213:
668:
38:
149:
634:. However, the N or C termini of the peptide are often crucial components in obtaining the desired folding pattern for the recombinant protein, making simple end-to-end conjoining of domains ineffective in this case. For this reason, a protein linker is often needed to maintain the functionality of the protein domains of interest.
544:
Novel recombinant technologies have made it possible to improve fusion protein design for use in fields as diverse as biodetection, paper and food industries, and biopharmaceuticals. Recent improvements have involved the fusion of single peptides or protein fragments to regions of existing proteins,
191:
fluorescent tag, but the development of Kikume green-red (KikGR) in 2005 offers a brighter signal and more efficient photoconversion. The advantage of using PCFP fluorescent tags is the ability to track the interaction of overlapping biochemical pathways in real time. The tag will change color from
675:
Protein linkers aid fusion protein design by providing appropriate spacing between domains, supporting correct protein folding in the case that N or C termini interactions are crucial to folding. Commonly, protein linkers permit important domain interactions, reinforce stability, and reduce steric
642:
This technique involves the fusion of consecutive protein domains by encoding desired structures into a single polypeptide chain, but sometimes may require insertion of a domain within another domain. This technique is typically regarding as more difficult to carry out than tandem fusion, due to
621:. Since then, a variety of fusion protein design techniques have been developed for applications as diverse as fluorescent protein tags to recombinant fusion protein drugs. Three commonly used design techniques include tandem fusion, domain insertion, and post-translational conjugation.
781:
There are also rare examples of naturally occurring polypeptides that appear to be a fusion of two clearly defined modules, in which each module displays its characteristic activity or function, independent of the other. Two major examples are: double PP2C chimera in
478:, linkers in protein or peptide fusions are sometimes engineered with cleavage sites for proteases or chemical agents that enable the liberation of the two separate proteins. This technique is often used for identification and purification of proteins, by fusing a
136:
Many whole gene fusions are fully functional and can still act to replace the original peptides. Some, however, experience interactions between the two proteins that can modify their functions. Beyond these effects, some gene fusions may cause
108:, tandem duplication, or retrotransposition creates a novel coding sequence containing parts of the coding sequences from two different genes. Naturally occurring fusion proteins are commonly found in cancer cells, where they may function as
132:
of the original proteins. However, other fusion proteins, especially those that occur naturally, combine only portions of coding sequences and therefore do not maintain the original functions of the parental genes that formed them.
473:
If the two entities are proteins, often linker (or "spacer") peptides are also added, which make it more likely that the proteins fold independently and behave as expected. Especially in the case where the linkers enable
629:
The proteins of interest are simply connected end-to-end via fusion of N or C termini between the proteins. This provides a flexible bridge structure allowing enough space between fusion partners to ensure proper
324:. TNFR provides specificity for the drug target and the antibody Fc segment is believed to add stability and deliverability of the drug. Additional chimeric proteins used for therapeutic applications include:
192:
green to red once the protein reaches a point of interest in the pathway, and the alternate colored protein can be monitored through the duration of pathway. This technique is especially useful when studying
570:: A common challenge in fusion protein design is the issue of insolubility of newly synthesized fusion proteins in the recombinant host, leading to an over-aggregation of the target protein in the cell.
580:: Singular peptides or protein fragments are typically added to reduce flexibility of either the N or C terminus of the target protein, which reinforces thermostability and stabilizes
171:
to proteins in a host cell is a widely popular technique used in experimental cell and biology research in order to track protein interactions in real time. The first fluorescent tag,
676:
hindrance, making them preferred for use in fusion protein design even when N and C termini can be fused. Three major types of linkers are flexible, rigid, and in vivo cleavable.
574:
that are able to aid in protein folding may be added, thereby better segregating hydrophobic and hydrophilic interactions in the solute to increase protein solubility.
181:
and is still used frequently in modern research. More recent derivations include photoconvertible fluorescent proteins (PCFPs), which were first isolated from
459:
843:
Schmidt A, Wiesner B, Schülein R, Teichmann A (2014). "Use of Kaede and Kikume Green–Red
Fusions for Live Cell Imaging of G Protein-Coupled Receptors".
605:
Immobilization: PHA synthase, an enzyme that allows for the immobilization of proteins of interest, is an important fusion tag in industrial research.
