1004:
1028:
210:
805:
1170:
1227:
1182:
1187:
31:
1222:
1217:
939:
1361:
342:
1293:
1208:
582:
197:. It is useful for understanding the stability of different isomers, for example, by taking into account the spatial orientation and through-space interactions of substituents. In addition, conformational analysis can be used to predict and explain product selectivity, mechanisms, and rates of reactions. Conformational analysis also plays an important role in rational, structure-based
866:, the two chair conformers interconvert with rapidly at room temperature, with cyclohexane itself undergoing the ring-flip at a rates of approximately 10 ring-flips/sec, with an overall energy barrier of 10 kcal/mol (42 kJ/mol), which precludes their separation at ambient temperatures. However, at low temperatures below the
265:
energy of the gauche conformer. The anti conformer is, therefore, the most stable (≈ 0 kcal/mol). The three eclipsed conformations with dihedral angles of 0°, 120°, and 240° are transition states between conformers. Note that the two eclipsed conformations have different energies: at 0° the
947:
The mechanism requires that the departing atoms or groups follow antiparallel trajectories. For open chain substrates this geometric prerequisite is met by at least one of the three staggered conformers. For some cyclic substrates such as cyclohexane, however, an antiparallel arrangement may not be
800:
are responsible for the relative stability of conformers and their transition states. The contributions of these factors vary depending on the nature of the substituents and may either contribute positively or negatively to the energy barrier. Computational studies of small molecules such as ethane
1122:
The importance of energy minima and energy maxima is seen by extension of these concepts to more complex molecules for which stable conformations may be predicted as minimum-energy forms. The determination of stable conformations has also played a large role in the establishment of the concept of
540:
Three isotherms are given in the diagram depicting the equilibrium distribution of two conformers at different temperatures. At a free energy difference of 0 kcal/mol, this gives an equilibrium constant of 1, meaning that two conformers exist in a 1:1 ratio. The two have equal free energy;
98:
between the local-minimum conformational isomers. Rotations about single bonds involve overcoming a rotational energy barrier to interconvert one conformer to another. If the energy barrier is low, there is free rotation and a sample of the compound exists as a rapidly equilibrating mixture of
1328:
of the other C-H bond. The energetic stabilization of this effect is maximized when the two orbitals have maximal overlap, occurring in the staggered conformation. There is no overlap in the eclipsed conformation, leading to a disfavored energy maximum. On the other hand, an analysis within
229:
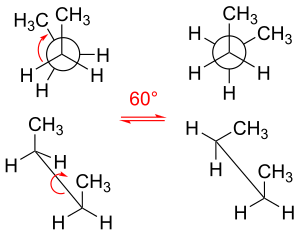
dihedral angle in the case of propane) equal to 60° (or approximately equal to 60° in the case of propane). The three eclipsed conformations, in which the dihedral angles are zero, are transition states (energy maxima) connecting two equivalent energy minima, the staggered conformers.
541:
neither is more stable, so neither predominates compared to the other. A negative difference in free energy means that a conformer interconverts to a thermodynamically more stable conformation, thus the equilibrium constant will always be greater than 1. For example, the Δ
185:
to break for interconversion. (Although the distinction is not always clear-cut, since certain bonds that are formally single bonds actually have double bond character that becomes apparent only when secondary resonance contributors are considered, like the C–N bonds of
1312:
for hydrogen of 120 pm, the hydrogen atoms in ethane are never in each other's way. The question of whether steric hindrance is responsible for the eclipsed energy maximum is a topic of debate to this day. One alternative to the steric hindrance explanation is based on
1085:
816:, which measure the energy difference when a substituent on cyclohexane in the axial as compared to the equatorial position. In large (>14 atom) rings, there are many accessible low-energy conformations which correspond to the strain-free diamond lattice.
854:
molecules. Protein side chains exhibit rotamers, whose distribution is determined by their steric interaction with different conformations of the backbone. This is evident from statistical analysis of the conformations of protein side chains in the
734:
573:), the equilibrium constant between two conformers can be increased by increasing the temperature, so that the amount of the less stable conformer present at equilibrium increases (although it always remains the minor conformer).
528:
908:. This is typical for situations where the conformational equilibration is much faster than reaction to form the product. The dependence of a reaction on the stereochemical orientation is therefore usually only visible in
260:
conformer, where the four carbon centres are coplanar and the substituents are 180° apart (refer to free energy diagram of butane). The energy difference between gauche and anti is 0.9 kcal/mol associated with the
942:
Base-induced bimolecular dehydrohalogenation (an E2 type reaction mechanism). The optimum geometry for the transition state requires the breaking bonds to be antiperiplanar, as they are in the appropriate staggered
537:" is useful in general for estimation of equilibrium constants at room temperature from free energy differences. At lower temperatures, a smaller energy difference is needed to obtain a given equilibrium constant.)
912:, in which a particular conformation is locked by substituents. Prediction of rates of many reactions involving the transition between sp2 and sp3 states, such as ketone reduction, alcohol oxidation or
971:-Bu group in the equatorial position, therefore the chloride group is not antiperiplanar with any vicinal hydrogen (it is gauche to all four). The thermodynamically unfavored conformation has the
213:
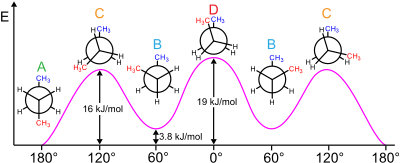
Relative conformation energy diagram of butane as a function of dihedral angle. A: antiperiplanar, anti or trans. B: synclinal or gauche. C: anticlinal or eclipsed. D: synperiplanar or cis.
2654:
Mo, Yirong; Wu, Wei; Song, Lingchun; Lin, Menghai; Zhang, Qianer; Gao, Jiali (2004-03-30). "The
Magnitude of Hyperconjugation in Ethane: A Perspective from Ab Initio Valence Bond Theory".
414:
1697:
565:
conformers at equilibrium. Conversely, a positive difference in free energy means the conformer already is the more stable one, so the interconversion is an unfavorable equilibrium (
107:(an isomer arising from hindered single-bond rotation). When the time scale for interconversion is long enough for isolation of individual rotamers (usually arbitrarily defined as a
353:, where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers:
266:
two methyl groups are eclipsed, resulting in higher energy (≈ 5 kcal/mol) than at 120°, where the methyl groups are eclipsed with hydrogens (≈ 3.5 kcal/mol).
