
952:10.1021/jacs.5b03510
830:10.1021/jacs.5b02203
491:diastereoselectivity
470:stretching frequency
408:Brønsted-Lowry bases
315:hexamethyldisilazide
79:donor-acceptor bonds
1501:(50): 15207–15212.
1235:10.1021/ja00854a077
1131:10.1021/ja00493a035
1100:10.1021/ja01477a052
1038:(16): 11118–11126.
1032:Inorganic Chemistry
495:Crabtree’s catalyst
413:potassium hydroxide
226:Carbodiphosphoranes
138:Carbodiphosphoranes
994:10.1039/C8CC10022E
559:
522:
462:
394:
354:
326:
287:
252:
167:
53:exist as unbonded
25:
16:Class of molecules
1896:
1895:
1879:adding categories
1817:(12): 6682–6690.
1784:10.1021/ja4120852
1692:(36): 8400–8403.
1661:(29): 5491–5494.
1655:Angewandte Chemie
1633:10.1021/om020753p
1627:(26): 5887–5900.
1585:Angewandte Chemie
1563:10.1021/om9804632
1457:(17): 3210–3214.
1413:(16): 2598–2601.
1384:978-3-642-04721-3
1337:(11): 1203–1204.
1229:(21): 6281–6282.
1163:(17): 3206–3209.
1125:(25): 8001–8002.
1094:(16): 3539–3540.
988:(13): 1967–1970.
946:(20): 6488–6491.
908:10.1021/ja502275w
902:(17): 6227–6230.
860:Angewandte Chemie
824:(16): 5582–5589.
780:(11): 3273–3289.
738:(45): 8695–8698.
684:(47): 8038–8042.
638:(11): 3260–3272.
301:-substituted and
291:hexafluoroacetone
99:proton affinities
85:with both σ- and
51:valence electrons
1915:
1908:Carbon compounds
1891:
1888:
1882:
1859:
1851:
1845:
1844:
1834:
1802:
1796:
1795:
1763:
1757:
1756:
1716:
1710:
1709:
1677:
1671:
1670:
1646:
1637:
1636:
1612:
1601:
1600:
1576:
1567:
1566:
1542:
1536:
1525:
1519:
1518:
1486:
1475:
1474:
1442:
1431:
1430:
1398:
1389:
1388:
1358:
1347:
1346:
1343:10.1039/A801805G
1326:
1317:
1316:
1282:
1273:
1272:
1248:
1239:
1238:
1214:
1208:
1207:
1187:
1181:
1180:
1148:
1135:
1134:
1110:
1104:
1103:
1079:
1064:
1063:
1023:
1014:
1013:
973:
964:
963:
931:
920:
919:
887:
876:
875:
866:(8): 2450–2454.
851:
842:
841:
809:
798:
797:
765:
756:
755:
723:
702:
701:
669:
656:
655:
623:
610:
609:
599:
575:
176:Crystallographic
148:resonance hybrid
129:.
111:transition metal
1923:
1922:
1918:
1917:
1916:
1914:
1913:
1912:
1898:
1897:
1892:
1886:
1883:
1872:
1860:
1849:
1848:
1804:
1803:
1799:
1765:
1764:
1760:
1718:
1717:
1713:
1679:
1678:
1674:
1648:
1647:
1640:
1621:Organometallics
1614:
1613:
1604:
1578:
1577:
1570:
1551:Organometallics
1544:
1543:
1539:
1526:
1522:
1488:
1487:
1478:
1444:
1443:
1434:
1400:
1399:
1392:
1385:
1360:
1359:
1350:
1328:
1327:
1320:
1313:
1284:
1283:
1276:
1250:
1249:
1242:
1216:
1215:
1211:
1189:
1188:
1184:
1150:
1149:
1138:
1112:
1111:
1107:
1081:
1080:
1067:
1025:
1024:
1017:
975:
974:
967:
933:
932:
923:
889:
888:
879:
853:
852:
845:
811:
810:
801:
767:
766:
759:
725:
724:
705:
671:
670:
659:
625:
624:
613:
577:
576:
569:
564:
527:
479:
435:carbon monoxide
422:
404:
399:
339:methyl triflate
331:
329:Carbodicarbenes
228:
223:
215:
211:
206:carbon suboxide
203:
198:
184:
182:Carbodicarbenes
142:In the initial
140:
135:
125:and main group
101:and are strong
60:
47:oxidation state
30:are a class of
23:Generic carbone
17:
12:
11:
5:
1921:
1919:
1911:
1910:
1900:
1899:
1894:
1893:
1863:
1861:
1854:
1847:
1846:
1797:
1778:(3): 914–917.