1151:"Identification and characterization of an unusual double serine/threonine protein phosphatase 2C in the malaria parasite Plasmodium falciparum"
354:
706:
are unique in that they are designed to allow the release of one or more fused domains under certain reaction conditions, such as a specific
617:
The earliest applications of recombinant protein design can be documented in the use of single peptide tags for purification of proteins in
423:
1293:
608:
Crystal quality: Crystal quality can be improved by adding covalent links between proteins, aiding in structure determination techniques.
470:
as a single protein. The protein can be engineered to include the full sequence of both original proteins, or only a portion of either.
860:
268:
304:
In addition to chimeric and humanized antibodies, there are other pharmaceutical purposes for the creation of chimeric constructs.
145:, the shuffling of different active sites and binding domains have the potential to result in new proteins with novel functions.
942:"Construction and use of a Cupriavidus necator H16 soluble hydrogenase promoter (PSH) fusion to gfp (green fluorescent protein)"
1006:
Yang H, Liu L, Xu F (October 2016). "The promises and challenges of fusion constructs in protein biochemistry and enzymology".
243:
was developed using mice and hence were initially "mouse" antibodies. As non-human proteins, mouse antibodies tend to evoke an
193:
321:
313:
298:
138:
1355:
659:
of the proteins of interest, in contrast to genetic fusion prior to translation used in other recombinant technologies.
644:
332:
117:
228:
is to impart properties from each of the "parent" proteins to the resulting chimeric protein. Several chimeric protein
309:
498:
by fusion to the original proteins of peptide domains that induce artificial protein di- or multimerization (e.g.,
251:
the replacement of segments of the antibody molecule that distinguish it from a human antibody. For example, human
75:
724:
527:
172:
105:
116:
is a well-known example of an oncogenic fusion protein, and is considered to be the primary oncogenic driver of
1326:
789:
732:
788:(the malaria parasite), in which each PP2C module exhibits protein phosphatase 2C enzymatic activity, and the
618:
595:
491:
378:
41:
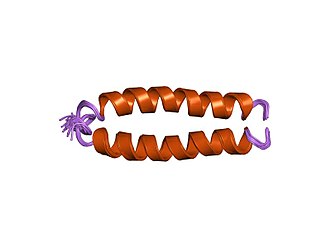
A chimeric protein including two subunits and a linker protein synthesized via recombinant fusion technology
31:
823:
1312:
768:
763:
758:
561:
154:
113:
56:(literally, made of parts from different sources) are proteins created through the joining of two or more
1335:
784:
740:
656:
285:
antibodies are obtained. Although not conceptually distinct from chimeras, this type is indicated using
260:
236:
897:
Baldo BA (May 2015). "Chimeric fusion proteins used for therapy: indications, mechanisms, and safety".
212:
571:
553:
475:
392:
336:
1322:
818:
813:
775:
736:
495:
439:
406:
396:
350:
248:
1031:
922:
557:
410:
368:
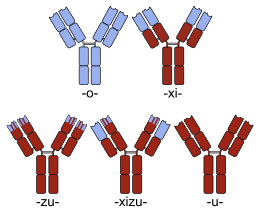
220:(bottom-left) monoclonal antibodies. Human parts are shown in brown, and non-human parts in blue.
84:
697:
residues, which can be helpful when highly specific spacing between domains must be maintained.
196:(GPCR) recycling pathways. The fates of recycled G-protein receptors may either be sent to the
1272:
1223:
1172:
1131:
1075:
1058:
Yu K, Liu C, Kim BG, Lee DY (2015-01-01). "Synthetic fusion protein design and applications".
1023:
973:
914:
866:
856:
847:. Methods in Molecular Biology. Vol. 1174. New York, NY: Humana Press. pp. 139–156.
494:
with nickel or cobalt resins. Di- or multimeric chimeric proteins can be manufactured through
450:
sequence coding for the first protein, then appending the cDNA sequence of the second protein
177:
30:
This article is about chimeric fusion proteins. For proteins involved in membrane fusion, see
1262:
1254:
1213:
1203:
1162:
1121:
1113:
1067:
1015:
963:
953:
906:
848:
317:
225:
259:
portions of the drug without altering its specificity for the intended therapeutic target.