2330:
Jensen, Frederick R.; Bushweller, C. Hackett (1969-06-01). "Separation of conformers. II. Axial and equatorial isomers of chlorocyclohexane and trideuteriomethoxycyclohexane".
603:
1169:
217:
Rotating their carbon–carbon bonds, the molecules ethane and propane have three local energy minima. They are structurally and energetically equivalent, and are called the
801:
suggest that electrostatic effects make the greatest contribution to the energy barrier; however, the barrier is traditionally attributed primarily to steric interactions.
585:
Boltzmann distribution % of lowest energy conformation in a two component equilibrating system at various temperatures (°C, color) and energy difference in kcal/mol (
2696:
2427:
1656:
828:
are conformational isomers which can be separated due to restricted rotation. The equilibrium between conformational isomers can be observed using a variety of
99:
multiple conformers; if the energy barrier is high enough then there is restricted rotation, a molecule may exist for a relatively long time period as a stable
967:-butylcyclohexyl chloride cannot easily eliminate but instead undergoes substitution (see diagram below) because the most stable conformation has the bulky
449:
870:
point one can directly monitor the equilibrium by NMR spectroscopy and by dynamic, temperature dependent NMR spectroscopy the barrier interconversion.
877:
spectroscopy at varying temperatures. The technique applies to barriers of 8–14 kcal/mol, and species exhibiting such dynamics are often called "
1494:
due to electrostatic repulsion of the fluorine atoms in the 1,3 positions. Evidence for the helix structure in the crystalline state is derived from
983:-Bu group "locks" the ring in the conformation where it is in the equatorial position and substitution reaction is observed. On the other hand,
2409:
2314:
2124:
1107:, exists as an infinite number of conformations with respect to rotation around the C–C bond. Two of these are recognised as energy minimum (
900:
Reaction rates are highly dependent on the conformation of the reactants. In many cases the dominant product arises from the reaction of the
533:
Thus, every 1.36 kcal/mol corresponds to a factor of about 10 in term of equilibrium constant at temperatures around room temperature. (The "
233:
The butane molecule is the simplest molecule for which single bond rotations result in two types of nonequivalent structures, known as the
2611:
Bickelhaupt, F. Matthias; Baerends, Evert Jan (2003-09-15). "The Case for Steric
Repulsion Causing the Staggered Conformation of Ethane".
161:
molecules have different handedness and optical activities, and can only be interconverted by breaking one or more bonds connected to the
2760:
2466:
2203:
1798:
1520:
1236:
groups are in closer proximity than the sum of their van der Waals radii. The interaction between the two methyl groups is repulsive (
1003:
856:
46:
conformation on the left is a transition state between conformers. Above: Newman projection; below: depiction of spatial orientation.
1877:
1734:
850:
constants as measured by NMR. The equation aids in the elucidation of protein folding as well as the conformations of other rigid
1251:
stored in butane conformers with greater steric hindrance than the 'anti'-conformer ground state is given by these values:
905:
1955:
1815:
269:
While simple molecules can be described by these types of conformations, more complex molecules require the use of the
1166:
conformation, which is the energy maximum for ethane. In the eclipsed conformation the torsional angle is minimised.
1115:) forms. The existence of specific conformations is due to hindered rotation around sigma bonds, although a role for
221:. For each molecule, the three substituents emanating from each carbon–carbon bond are staggered, with each H–C–C–H
1052:-butyl group and hydrogen atoms in the 1,3-diaxial position is so strong that the cyclohexane ring will revert to a
345:
Equilibrium distribution of two conformers at different temperatures given the free energy of their interconversion.
1037:
isomer can attain antiperiplanarity only via the unfavored axial conformer; therefore, it does not eliminate. The
804:
359:
209:
1558:
298:
2765:
1535:
1330:
1053:
991:-butylcyclohexyl chloride undergoes elimination because antiperiplanarity of Cl and H can be achieved when the
909:
283:
83:
1553:
1350:
270:
166:
2574:"Rebuttal to the Bickelhaupt–Baerends Case for Steric Repulsion Causing the Staggered Conformation of Ethane"
1360:
1207:
1689:, Vol. 14 (N. L. Allinger, E. L. Eliel and S. H. Wilen, Eds.), Hoboken, NJ:John Wiley & Sons, pp. 1-82;
1563:
1487:
867:
812:
In the case of cyclic systems, the steric effect and contribution to the free energy can be approximated by
138:
1300:
The textbook explanation for the existence of the energy maximum for an eclipsed conformation in ethane is
1041:
isomer is already in the correct geometry in its most stable conformation; therefore, it eliminates easily.
1027:
553:
conformer is −0.47 kcal/mol at 298 K. This gives an equilibrium constant is about 2.2 in favor of the
141:
isomers) where interconversion necessarily involves breaking and reforming of chemical bonds. For example,
1226:
1108:
594:
824:
The short timescale of interconversion precludes the separation of conformational isomers in most cases.
165:
atom and reforming a similar bond in a different direction or spatial orientation. They also differ from
1495:
1181:
1112:
772:
is the molar ideal gas constant (approximately equal to 8.314 J/(mol·K) or 1.987 cal/(mol·K)), and
729:{\displaystyle {\frac {N_{i}}{N_{\text{total}}}}={\frac {e^{-E_{i}/RT}}{\sum _{k=1}^{M}e^{-E_{k}/RT}}}.}
190:, for instance.) Due to rapid interconversion, conformers are usually not isolable at room temperature.
162:
1694:
1186:
2141:
2004:
forms, there is a statistical factor that needs to be taken into account as an entropic term. Thus, Δ
2620:
2073:
1827:
1476:
1309:
1237:
1196:, the two staggered conformations are no longer equivalent and represent two distinct conformers:the
1124:
924:
777:
2483:
2116:
1751:
1568:
1334:
1325:
1073:
843:
350:
318:
30:
888:
is used to measure conformer ratios. For the axial and equatorial conformer of bromocyclohexane, ν
2755:
2164:
1639:
1583:
1573:
1503:
1453:
917:
878:
851:
262:
2039:
1221:
1056:
conformation. The strain in cyclic structures is usually characterized by deviations from ideal
2401:
1790:
2729:
2679:
2671:
2636:
2593:
2554:
2546:
2511:
2503:
2462:
2405:
2347:
2310:
2284:
2266:
2199:
2120:
2089:
2064:
Liu, Shubin (7 February 2013). "Origin and Nature of Bond
Rotation Barriers: A Unified View".