1758:
1731:(1): 590–598.
1711:
1672:
1638:
1602:
1591:(9): 753–755.
1568:
1557:(4): 619–626.
1537:
1520:
1476:
1432:
1390:
1383:
1348:
1318:
1311:
1274:
1240:
1209:
1182:
1136:
1105:
1065:
1015:
965:
921:
877:
843:
799:
757:
703:
657:
611:
590:(4): 597–614.
566:
565:
563:
560:
548:ring expansion
526:
523:
503:Suzuki-Miyaura
478:
475:
421:
418:
403:
400:
398:
395:
374:n-butyllithium
364:, followed by
330:
327:
268:sodium hydride
232:reducing agent
227:
224:
222:
219:
213:
209:
201:
197:
194:
183:
180:
139:
136:
134:
131:
69:, typically a
58:
45:with a formal
15:
13:
10:
9:
6:
4:
3:
2:
1920:
1909:
1906:
1905:
1903:
1890:
1887:November 2022
1880:
1876:
1870:
1869:
1864:This article
1862:
1858:
1853:
1852:
1842:
1838:
1833:
1828:
1824:
1820:
1816:
1812:
1808:
1801:
1798:
1793:
1789:
1785:
1781:
1777:
1773:
1769:
1762:
1759:
1754:
1750:
1746:
1742:
1738:
1734:
1730:
1726:
1722:
1715:
1712:
1707:
1703:
1699:
1695:
1691:
1687:
1683:
1676:
1673:
1668:
1664:
1660:
1656:
1652:
1645:
1643:
1639:
1634:
1630:
1626:
1622:
1618:
1611:
1609:
1607:
1603:
1598:
1594:
1590:
1586:
1582:
1575:
1573:
1569:
1564:
1560:
1556:
1552:
1548:
1541:
1538:
1534:
1530:
1524:
1521:
1516:
1512:
1508:
1504:
1500:
1496:
1492:
1485:
1483:
1481:
1477:
1472:
1468:
1464:
1460:
1456:
1452:
1448:
1441:
1439:
1437:
1433:
1428:
1424:
1420:
1416:
1412:
1408:
1404:
1397:
1395:
1391:
1386:
1380:
1376:
1372:
1368:
1364:
1357:
1355:
1353:
1349:
1344:
1340:
1336:
1332:
1325:
1323:
1319:
1314:
1312:9783131186812
1308:
1304:
1300:
1296:
1292:
1288:
1281:
1279:
1275:
1270:
1266:
1262:
1258:
1254:
1247:
1245:
1241:
1236:
1232:
1228:
1224:
1220:
1213:
1210:
1205:
1201:
1198:: 1703–1706.