1339:
631:
577:
503:
463:
252:
244:
197:
188:
168:
129:
731:
of one gene with intact exons from a second gene. This creates a single gene that can be
1267:
1242:
1218:
1191:
1126:
1101:
968:
941:
467:
256:
1349:
1307:
1071:
793:
588:
533:
451:
340:
142:
1035:
926:
556:: Fusion of certain peptides allow for greater catalytic efficiency by altering the
599:
499:
422:
93:
79:
67:
331:: A human recombinant protein that aids in the treatment of oxaliplatin-resistant
792:
that occur in a number of unicellular organisms (such as protozoan parasites and
852:
797:
711:
374:
328:
109:
62:
17:
1117:
514:
attached to them in order to study disease development. Hydrogenase promoter, P
1258:
1019:
910:
567:
546:
511:
388:
346:
305:
290:
1243:"On the role, ecology, phylogeny, and structure of dual-family immunophilins"
1167:
1150:
804:
chaperone modules. The evolutionary origin of such chimera remains unclear.
400:
384:
360:
281:
272:
217:
1276:
1227:
1208:
1135:
1079:
1027:
977:
918:
870:
391:: Interferes with T-cell co-stimulation to treat autoimmune disorders like
1176:
667:
602:), serve to enhance enzyme expression and secretion of the target protein.
37:
748:
483:
279:). If parts of the variable domains are also replaced by human portions,
201:
183:
101:
958:
694:
687:
residues, giving them the ability curl into a dynamic, adaptable shape.
684:
487:
479:
435:
426:
Fusion of two genes (BCR-ABL) to encode a recombinant oncogenic protein
240:
200:
to be recycled, marked by a green fluorescent tag, or may be sent to a
70:
with functional properties derived from each of the original proteins.
128:
Some fusion proteins combine whole peptides and therefore contain all
744:
455:
364:
367:
responses by selectively targeting effector memory T-cells to treat
148:
27:
Protein created by joining other proteins into a single polypeptide
723:
Naturally occurring fusion genes are most commonly created when a
666:
594:
Expression levels: Addition of numerous fusion fragments, such as
507:
443:
60:
that originally coded for separate proteins. Translation of this
801:
728:
447:
229:
57:
255:
can be introduced, thereby eliminating most of the potentially
1342:: The Server Protein–Protein Interaction of Chimeric Proteins.
1332:
247:
if administered to humans. The chimerization process involves
152:
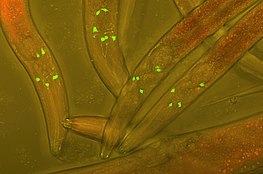
Green fluorescent protein (GFP) inserted into the neurons of
1149:
Mamoun CB, Sullivan DJ, Banerjee R, Goldberg DE (May 1998).
1102:"Fusion protein linkers: property, design and functionality"
442:
of a fusion gene. This typically involves removing the stop
707:
581:
587:
Enzyme activity: Fusion that involves the introduction of
655:
This technique fuses protein domains following ribosomal
96:
having different functions or physico-chemical patterns.
940:
Jugder BE, Welch J, Braidy N, Marquis CP (2016-07-26).
743:
to produce a functional fusion protein. Many important
549:, and are known to increase the following properties:
216:
Sketches of mouse (top-left), chimeric (top-right) and
1190:
Adams B, Musiyenko A, Kumar R, Barik S (July 2005).
671:
A protein used as a linker in fusion protein design
349:: Reduces inflammation by preventing activation of
204:for degradation, marked by a red fluorescent tag.
710:gradient, or when coming in contact with another
506:). Fusion proteins can also be manufactured with
263:indicates this type of modification by inserting
141:that alter when and where these genes act. For
591:may be used to expand overall enzyme activity.
312:blocker created through the combination of a
8:
1192:"A novel class of dual-family immunophilins"
774:Antibodies are fusion proteins produced by
224:The purpose of creating fusion proteins in
92:usually designate hybrid proteins made of
1325:at the U.S. National Library of Medicine
1266:
1217:
1207:
1166:
1125:
1100:Chen X, Zaro JL, Shen WC (October 2013).
967:
957:
490:(6xHis-tag), which can be isolated using
232:are currently available for medical use.
598:(MBP) or small ubiquitin-like molecule (
421:
211:
147:
36:
835:
751:are fusion genes produced in this way.