1936:
1873:
1843:
1794:
1730:
1631:
1317:
as analyzed within the
Natural Bond Orbital framework. In the staggered conformation, one C-H
1296:
Relative energies of conformations of butane with respect to rotation of the central C-C bond.
1241:
1174:
1139:
321:, including the secondary and tertiary structure of biopolymers (nucleic acids and proteins).
309:– energetics related to rotation about the single bond between an sp carbon and an sp carbon.
2710:
2663:
2628:
2585:
2538:
2495:
2441:
2393:
2339:
2274:
2258:
2191:
2156:
2108:
2081:
1928:
1835:
1782:
1722:
1714:
1670:
1621:
1499:
1314:
1301:
1282:
1248:
1151:
1116:
839:
797:
158:
95:
1216:
2378:
1515:
1437:
1399:
1354:
1346:
1333:
shows that 2-orbital-4-electron (steric) repulsions are dominant over hyperconjugation. A
1321:
1232:
Both conformations are free of torsional strain, but, in the gauche conformation, the two
1128:
1065:
885:
835:
324:
134:
59:
2109:
1894:
1709:
Alkorta, Ibon; Jose
Elguero; Christian Roussel; Nicolas Vanthuyne; Patrick Piras (2012).
948:
attainable depending on the substituents which might set a conformational lock. Adjacent
2624:
2220:
2077:
1831:
975:-Bu group in the axial position, which is higher in energy by more than 5 kcal/mol (see
938:
341:
2279:
2246:
1718:
1578:
793:
789:
312:
306:
222:
1932:
2749:
2573:
2394:
1783:
1543:
1069:
1061:
928:
523:{\displaystyle K\approx 10^{-\Delta G^{\circ }/(1.36{\text{ kcal}}/\mathrm {mol} )}.}
17:
2706:
2437:
2168:
1666:
1643:
2365:
1963:
1278:
1084:
913:
829:
432:
2484:"Hyperconjugation not steric repulsion leads to the staggered structure of ethane"
956:
positions (that is, both are in axial position, one going up and one going down).
873:
The dynamics of conformational (and other kinds of) isomerism can be monitored by
581:
427:
is the difference in standard free energy between the two conformers in kcal/mol,
1839:
2701:
2432:
1661:
1530:
1525:
1305:
1292:
1159:
1096:
1057:
952:
on a cyclohexane ring can achieve antiperiplanarity only when they occupy trans
949:
863:
825:
293:
289:
198:
187:
182:
127:
119:
67:
2728:
Kenji Monde, Nobuaki Miura, Mai
Hashimoto, Tohru Taniguchi, and Tamotsu Inabe
2160:
1318:
1100:
847:
253:
193:
The study of the energetics between different conformations is referred to as
94:. Conformations that correspond to local maxima on the energy surface are the
2705:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
2675:
2640:
2597:
2550:
2507:
2436:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
2364:
Schneider, H.-J.; Schmidt, G.; Thomas F. J. Am. Chem. Soc., 1983, 105, 3556.
2351:
2270:
1635:
2714:
2445:
2195:
1674:
1626:
1609:
1465:
123:
108:
51:
2683:
2667:
2632:
2589:
2558:
2515:
2262:
2093:
1940:
1847:
276:
More specific examples of conformational isomerism are detailed elsewhere:
78:
that differ by rotation about single bonds can be referred to as different
2288:
953:
916:
is possible if all conformers and their relative stability ruled by their
2247:"Bayesian statistical analysis of protein side-chain rotamer preferences"
1483:
1383:
a torsion angle between 30° and 150° or between −30° and −150° is called
1286:
1163:
796:
interactions of the substituents as well as orbital interactions such as
593:
The fractional population distribution of different conformers follows a
569: < 1). Even for highly unfavorable changes (large positive Δ
75:
70:(refer to figure on single bond rotation). While any two arrangements of
27:
Different molecular structures formed only by rotation about single bonds
2343:
2183:
838:
also generates stable conformational isomers which can be observed. The
2737:
1726:
976:
813:
244:
For example, butane has three conformers relating to its two methyl (CH
178:
2726:
Conformational
Analysis of Chiral Helical Perfluoroalkyl Chains by VCD
2085:
1665:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1996) "
1150:
with respect to the nearest hydrogen atom on the other carbon so that
111:
of interconversion of 1000 seconds or longer), the isomers are termed
2542:
2499:
1713:. Advances in Heterocyclic Chemistry. Vol. 105. pp. 1–188.
1548:
1472:
1233:
1193:
1135:
1104:
1089:
440:
63:
35:
301:, which includes cyclohexane conformations as well as other details.
2400:(Dodr. ed.). Sausalito, CA: University Science Books. p.
1610:"Basic terminology of stereochemistry (IUPAC Recommendations 1996)"
133:
Conformational isomers are thus distinct from the other classes of
1491:
1490:
changes the stereochemistry from the zigzag geometry to that of a
1456:
or "Pitzer strain" refers to resistance to twisting about a bond.
1291:
1155:
1083:
937:
803:
580:
1428:
a torsion angle between 90° and 150° or −90° and −150° is called
1390:
a torsion angle between 0° and ±30° or ±150° and 180° is called
1154:
is minimised. The staggered conformation is more stable by 12.5
1021:-Bu group is in the axial position exerting 7-atom interactions.
71:
780:. The denominator of the right side is the partition function.
1919:
Dunbrack, R. (2002). "Rotamer
Libraries in the 21st Century".
1711:
Atropisomerism and Axial
Chirality in Heteroaromatic Compounds
1414:
a torsion angle between 30° to 90° and −30° to −90° is called
874:
177:) isomers, another class of stereoisomers, which require the
130:
constitutes another common form of conformational isomerism.
1685:Ōki, Michinori (1983) Recent Advances in Atropisomerism, in
1359:
1206:
1173:
staggered conformation left, eclipsed conformation right in
1168:
747:
conformers in thermodynamic equilibrium. On the right side,
927:, which involve the simultaneous removal of a proton and a
286:, including with chair and boat conformations among others.
2537:(6837). Springer Science and Business Media LLC: 539–541.