1197:
1193:
1186:
1183:
1178:
1174:
1170:
1166:
1162:
1158:
1154:
1147:
1145:
1143:
1141:
1137:
1132:
1128:
1124:
1120:
1116:
1109:
1106:
1101:
1097:
1093:
1089:
1085:
1078:
1076:
1074:
1072:
1070:
1066:
1061:
1057:
1053:
1049:
1045:
1041:
1037:
1033:
1029:
1022:
1020:
1016:
1011:
1007:
1003:
999:
995:
991:
987:
983:
979:
972:
970:
966:
961:
957:
953:
949:
945:
941:
937:
930:
928:
926:
922:
917:
913:
909:
905:
901:
897:
893:
886:
884:
882:
878:
873:
869:
865:
861:
857:
850:
848:
844:
839:
835:
831:
827:
823:
819:
815:
808:
806:
804:
800:
795:
791:
787:
783:
779:
775:
771:
764:
762:
758:
753:
749:
745:
741:
737:
733:
729:
722:
720:
718:
716:
714:
712:
710:
708:
704:
699:
695:
691:
687:
683:
679:
675:
668:
666:
664:
662:
658:
653:
649:
645:
641:
637:
633:
629:
622:
620:
618:
616:
612:
607:
603:
598:
593:
589:
585:
581:
574:
572:
568:
561:
555:
551:
549:
545:
541:
537:
536:borenium ions
533:
524:
518:
514:
512:
508:
507:Heck-Mizoroki
504:
500:
496:
492:
488:
484:
476:
474:
471:
467:
458:
454:
452:
448:
444:
440:
436:
432:
428:
419:
417:
414:
409:
401:
396:
390:
386:
384:
380:
375:
371:
370:dimethylamine
367:
363:
359:
350:
346:
344:
340:
336:
328:
322:
318:
316:
312:
308:
304:
300:
296:
292:
283:
279:
277:
273:
269:
265:
261:
257:
256:deprotonation
248:
244:
242:
238:
233:
225:
220:
218:
207:
204:P=C=C=O) and
195:
193:
190:
181:
179:
177:
172:
163:
159:
157:
153:
149:
145:
137:
132:
130:
128:
124:
120:
116:
112:
108:
104:
100:
96:
92:
88:
84:
80:
76:
72:
68:
64:
56:
52:
48:
44:
43:excited state
40:
37:
34:containing a
33:
29:
21:
1884:
1865:
1814:
1810:
1800:
1775:
1771:
1761:
1728:
1724:
1714:
1689:
1685:
1675:
1658:
1654:
1624:
1620:
1588:
1584:
1554:
1550:
1540:
1528:
1523:
1498:
1494:
1454:
1450:
1410:
1406:
1366:
1334:
1294:
1290:
1260:
1256:
1226:
1222:
1212:
1195:
1185:
1160:
1156:
1122:
1118:
1108:
1091:
1087:
1035:
1031:
985:
981:
943:
939:
899:
895:
863:
859:
821:
817:
777:
773:
735:
731:
681:
677:
635:
631:
587:
583:
528:
480:
463:
423:
405:
355:
332:
311:benzonitrile
288:
272:sodium amide
253:
229:
199:
185:
168:
156:zwitterionic
154:atoms and a
141:
115:reactivities
103:nucleophiles
27:
26:
1263:: 152–158.
335:methylation
295:thioacetone
260:elimination
95:π-backdonor
93:and strong
91:Lewis bases
1868:categories
1297:: 65–320.
562:References
397:Reactivity
152:phosphorus
107:main group
65:-donating
55:lone pairs
1753:245713340
1060:199437238
544:beryllium
499:Palladium
483:ruthenium
383:thioether
237:potassium
221:Synthesis
144:syntheses
127:reactions
71:phosphine
41:in the D
32:molecules
1902:Category
1875:help out
1841:33290596
1792:24383448
1745:35016509
1706:21761533
1515:26489967
1471:18348113
1427:16534821
1177:18311741
1052:31380626
1010:59252440
1002:30681680
960:25961506
916:24742315
838:25855868
794:18318021
752:17924383
698:17075933
652:18318020
606:98257123
497:.