355:cryopyrin-associated periodic syndromes
1008:Applied Microbiology and Biotechnology
187:. The most commonly used PCFP is the
7:
1095:
1093:
1091:
1089:
1053:
1051:
1049:
1047:
1045:
1001:
999:
997:
995:
993:
991:
989:
987:
892:
890:
888:
886:
884:
882:
880:
1196:The Journal of Biological Chemistry
1155:The Journal of Biological Chemistry
158:worms to track neuronal development
643:difficulty finding an appropriate
462:. That DNA sequence will then be
78:for use in biological research or
25:
1072:10.1016/j.biotechadv.2014.11.005
235:Many chimeric protein drugs are
66:results in a single or multiple
693:may be formed of large, cyclic
100:occur naturally when a complex
1106:Advanced Drug Delivery Reviews
651:Post-translational conjugation
647:site in the gene of interest.
518:, was studied constructing a P
314:tumor necrosis factor receptor
1:
299:list of monoclonal antibodies
1313:Resources in other libraries
1247:Cell Stress & Chaperones
333:metastatic colorectal cancer
118:chronic myelogenous leukemia
74:are created artificially by
853:10.1007/978-1-4939-0944-5_9
528:green fluorescent protein (
72:Recombinant fusion proteins
1372:
1118:10.1016/j.addr.2012.09.039
845:Exocytosis and Endocytosis
796:) and contain full-length
683:may consist of many small
613:Recombinant protein design
432:recombinant fusion protein
377:: A peptibody that treats
194:G-protein coupled receptor
76:recombinant DNA technology
29:
1308:Resources in your library
1259:10.1007/s12192-017-0813-x
1241:Barik S (November 2017).
1020:10.1007/s00253-016-7795-y
911:10.1007/s40264-015-0285-9
790:dual-family immunophilins
725:chromosomal translocation
540:Recombinant functionality
175:(GFP), was isolated from
173:green fluorescent protein
106:chromosomal translocation
1327:Medical Subject Headings
1323:Mutant+Chimeric+Proteins
1168:10.1074/jbc.273.18.11241
239:whose specificity for a
163:Fluorescent protein tags
98:Chimeric mutant proteins
619:affinity chromatography
596:maltose binding protein
492:affinity chromatography
379:immune thrombocytopenia
32:membrane fusion protein
1209:10.1074/jbc.M500990200
1060:Biotechnology Advances
769:Tpr-met fusion protein
764:Bcr-abl fusion protein
759:Gag-onc fusion protein
727:replaces the terminal
672:
564:of the target protein.
427:
418:Recombinant technology
221:
208:Chimeric protein drugs
159:
155:Caenorhabditis elegans
114:bcr-abl fusion protein
42:
785:Plasmodium falciparum
670:
460:overlap extension PCR
425:
261:Antibody nomenclature
237:monoclonal antibodies
215:
151:
40:
572:Molecular chaperones
562:quaternary structure
554:Catalytic efficiency
476:protein purification
393:rheumatoid arthritis
337:macular degeneration
308:, for example, is a
269:non-proprietary name
143:partial gene fusions
1356:Engineered proteins
819:Protein engineering
814:Genetic engineering
776:V(D)J recombination
496:genetic engineering
440:genetic engineering
407:Denileukin-diftitox
397:psoriatic arthritis
301:for more examples.
1338:2021-11-10 at the
959:10.7717/peerj.2269
824:Cell–cell fusogens
754:Examples include:
719:Natural occurrence
673:
428:
411:cutaneous lymphoma
369:psoriasis vulgaris
222:
160:
139:regulatory changes
130:functional domains
43:
1294:Library resources
704:cleavable linkers
178:Aequorea victoria
16:(Redirected from
1363:
1281:
1280:
1270:
1238:
1232:
1231:
1221:
1211:
1202:(26): 24308–14.
1187:
1181:
1180:
1170:
1146:
1140:
1139:
1129:
1097:
1084:
1083:
1055:
1040:
1039:
1003:
982:
981:
971:
961:
937:
931:
930:
894:
875:
874:
840:
681:Flexible linkers
638:Domain insertion
526:fusion by using
488:hexa-his peptide
438:created through
318:immunoglobulin G
316:(TNFR) with the
253:constant domains
226:drug development
169:fluorescent tags
21:
18:Chimeric protein
1371:
1370:
1366:
1365:
1364:
1362:
1361:
1360:
1346:
1345:
1340:Wayback Machine
1319:
1318:
1317:
1302:
1301:
1297:
1290:
1285:
1284:
1240:
1239:
1235:
1189:
1188:
1184:
1161:(18): 11241–7.