2529:
Weinhold, Frank (2001). "A new twist on molecular shape".
2028:–0.88 kcal/mol + 0.41 kcal/mol = –0.47 kcal/mol, at 298 K.
1337:
study also emphasizes the importance of steric effects.
923:
One example with configurational isomers is provided by
1281:
exert a greater steric strain because of their greater
82:, conformations that correspond to local minima on the
66:
can be interconverted just by rotations about formally
1667:
Free rotation (hindered rotation, restricted rotation)
252:
conformers, which have the methyls ±60° apart and are
784:
Factors contributing to the free energy of conformers
606:
452:
362:
1872:(8 ed.). Belmont, CA: Brooks/Cole. p. 98.
935:
periplanar positions under the influence of a base.
332:
Free energy and equilibria of conformational isomers
42:
conformation on the right is a conformer, while the
995:-Bu group is in the favorable equatorial position.
2305:Eliel, E. L.; Wilen, S. H.; Mander, L. N. (1994).
1353:for specifying angles (called either torsional or
820:Isolation or observation of conformational isomers
739:The left hand side is the proportion of conformer
728:
522:
408:
38:to interconvert one conformation to another. The
1435:a torsion angle between ±150° and 180° is called
1103:. The smallest alkane with such a chemical bond,
2366:https://pubs.acs.org/doi/pdf/10.1021/ja00349a031
1376:a torsion angle between ±90° and 180° is called
2482:Pophristic, Vojislava; Goodman, Lionel (2001).
2115:(8th ed.). New York: McGraw-Hill. p.
1996:is –0.88 kcal/mol. However, because there are
1574:Molecular Symmetry § Molecular nonrigidity
1142:, a hydrogen atom on one carbon atom has a 60°
2392:Dougherty, Eric V. Anslyn; Dennis, A. (2006).
1397:a torsion angle between 0° and ±30° is called
1369:a torsion angle between 0° and ±90° is called
327:– due to restricted inversion of a bond angle.
2190:. Oxford: Blackwell Scientific Publications.
1357:) between substituents around a single bond:
1095:Alkane conformers arise from rotation around
8:
1009:Thermodynamically unfavored conformation of
409:{\displaystyle K=e^{-\Delta G^{\circ }/RT},}
1816:"The Principles of Conformational Analysis"
1776:
1774:
1772:
1345:Naming alkanes per standards listed in the
1131:of reactions controlled by steric effects.
2423:
2421:
808:Contributions to rotational energy barrier
545:for the transformation of butane from the
315:– due to restricted rotation about a bond.
292:conformations, including medium rings and
2278:
2142:"Conformational analysis of cycloalkanes"
1826:(3945). Elsevier Publishing Co.: 539–44.
1625:
959:One consequence of this analysis is that
707:
701:
693:
683:
672:
654:
648:
640:
634:
623:
613:
607:
605:
501:
496:
491:
480:
474:
463:
451:
390:
384:
373:
361:
2332:Journal of the American Chemical Society
2300:
2298:
2188:IUPAC Compendium of Chemical Terminology
340:
208:
29:
2656:Angewandte Chemie International Edition
2578:Angewandte Chemie International Edition
1781:Anslyn, Eric; Dennis Dougherty (2006).
1595:
2245:Dunbrack, R. L.; Cohen, F. E. (1997).
1693:, DOI: 10.1002/9780470147238.ch1, see
273:to describe the different conformers.
1921:Current Opinion in Structural Biology
577:Population distribution of conformers
7:
2307:Stereochemistry Of Organic Compounds
1603:
1601:
1599:
1017:-butylcyclohexyl chloride where the
2066:The Journal of Physical Chemistry A
1119:is proposed by a competing theory.
2702:Compendium of Chemical Terminology
2433:Compendium of Chemical Terminology
1719:10.1016/B978-0-12-396530-1.00001-2
1662:Compendium of Chemical Terminology
1521:Backbone-dependent rotamer library
857:Backbone-dependent rotamer library
508:
505:
502:
467:
377:
349:Conformational isomers exist in a
25:
2396:Modern Physical Organic Chemistry
1960:University of Illinois at Chicago
1785:Modern Physical Organic Chemistry
557:conformer, or a 31:69 mixture of
443:. In units of kcal/mol at 298 K,
1895:"Butane Conformational Analysis"
1324:donates electron density to the
1225:
1220:
1215:
1185:
1180:
1026:
1002:
896:Conformation-dependent reactions
435:(1.987×10 kcal/mol K), and
1127:and the ability to predict the
1048:The repulsion between an axial
743:in an equilibrating mixture of
439:is the system's temperature in
2572:Weinhold, Frank (2003-09-15).
1984:The standard enthalpy change Δ
1789:. University Science. p.
1255:Gauche, conformer – 3.8 kJ/mol
892:differs by almost 50 cm.
842:relates the dihedral angle of
512:
485:
423:is the equilibrium constant, Δ
34:Rotation about single bond of
1:
2225:Chemical and Engineering News
1933:10.1016/S0959-440X(02)00344-5
1475:groups experience additional
764:) is the energy of conformer
2140:Dragojlovic, Veljko (2015).
1840:10.1126/science.169.3945.539
1211:anti vs gauche conformations
1134:In the example of staggered
904:conformer, by virtue of the
2461:(6 ed.). Brooks Cole.
2227:. American Chemical Society
1200:(left-most, below) and the
2782:
2761:Physical organic chemistry
2381:. Imperial College London.
2107:Carey, Francis A. (2011).
1614:Pure and Applied Chemistry
884:Besides NMR spectroscopy,
2161:10.1007/s40828-015-0014-0
2040:"Conformational Analysis"
1956:"Chapter 6: Conformation"
1687:Topics in Stereochemistry
1559:Macrocyclic stereocontrol
1536:Cyclohexane conformations
1349:is done according to the
1099:hybridised carbon–carbon
914:nucleophilic substitution
337:Equilibrium of conformers
299:Carbohydrate conformation
284:Cyclohexane conformations
241:conformers (see figure).
2736:; 128(18) pp 6000–6001;
2662:(15). Wiley: 1986–1990.
2377:Rzepa, Henry S. (2014).
1699:, accessed 12 June 2014.