427:tungsten
402:Basicity
297:to form
266:such as
121:various
119:catalyse
83:orbitals
28:Carbones
1873:Please
1832:7986408
540:bismuth
466:rhodium
451:geminal
420:Ligands
358:iminium
241:diglyme
123:organic
1839:
1829:
1790:
1751:
1743:
1704:
1513:
1469:
1425:
1381:
1309:
1175:
1058:
1050:
1008:
1000:
958:
914:
836:
792:
750:
696:
650:
604:
511:dienes
487:reduce
445:, and
443:silver
439:copper
431:nickel
379:olefin
307:Cyclic
276:halide
189:allene
67:ligand
36:carbon
1749:S2CID
1056:S2CID
1006:S2CID
602:S2CID
532:boron
368:with
1837:PMID
1788:PMID
1741:PMID
1702:PMID
1511:PMID
1467:PMID
1423:PMID
1379:ISBN
1307:ISBN
1173:PMID
1048:PMID
998:PMID
956:PMID
912:PMID
834:PMID
790:PMID
748:PMID
694:PMID
648:PMID
513:.
505:and
447:gold
429:and
264:base
117:and
109:and
39:atom
1877:by
1827:PMC
1819:doi
1780:doi
1776:136
1733:doi
1729:144
1694:doi
1663:doi
1659:120
1629:doi
1593:doi
1559:doi
1503:doi
1459:doi
1415:doi
1371:doi
1339:doi
1299:doi
1265:doi
1261:468
1231:doi
1200:doi
1165:doi
1127:doi
1123:100
1096:doi
1040:doi
990:doi
948:doi
944:137
904:doi
900:136
868:doi
864:127
826:doi
822:137
782:doi
740:doi
686:doi
640:doi
592:doi
293:or
270:or
258:or
1904::
1835:.
1825:.
1815:60
1813:.
1809:.
1786:.
1774:.
1770:.
1747:.
1739:.
1727:.
1723:.
1700:.
1690:50
1688:.
1684:.
1657:.
1653:.
1641:^
1625:21
1623:.
1619:.
1605:^
1589:95
1587:.
1583:.
1571:^
1555:18
1553:.
1549:.
1509:.
1499:54
1497:.
1493:.
1479:^
1465:.
1455:47
1453:.
1449:.
1435:^
1421:.
1411:45
1409:.
1405:.
1393:^
1377:.
1365:.
1351:^
1333:.
1321:^
1305:.
1295:18
1293:.
1289:.
1277:^
1259:.
1255:.
1243:^
1227:97
1225:.
1221:.
1194:.
1171:.
1161:47
1159:.
1155:.
1139:^
1121:.
1117:.
1092:83
1090:.
1086:.
1068:^
1054:.
1046:.
1036:58
1034:.
1030:.
1018:^
1004:.
996:.
986:55
984:.
980:.
968:^
954:.
942:.
938:.
924:^
910:.
898:.
894:.
880:^
862:.
858:.
846:^
832:.
820:.
816:.
802:^
788:.
778:14
776:.
772:.
760:^
746:.
736:46
734:.
730:.
706:^
692:.
682:45
680:.
676:.
660:^
646:.
636:14
634:.
630:.
614:^
600:.
588:81
586:.
582:.
570:^
550:.
441:,
317:.
212:PC
1889:)
1885:(
1871:.
1843:.
1821::
1794:.
1782::
1755:.
1735::
1708:.
1696::
1669:.
1665::
1635:.
1631::
1599:.
1595::
1565:.
1561::
1535:.
1517:.
1505::
1473:.
1461::
1429:.
1417::
1387:.
1373::
1345:.
1341::
1315:.
1301::
1271:.
1267::
1237:.
1233::
1206:.
1202::
1179:.
1167::
1133:.
1129::
1102:.
1098::
1062:.
1042::
1012:.
992::
962:.
950::
918:.
906::
874:.
870::
840:.
828::
796:.
784::
754:.
742::
700:.
688::
654:.
642::
608:.
594::
303:S
299:O
214:2
210:3
202:3
87:π
63:σ
59:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.