1148:
1147:
1143:
1112:(10): 1357–69.
1099:
1098:
1087:
1057:
1056:
1043:
1014:(19): 8273–81.
1005:
1004:
985:
939:
938:
934:
896:
895:
878:
863:
842:
841:
837:
832:
810:
721:
665:
663:Protein linkers
653:
640:
627:
615:
578:Thermostability
547:N and C termini
542:
521:
517:
504:leucine zippers
420:
335:, neo-vascular
245:immune reaction
241:target molecule
210:
198:plasma membrane
165:
126:
46:Fusion proteins
35:
28:
23:
22:
15:
12:
11:
5:
1369:
1367:
1359:
1358:
1348:
1347:
1344:
1343:
1330:
1316:
1315:
1310:
1304:
1303:
1299:Fusion protein
1292:
1291:
1289:
1288:External links
1286:
1283:
1282:
1253:(6): 833–845.
1233:
1182:
1141:
1085:
1066:(1): 155–164.
1041:
983:
932:
876:
861:
834:
833:
831:
828:
827:
826:
821:
816:
809:
806:
772:
771:
766:
761:
720:
717:
716:
715:
698:
688:
664:
661:
652:
649:
639:
636:
626:
623:
614:
611:
610:
609:
606:
603:
592:
589:hydrogen bonds
585:
575:
565:
541:
538:
519:
515:
419:
416:
415:
414:
404:
382:
372:
358:
351:IL-1 receptors
344:
209:
206:
167:The fusion of
164:
161:
125:
122:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1368:
1357:
1354:
1353:
1351:
1341:
1337:
1334:
1331:
1328:
1324:
1321:
1320:
1314:
1311:
1309:
1306:
1305:
1300:
1295:
1287:
1278:
1274:
1269:
1264:
1260:
1256:
1252:
1248:
1244:
1237:
1234:
1229:
1225:
1220:
1215:
1210:
1205:
1201:
1197:
1193:
1186:
1183:
1178:
1174:
1169:
1164:
1160:
1156:
1152:
1145:
1142:
1137:
1133:
1128:
1123:
1119:
1115:
1111:
1107:
1103:
1096:
1094:
1092:
1090:
1086:
1081:
1077:
1073:
1069:
1065:
1061:
1054:
1052:
1050:
1048:
1046:
1042:
1037:
1033:
1029:
1025:
1021:
1017:
1013:
1009:
1002:
1000:
998:
996:
994:
992:
990:
988:
984:
979:
975:
970:
965:
960:
955:
951:
947:
943:
936:
933:
928:
924:
920:
916:
912:
908:
905:(5): 455–79.
904:
900:
893:
891:
889:
887:
885:
883:
881:
877:
872:
868:
864:
862:9781493909438
858:
854:
850:
846:
839:
836:
829:
825:
822:
820:
817:
815:
812:
811:
807:
805:
803:
799:
795:
794:Flavobacteria
791:
787:
786:
779:
777:
770:
767:
765:
762:
760:
757:
756:
755:
752:
750:
746:
742:
738:
734:
730:
726:
718:
713:
709:
705:
703:
699:
696:
692:
691:Rigid linkers
689:
686:
682:
679:
678:
677:
669:
662:
660:
658:
650:
648:
646:
637:
635:
633:
625:Tandem fusion
624:
622:
620:
612:
607:
604:
601:
597:
593:
590:
586:
583:
579:
576:
573:
569:
566:
563:
559:
555:
552:
551:
550:
548:
539:
537:
535:
534:reporter gene
532:
531:
525:
513:
509:
505:
501:
497:
493:
489:
485:
481:
477:
471:
469:
465:
461:
457:
453:
449:
445:
441:
437:
433:
424:
417:
412:
408:
405:
402:
398:
394:
390:
386:
383:
380:
376:
373:
370:
366:
362:
359:
356:
352:
348:
345:
342:
341:macular edema
338:
334:
330:
327:
326:
325:
323:
319:
315:
311:
307:
302:
300:
296:
294:
288:
284:
283:
278:
276:
270:
266:
262:
258:
254:
250:
246:
242:
238:
233:
231:
227:
219:
214:
207:
205:
203:
199:
195:
190:
186:
185:
180:
179:
174:
170:
162:
157:
156:
150:
146:
144:
140:
134:
131:
123:
121:
119:
115:
111:
107:
103:
99:
95:
91:
87:
86:
81:
77:
73:
69:
65:
64:
59:
55:
52:(kī-ˈmir-ik)
51:
47:
39:
33:
19:
1298:
1250:
1246:
1236:
1199:
1195:
1185:
1158:
1154:
1144:
1109:
1105:
1063:
1059:
1011:
1007:
949:
945:
935:
902:
898:
844:
838:
783:
780:
773:
753:
722:
714:in the cell.