1691:published online in 2007
1331:molecular orbital theory
906:Curtin-Hammett principle
830:spectroscopic techniques
86:are specifically called
84:potential energy surface
56:conformational isomerism
2715:10.1351/goldbook.G02593
2446:10.1351/goldbook.T06406
2196:10.1351/goldbook.A00511
2044:Imperial College London
1675:10.1351/goldbook.F02520
1627:10.1351/pac199668122193
1608:Moss, GP (1996-01-01).
1564:Molecular configuration
1488:polytetrafluoroethylene
1076:(Prelog) interactions.
920:is taken into account.
910:configurational isomers
864:cyclohexane derivatives
195:conformational analysis
2668:10.1002/anie.200352931
2633:10.1002/ange.200350947
2590:10.1002/anie.200351777
2457:McMurry, J.E. (2003).
2263:10.1002/pro.5560060807
1814:Barton, Derek (1970).
1482:Replacing hydrogen by
1365:
1297:
1212:
1177:
1111:) and energy maximum (
1109:staggered conformation
1092:
1080:Alkane stereochemistry
944:
809:
730:
688:
595:Boltzmann distribution
590:
524:
410:
346:
214:
88:conformational isomers
47:
2309:. J. Wiley and Sons.
1756:University of Calgary
1496:X-ray crystallography
1363:
1295:
1210:
1204:(right-most, below).
1172:
1113:eclipsed conformation
1087:
941:
925:elimination reactions
807:
731:
668:
584:
525:
411:
344:
212:
33:
18:Chemical conformation
1477:pentane interference
1364:syn/anti peri/clinal
1310:Van der Waals radius
1238:van der Waals strain
1125:asymmetric induction
979:). As a result, the
778:absolute temperature
604:
450:
360:
219:staggered conformers
157:- configurations of
2625:2003AngCh.115.4315B
2344:10.1021/ja01040a022
2078:2013JPCA..117..962L
1966:on 11 November 2013
1899:University of Texas
1868:J, McMurry (2012).
1832:1970Sci...169..539B
1820:Nobel Media AB 2013
1569:Molecular modelling
1554:Klyne–Prelog system
1351:Klyne–Prelog system
1335:valence bond theory
1326:antibonding orbital
1202:gauche conformation
351:dynamic equilibrium
271:Klyne–Prelog system
2738:Graphical abstract
2221:"Karplus Equation"
1584:Strain (chemistry)
1504:circular dichroism
1441:(ap), also called
1418:(sc), also called
1403:(sp), also called
1366:
1304:, but, with a C-C
1298:
1213:
1178:
1093:
945:
810:
726:
591:
520:
406:
347:
280:Ring conformation
215:
48:
2730:J. Am. Chem. Soc.
2619:(35): 4315–4320.
2613:Angewandte Chemie
2584:(35): 4188–4194.
2494:(6837): 565–568.
2459:Organic Chemistry
2411:978-1-891389-31-3
2338:(12): 3223–3225.
2316:978-0-471-01670-0
2182:McNaught (1997).
2126:978-0-07-340261-1
2111:Organic chemistry
2086:10.1021/jp312521z
1870:Organic chemistry
1752:"Stereochemistry"
1620:(12): 2193–2222.
1285:compared to lone
1258:Eclipsed H and CH
1247:A measure of the
1198:anti-conformation
1175:Newman projection
1140:Newman projection
1088:Conformations of
846:protons to their
721:
629:
626:
549:conformer to the
494:
431:is the universal
148:
144:
101:rotational isomer
96:transition states
16:(Redirected from
2773:
2740:
2723:
2717:
2694:
2688:
2687:
2651:
2645:
2644:
2608:
2602:
2601:
2569:
2563:
2562:
2543:10.1038/35079225
2526:
2520:
2519:
2500:10.1038/35079036
2479:
2473:
2472:
2454:
2448:
2425:
2416:
2415:
2399:
2389:
2383:
2382:
2374:
2368:
2362:
2356:
2355:
2327:
2321:
2320:
2302:
2293:
2292:
2282:
2257:(8): 1661–1681.
2242:
2236:
2235:
2233:
2232:
2219:Dalton, Louisa.
2216:
2210:
2209:
2179:
2173:
2172:
2146:
2137:
2131:
2130:
2114:
2104:
2098:
2097:
2061:
2055:
2054:
2052:
2050:
2035:
2029:
1982:
1976:
1975:
1973:
1971:
1962:. Archived from
1951:
1945:
1944:
1916:
1910:
1909:
1907:
1905:
1890:
1884:
1883:
1865:
1859:
1858:
1856:
1854:
1811:
1805:
1804:
1788:
1778:
1767:
1766:
1764:
1762:
1747:
1741:
1740:
1706:
1700:
1683:
1677:
1654:
1648:
1647:
1629:
1605:
1500:NMR spectroscopy
1454:Torsional strain
1315:hyperconjugation
1308:of 154 pm and a
1302:steric hindrance
1283:electron density
1249:potential energy
1229:
1224:
1219:
1189:
1184:
1152:steric hindrance
1117:hyperconjugation
1066:torsional angles
1030:
1006:
931:from vicinal or
840:Karplus equation
798:hyperconjugation
735:
733:
732:
727:
722:
720:
719:
718:
711:
706:
705:
687:
682:
666:
665:
658:
653:
652:
635:
630:
628:
627:
624:
618:
617:
608:
529:
527:
526:
521:
516:
515:
511:
500:
495:
492:
484:
479:
478:
415:
413:
412:
407:
402:
401:
394:
389:
388:
146:
142:
21:
2781:
2780:
2776:
2775:
2774:
2772:
2771:
2770:
2766:Stereochemistry
2746:
2745:
2744:
2743:
2724:
2720:
2695:
2691:
2653:
2652:
2648:
2610:
2609:
2605:
2571:
2570:
2566:
2528:
2527:
2523:
2481:
2480:
2476:
2469:
2456:
2455:
2451:
2426:
2419:
2412:
2391:
2390:
2386:
2376:
2375:
2371:
2363:
2359:
2329:
2328:
2324:
2317:
2304:
2303:
2296:
2251:Protein Science
2244:
2243:
2239:
2230:
2228:
2218:
2217:
2213:
2206:
2181:
2180:
2176:
2144:
2139:
2138:
2134:
2127:
2106:
2105:
2101:
2063:
2062:
2058:
2048:
2046:
2037:
2036:
2032:
1983:
1979:
1969:
1967:
1954:Bruzik, Karol.