701:
700:
690:
680:
674:
654:
641:
628:
616:
543:
529:
523:
500:streptavidin
484:FLAG peptide
472:
431:
429:
363:: Regulated
303:
292:
286:
280:
274:
264:
234:
223:
182:
176:
166:
153:
135:
127:
110:oncoproteins
104:, such as a
97:
94:polypeptides
89:
83:
80:therapeutics
71:
68:polypeptides
61:
53:
49:
45:
44:
899:Drug Safety
798:cyclophilin
747:-promoting
733:transcribed
712:biomolecule
657:translation
480:GST protein
375:Romiplostim
329:Aflibercept
289:such as in
257:immunogenic
249:engineering
63:fusion gene
830:References
741:translated
568:Solubility
512:antibodies
389:Belatacept
347:Rilonacept
322:Fc segment
306:Etanercept
297:. See the
952:: e2269.
749:oncogenes
522:promoter-
464:expressed
409:: Treats
401:psoriasis
385:Abatacept
361:Alefacept
353:to treat
282:humanized
267:into the
218:humanized
124:Functions
1350:Category
1336:Archived
1277:28567569
1228:15845546
1136:23026637
1080:25450191
1036:14316893
1028:27541749
978:27547572
927:23852865
919:25832756
871:24947379
808:See also
645:ligation
582:pH range
558:tertiary
545:such as
456:ligation
454:through
452:in frame
202:lysosome
184:Anthozoa
102:mutation
85:Chimeric
54:proteins
50:chimeric
1268:5655371
1219:2270415
1177:9556615
1127:3726540
969:4974937
737:spliced
702:In vivo
695:proline
685:glycine
632:folding
486:, or a
446:from a
436:protein
357:(CAPS).
271:(e.g.,
90:chimera
1333:ChiPPI
1329:(MeSH)
1296:about
1275:
1265:
1226:
1216:
1175:
1134:
1124:
1078:
1034:
1026:
976:
966:
925:
917:
869:
859:
745:cancer
739:, and
508:toxins
399:, and
365:T-cell
339:, and
291:dacli-
112:. The
1032:S2CID
946:PeerJ
923:S2CID
729:exons
466:by a
444:codon
434:is a
273:abci-
230:drugs
189:Kaede
58:genes
1273:PMID
1224:PMID
1173:PMID
1132:PMID
1076:PMID
1024:PMID
974:PMID
915:PMID
867:PMID
857:ISBN
802:FKBP
800:and
600:SUMO
560:and
530:gfp)
468:cell
448:cDNA
310:TNFα
295:-mab
287:-zu-
277:-mab
265:-xi-
1263:PMC
1255:doi
1214:PMC
1204:doi
1200:280
1163:doi
1159:273
1122:PMC
1114:doi
1068:doi
1016:doi
1012:100
964:PMC
954:doi
907:doi
849:doi
524:gfp
510:or
502:or
458:or
88:or
48:or
1352::
1271:.
1261:.
1251:22
1249:.
1245:.
1222:.
1212:.
1198:.
1194:.
1171:.
1157:.
1153:.
1130:.
1120:.
1110:65
1108:.
1104:.
1088:^
1074:.
1064:33
1062:.
1044:^
1030:.
1022:.
1010:.
986:^
972:.
962:.
948:.
944:.
921:.
913:.
903:38
901:.
879:^
865:.
855:.
778:.
735:,
708:pH
536:.
520:SH
516:SH
482:,
430:A
395:,
320:1
293:zu
275:xi
120:.
82:.
1279:.
1257::
1230:.
1206::
1179:.
1165::
1138:.
1116::
1082:.
1070::
1038:.
1018::
980:.
956::
950:4
929:.
909::
873:.
851::
584:.
413:.
403:.
387:/
381:.
371:.
343:.
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.