1953:
1952:
1948:
1918:
1917:
1913:
1903:
1901:
1893:Bauld, Nathan.
1892:
1891:
1887:
1880:
1867:
1866:
1862:
1852:
1850:
1813:
1812:
1808:
1801:
1780:
1779:
1770:
1760:
1758:
1749:
1748:
1744:
1737:
1708:
1707:
1703:
1684:
1680:
1655:
1651:
1607:
1606:
1597:
1592:
1516:Anomeric effect
1512:
1471:, the terminal
1462:
1355:dihedral angles
1347:IUPAC Gold Book
1343:
1322:bonding orbital
1272:
1268:
1261:
1230:
1190:
1144:torsional angle
1129:stereochemistry
1082:
1046:
1045:
1044:
1043:
1042:
1031:
1023:
1022:
1007:
898:
891:
886:IR spectroscopy
836:Protein folding
822:
788:The effects of
786:
755:
697:
689:
667:
644:
636:
619:
609:
602:
601:
579:
470:
459:
448:
447:
380:
369:
358:
357:
339:
334:
325:Akamptisomerism
247:
228:
207:
139:configurational
126:of substituted
60:stereoisomerism
28:
23:
22:
15:
12:
11:
5:
2779:
2777:
2769:
2768:
2763:
2758:
2748:
2747:
2742:
2741:
2718:
2689:
2646:
2603:
2564:
2521:
2474:
2468:978-0534000134
2467:
2449:
2417:
2410:
2384:
2379:"Cycloalkanes"
2369:
2357:
2322:
2315:
2294:
2237:
2211:
2205:978-0967855097
2204:
2184:"Atropisomers"
2174:
2132:
2125:
2099:
2072:(5): 962–965.
2056:
2038:Rzepa, Henry.
2030:
1977:
1946:
1927:(4): 431–440.
1911:
1885:
1878:
1860:
1806:
1800:978-1891389313
1799:
1768:
1742:
1735:
1701:
1678:
1649:
1594:
1593:
1591:
1588:
1587:
1586:
1581:
1579:Steric effects
1576:
1571:
1566:
1561:
1556:
1551:
1546:
1541:
1540:
1539:
1528:
1523:
1518:
1511:
1508:
1461:
1458:
1451:
1450:
1438:antiperiplanar
1433:
1426:
1412:
1395:
1388:
1381:
1374:
1342:
1339:
1275:
1274:
1270:
1266:
1263:
1259:
1256:
1242:energy barrier
1214:
1179:
1081:
1078:
1032:
1025:
1024:
1008:
1001:
1000:
999:
998:
997:
902:less prevalent
897:
894:
889:
821:
818:
785:
782:
751:
737:
736:
725:
717:
714:
710:
704:
700:
696:
692:
686:
681:
678:
675:
671:
664:
661:
657:
651:
647:
643:
639:
633:
622:
616:
612:
578:
575:
531:
530:
519:
514:
510:
507:
504:
499:
490:
487:
483:
477:
473:
469:
466:
462:
458:
455:
417:
416:
405:
400:
397:
393:
387:
383:
379:
376:
372:
368:
365:
338:
335:
333:
330:
329:
328:
322:
316:
313:Atropisomerism
310:
307:Allylic strain
304:
303:
302:
296:
287:
248:) groups: two
245:
226:
223:dihedral angle
206:
203:
120:atropisomerism
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2778:
2767:
2764:
2762:
2759:
2757:
2754:
2753:
2751:
2739:
2735:
2731:
2727:
2722:
2719:
2716:
2712:
2708:
2704:
2703:
2698:
2693:
2690:
2685:
2681:
2677:
2673:
2669:
2665:
2661:
2657:
2650:
2647:
2642:
2638:
2634:
2630:
2626:
2622:
2618:
2615:(in German).
2614:
2607:
2604:
2599:
2595:
2591:
2587:
2583:
2579:
2575:
2568:
2565:
2560:
2556:
2552:
2548:
2544:
2540:
2536:
2532:
2525:
2522:
2517:
2513:
2509:
2505:
2501:
2497:
2493:
2489:
2485:
2478:
2475:
2470:
2464:
2460:
2453:
2450:
2447:
2443:
2439:
2438:torsion angle
2435:
2434:
2429:
2424:
2422:
2418:
2413:
2407:
2403:
2398:
2397:
2388:
2385:
2380:
2373:
2370:
2367:
2361:
2358:
2353:
2349:
2345:
2341:
2337:
2333:
2326:
2323:
2318:
2312:
2308:
2301:
2299:
2295:
2290:
2286:
2281:
2276:
2272:
2268:
2264:
2260:
2256:
2252:
2248:
2241:
2238:
2226:
2222:
2215:
2212:
2207:
2201:
2197:
2193:
2189:
2185:
2178:
2175:
2170:
2166:
2162:
2158:
2154:
2150:
2143:
2136:
2133:
2128:
2122:
2118:
2113:
2112:
2103:
2100:
2095:
2091:
2087:
2083:
2079:
2075:
2071:
2067:
2060:
2057:
2045:
2041:
2034:
2031:
2027:
2023:
2019:
2015:
2011:
2007:
2003:
1999:
1995:
1991:
1987:
1981:
1978:
1965:
1961:
1957:
1950:
1947:
1942:
1938:
1934:
1930:
1926:
1922:
1915:
1912:
1900:
1896:
1889:
1886:
1881:
1879:9780840054449
1875:
1871:
1864:
1861:
1849:
1845:
1841:
1837:
1833:
1829:
1825:
1821:
1817:
1810:
1807:
1802:
1796:
1792:
1787:
1786:
1777:
1775:
1773:
1769:
1757:
1753:
1746:
1743:
1738:
1736:9780123965301
1732:
1728:
1724:
1720:
1716:
1712:
1705:
1702:
1698:
1695:
1692:
1688:
1682:
1679:
1676:
1672:
1668:
1664:
1663:
1658:
1653:
1650:
1645:
1641:
1637:
1633:
1628:
1623:
1619:
1615:
1611:
1604:
1602:
1600:
1596:
1589:
1585:
1582:
1580:
1577:
1575:
1572:
1570:
1567:
1565:
1562:
1560:
1557:
1555:
1552:
1550:
1547:
1545:
1544:Gauche effect
1542:
1537:
1534:
1533:
1532:
1529:
1527:
1524:
1522:
1519:
1517:
1514:
1513:
1509:
1507:
1506:in solution.
1505:
1501:
1497:
1493:
1489:
1485:
1480:
1478:
1474:
1470:
1468:
1460:Special cases
1459:
1457:
1455:
1448:
1444:
1440:
1439:
1434:
1431:
1427:
1425:
1421:
1417:
1413:
1410:
1406:
1402:
1401:
1400:synperiplanar
1396:
1393:
1389:
1386:
1382:
1379:
1375:
1372:
1368:
1367:
1362:
1358:
1356:
1352:
1348:
1340:
1338:
1336:
1332:
1329:quantitative
1327:
1323:
1320:
1316:
1311:
1307:
1303:
1294:
1290:
1288:
1284:
1280:
1279:methyl groups
1277:The eclipsed
1264:
1257:
1254:
1253:
1252:
1250:
1245:
1243:
1239:
1235:
1228:
1223:
1218:
1209:
1205:
1203:
1199:
1195:
1188:
1183:
1176:
1171:
1167:
1165:
1161:
1157:
1153:
1149:
1148:torsion angle
1145:
1141:
1137:
1132:
1130:
1126:
1120:
1118:
1114:
1110:
1106:
1102:
1098:
1091:
1086:
1079:
1077:
1075:
1071:
1070:Pitzer strain
1067:
1063:
1062:Baeyer strain
1059:
1055:
1051:
1040:
1036:
1029:
1020:
1016:
1012:
1005:
996:
994:
990:
986:
982:
978:
974:
970:
966:
962:
957:
955:
951:
940:
936:
934:
930:
929:leaving group
926:
921:
919:
915:
911:
907:
903:
895:
893:
887:
882:
880:
876:
871:
869:
865:
860:
858:
853:
849:
845:
841:
837:
833:
831:
827:
819:
817:
815:
806:
802:
799:
795:
791:
790:electrostatic
783:
781:
779:
775:
771:
767:
763:
760:= 1, 2, ...,
759:
754:
750:
746:
742:
723:
715:
712:
708:
702:
698:
694:
690:
684:
679:
676:
673:
669:
662:
659:
655:
649:
645:
641:
637:
631:
620:
614:
610:
600:
599:
598:
596:
588:
583:
576:
574:
572:
568:
564:
560:
556:
552:
548:
544:
538:
536:
517:
497:
488:
481:
475:
471:
464:
460:
456:
453:
446:
445:
444:
442:
438:
434:
430:
426:
422:
403:
398:
395:
391:
385:
381:
374:
370:
366:
363:
356:
355:
354:
352:
343:
336:
331:
326:
323:
320:
317:
314:
311:
308:
305:
300:
297:
295:
291:
288:
285:
282:
281:
279:
278:
277:
274:
272:
267:
264:
259:
255:
251:
242:
240:
236:
231:
225:(and H–C–C–CH
224:
220:
211:
204:
202:
200:
196:
191:
189:
184:
180:
176:
172:
168:
164:
160:
156:
152:
140:
136:
135:stereoisomers
131:
129:
125:
121:
118:
114:
110:
106:
102:
97:
93:
89:
85:
81:
80:conformations
77:
73:
69:
65:
62:in which the
61:
58:is a form of
57:
53:
45:
41:
37:
32:
19:
2733:
2725:
2721:
2700:
2692:
2659:
2655:
2649:
2616:
2612:
2606:
2581:
2577:
2567:
2534:
2530:
2524:
2491:
2487:
2477:
2458:
2452:
2431:
2395:
2387:
2372:
2360:
2335:
2331:
2325:
2306:
2254:
2250:
2240:
2229:. Retrieved
2224:
2214:
2187:
2177:
2152:
2148:
2135:
2110:
2102:
2069:
2065:
2059:
2047:. Retrieved
2043:
2033:
2025:
2021:
2017:
2013:
2009:
2005:
2001:
1997:
1993:
1989:
1985:
1980:
1968:. Retrieved
1964:the original
1959:
1949:
1924:
1920:
1914:
1902:. Retrieved
1898:
1888:
1869:
1863:
1851:. Retrieved
1823:
1819:
1809:
1784:
1759:. Retrieved
1755:
1745:
1710:
1704:
1690:
1686:
1681:
1660:
1652:
1617:
1613:
1481:
1466:
1463:
1452:
1449:conformation
1446:
1442:
1436:
1429:
1423:
1419:
1415:
1411:conformation
1408:
1404:
1398:
1391:
1384:
1377:
1370:
1344:
1341:Nomenclature
1299:
1276:
1273:– 19 kJ/mol.
1246:
1231:
1201:
1197:
1191:
1147:
1143:
1133:
1121:
1094:
1074:transannular
1054:twisted boat
1049:
1047:
1038:
1034:
1018:
1014:
1010:
992:
988:
984:
980:
972:
968:
964:
960:
958:
950:substituents
946:
943:conformation
932:
922:
901:
899:
883:
872:
861:
834:
826:Atropisomers
823:
811:
787:
773:
769:
765:
761:
757:
752:
748:
744:
740:
738:
592:
586:
570:
566:
562:
558:
554:
550:
546:
542:
539:
534:
532:
436:
433:gas constant
428:
424:
420:
418:
348:
275:
268:
257:
254:enantiomeric
249:
243:
238:
234:
232:
218:
216:
194:
192:
183:double bonds
174:
170:
154:
150:
132:
128:cyclohexanes
116:
113:atropisomers
112:
104:
100:
91:
87:
79:
68:single bonds
55:
49:
43:
39:
2049:11 November
1970:10 November
1853:10 November
1750:Hunt, Ian.
1727:10261/62060
1531:Cyclohexane
1526:Cycloalkane
1306:bond length
1265:Eclipsed CH
1262:– 16 kJ/mol
1101:sigma bonds
1058:bond angles
868:coalescence
294:macrocycles
290:Cycloalkane
199:drug design
179:π-component
2750:Categories
2231:2013-10-27
1904:28 October
1761:28 October
1590:References
1430:anticlinal
1392:periplanar
1240:), and an
848:J-coupling
493: kcal
92:conformers
2756:Isomerism
2676:1433-7851
2641:0044-8249
2598:1433-7851
2551:0028-0836
2508:1476-4687
2352:0002-7863
2271:0961-8368
2149:Chemtexts
2000:possible
1636:1365-3075
1498:and from
1416:synclinal
1244:results.
1162:than the
1064:), ideal
879:fluxional
852:aliphatic
695:−
670:∑
642:−
535:1.36 rule
476:∘
468:Δ
465:−
457:≈
386:∘
378:Δ
375:−
256:, and an
167:geometric
124:ring-flip
109:half-life
52:chemistry
2684:15065281
2559:11385553
2516:11385566
2169:94348487
2094:23327680
1941:12163064
1848:17746022
1644:98272391
1510:See also
1484:fluorine
1469:-pentane
1287:hydrogen
1164:eclipsed
814:A values
76:molecule
44:eclipsed
2621:Bibcode
2289:9260279
2280:2143774
2074:Bibcode
2022:H° + RT
1988:° from
1828:Bibcode
1289:atoms.
977:A value
954:diaxial
844:vicinal
776:is the
441:kelvins
319:Folding
239:gauche-
159:organic
137:(i. e.
122:). The
105:rotamer
64:isomers
2707:gauche
2682:
2674:
2639:
2596:
2557:
2549:
2531:Nature
2514:
2506:
2488:Nature
2465:
2408:
2350:
2313:
2287:
2277:
2269:
2202:
2167:
2123:
2092:
2008:° = Δ
2002:gauche
1990:gauche
1939:
1876:
1846:
1797:
1733:
1642:
1634:
1549:Isomer
1473:methyl
1447:trans-
1420:gauche
1385:clinal
1269:and CH
1234:methyl
1194:butane
1136:ethane
1105:ethane
1090:Ethane
918:strain
794:steric
589:-axis)
559:gauche
547:gauche
419:where
263:strain
250:gauche
237:- and
188:amides
163:chiral
149:- and
40:gauche
36:butane
2697:IUPAC
2428:IUPAC
2165:S2CID
2155:(3).
2145:(PDF)
2024:ln 2
1657:IUPAC
1640:S2CID
1492:helix
1443:anti-
1319:sigma
1072:) or
1035:trans
1011:trans
961:trans
625:total
205:Types
175:trans
74:in a
72:atoms
2734:2006
2680:PMID
2672:ISSN
2637:ISSN
2594:ISSN
2555:PMID
2547:ISSN
2512:PMID
2504:ISSN
2463:ISBN
2406:ISBN
2348:ISSN
2311:ISBN
2285:PMID
2267:ISSN
2200:ISBN
2121:ISBN
2090:PMID
2051:2013
2018:S° =
2012:° –
1994:anti
1972:2013
1937:PMID
1906:2013
1874:ISBN
1855:2013
1844:PMID
1795:ISBN
1763:2013
1731:ISBN
1696:and
1632:ISSN
1502:and
1432:(ac)
1424:skew
1409:cis-
1405:syn-
1378:anti
1033:The
1015:tert
989:tert
965:tert
933:anti
792:and
563:anti
555:anti
551:anti
489:1.36
258:anti
235:anti
117:see:
2711:doi
2709:".
2664:doi
2629:doi
2617:115
2586:doi
2539:doi
2535:411
2496:doi
2492:411
2442:doi
2440:".
2402:104
2340:doi
2275:PMC
2259:doi
2192:doi
2157:doi
2117:105
2082:doi
2070:117
1998:two
1992:to
1929:doi
1836:doi
1824:169
1723:hdl
1715:doi
1671:doi
1669:".
1622:doi
1486:in
1464:In
1445:or
1422:or
1407:or
1394:(p)
1387:(c)
1380:(a)
1373:(s)
1371:syn
1192:In
1160:mol
1146:or
1138:in
1039:cis
1013:-4-
987:-4-
985:cis
963:-4-
890:CBr
881:".
875:NMR
862:In
597::
181:of
171:cis
103:or
90:or
50:In
2752::
2732:;
2699:,
2678:.
2670:.
2660:43
2658:.
2635:.
2627:.
2592:.
2582:42
2580:.
2576:.
2553:.
2545:.
2533:.
2510:.
2502:.
2490:.
2486:.
2430:,
2420:^
2404:.
2346:.
2336:91
2334:.
2297:^
2283:.
2273:.
2265:.
2253:.
2249:.
2223:.
2198:.
2186:.
2163:.
2151:.
2147:.
2119:.
2088:.
2080:.
2068:.
2042:.
1958:.
1935:.
1925:12
1923:.
1897:.
1842:.
1834:.
1822:.
1818:.
1793:.
1791:95
1771:^
1754:.
1729:.
1721:.
1659:,
1638:.
1630:.
1618:68
1616:.
1612:.
1598:^
1479:.
1156:kJ
1097:sp
859:.
832:.
768:,
571:G°
543:G°
461:10
425:G°
201:.
54:,
2713::
2686:.
2666::
2643:.
2631::
2623::
2600:.
2588::
2561:.
2541::
2518:.
2498::
2471:.
2444::
2414:.
2354:.
2342::
2319:.
2291:.
2261::
2255:6
2234:.
2208:.
2194::
2171:.
2159::
2153:1
2129:.
2096:.
2084::
2076::
2053:.
2026:=
2020:Δ
2016:Δ
2014:T
2010:H
2006:G
1986:H
1974:.
1943:.
1931::
1908:.
1882:.
1857:.
1838::
1830::
1803:.
1765:.
1739:.
1725::
1717::
1673::
1646:.
1624::
1538:.
1467:n
1271:3
1267:3
1260:3
1158:/
1068:(
1060:(
1050:t
1019:t
993:t
981:t
973:t
969:t
774:T
770:R
766:k
762:M
758:k
756:(
753:k
749:E
745:M
741:i
724:.
716:T
713:R
709:/
703:k
699:E
691:e
685:M
680:1
677:=
674:k
663:T
660:R
656:/
650:i
646:E
638:e
632:=
621:N
615:i
611:N
587:x
567:K
561::
518:.
513:)
509:l
506:o
503:m
498:/
486:(
482:/
472:G
454:K
437:T
429:R
421:K
404:,
399:T
396:R
392:/
382:G
371:e
367:=
364:K
246:3
227:3
173:/
169:(
155:S
153:/
151:R
147:D
145:/
143:L
115:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.