73:
314:
publication of a manuscript in 1984 entitled, "Stereochemistry, a basis of sophisticated nonsense in pharmacokinetics and clinical pharmacology" by Ariëns. This article, and the series of articles that followed, criticized the practice of conducting pharmacokinetic and pharmacodynamic studies on racemic drugs and ignoring the separate contributions of the individual enantiomers. These papers have served to crystallize some of the important issues surrounding racemic drugs and stimulated much discussion in industry, government and academia.
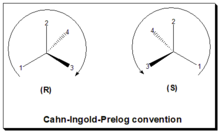
161:(International Union of Pure and Applied Chemistry) rules of nomenclature. In this approach: identify the chiral center, label the four atoms directly attached to the stereogenic center in question, assign priorities according to the sequence rule ( from 1 to 4), rotate the molecule until the lowest priority (number 4) substituent is away from the observer/viewer, draw a curve from number 1 to number 2 to number 3 substituent. If the curve is clockwise, the chiral center is of R-absolute configuration, "R" (Latin,
377:
active. The other isomer, the distomer, should be regarded as impurity or isomeric ballast, a term coined by Ariëns, not contributing to the effects aimed at. In contrast to the pharmacokinetic properties of an enantiomeric pair, differences in pharmacodynamic activity tend to be more obvious. There is a wide spectrum of possibilities of distomer actions, many of which are confirmed experimentally. Selected examples of the distomer actions (viz. equipotent, less active, inactive, antagonistic,
173:
88:, a famous student of Biot's, made a series of observations that led him to suggest that the optical activity of some substances is caused by their molecular asymmetry, which makes nonsuperimposable mirror-images. In 1848, Pasteur grew two different kinds of crystals from the racemic sodium ammonium salt of tartaric acid. He was the first person to separate enantiomeric crystals by hand. In fact Pasteur laid the foundations of stereochemistry and chirality.
153:
attached to the highest chiral carbon is on the right-hand side it is referred to as D-series and if on the left-hand side it is called L-series. This nomenclature system has also become obsolete. But D-/L-system of naming is still employed to designate the configuration of amino acids and sugars. In general the D/L system of nomenclature is superseded by the Cahn-Ingold-Prelog (CIP) rule to describe the configuration of a stereogenic/chiral center.
781:
755:
1156:, enantiomer purity, enantiomeric purity, and optical purity. Optical purity is an obsolete term since today most of the chiral purity measurements are done using chromatographic techniques (not based on optical principles). Enantiomeric excess tells the extent (in %) to which the chiral substance contains one enantiomer over the other. For a racemic drug the enantiomeric excess will be 0%. There are number of
721:
357:
344:
body, a classic bio-environment, is inherently handed as it is filled with chiral discriminators like amino acids, enzymes, carbohydrates, lipids, nucleic acids, etc. Hence when a racemic therapeutic gets exposed to biological system the component enantiomers will be acted upon stereoselectively. For drugs, chiral discrimination can take place either in the pharmacokinetic or pharmacodynamic phase.
688:
667:
738:
331:. The member of the chiral twin that has greater physiological activity is referred to as the eutomer and the other one with lesser activity is referred to as distomer. It is generally understood that this reference is necessarily to a single activity being studied. The eutomer for one effect may well be the distomer when another is studied. The eutomer/distomer ratio is called the
3872:
1144:
pair are different but supplementary, distomer is inactive, but separation is exorbitant. Insignificant/low toxicity of the distomer, high therapeutic index, mutually beneficial, pharmacological activities of both the enantiomers, and if the development of an enantiomer takes huge amount of time for a drug of emergency need e.g., cancer, AIDS, etc.
353:
surface (good fit), Figure. A., where as the less active enantiomer (distomer) interacts at two sites only (bad fit), Figure B. . Thus the "fit" of the individual enantiomers to the receptor site differs, as does the energy of interaction. This is a simplistic model but used to explain the biological discrimination between enantiomeric pairs.
323:
field has grown itself into a specialized discipline concerned with the three-dimensional aspects of drug action and disposition. This approach essentially views each version of the chiral twins as separate chemical species. To express the pharmacological activities of each of the chiral twins two technical terms have been coined,
945:
Globally drug companies and regulatory agencies have an inclination towards the development of unichiral drugs as a consequence of the increased understanding of the differing biological properties of individual enantiomers of a racemic therapeutics. Most of these unichiral drugs are the consequence
59:
illustrates the potential for extreme consequences resulting from the administration of a racemate drug that exhibits multiple effects attributable to individual enantiomers. With the advancements in chiral technology and the increased awareness about three-dimensional consequences of drug action and
352:
Easson and
Stedman (1933) advanced a drug-receptor interaction model to account for the differential pharmacodynamic activity between enantiomeric pairs. In this model the more active enantiomer (the eutomer) take part in a minimum of three simultaneous intermolecular interactions with the receptor
1143:
A company may go in for developing a racemic drug against an enantiomer by providing adequate reasoning. The rationale why a company might pursue developing racemic drugs could include expensive separation of enantiomers, eutomer racemizes in solution (e.g. oxazepam), activities of the enantiomeric
376:
in the accepted sense of two or more co-formulated therapeutic agents, but combinations of isomeric substances whose pharmacological activity may reside predominantly in one specific enantiomeric form. In case of stereoselectivity in action only one of the components in the racemic mixture is truly
313:
have been blind to the three-dimensional consequences of stereochemistry, chiefly due to the lack of technology for making enantioselective investigations. Besides the thalidomide tragedy, another event that raised the importance of issues of stereochemistry in drug research and development was the
54:
Many medicinal agents important to life are combinations of mirror-image twins. Despite the close resemblance of such twins, the differences in their biological properties can be profound. In other words, the component enantiomers of a racemic chiral drug may differ wildly in their pharmacokinetic,
771:
form. The drug had initially been introduced for clinical use as the racemate and was changed to the (S,S)-enantiomer, as a result of optic neuritis leading to blindness. Toxicity is related to both dose and duration of treatment. All the three stereoisomers were almost equipotent with respect to
745:
The initial use of racemic dopa for the treatment of
Parkinson's disease resulted in a number of adverse effects viz. nausea, vomiting, anorexia, involuntary movements and granulocytopenia. The use of L-dopa resulted in reducing the required dose, and adverse effects. The granulocytopenia was not
648:
Since there is a frequent large pharmacokinetic and pharmacodynamic differences between enantiomers of a chiral drug it is not surprising that enantiomers may result in stereoselective toxicity. They can reside in the pharmacologically active enantiomer (eutomer) or in the inactive one (distomer).
1160:
tools such as polarimetry, NMR spectroscopy with the use of chiral shift reagents, chiral GC (gas chromatography), chiral HPLC (high performance liquid chromatography), chiral TLC (thin-layer chromatography) and other chiral chromatographic techniques, that are employed to evaluate chiral purity.
343:
The behavior of the chiral twins depends mainly on the nature of the environment (achiral/chiral) in which they are present. An achiral environment does not differentiate the molecular twins whereas a chiral environment does distinguish the left-handed version from the right-handed version. Human
322:
As a result of these criticisms and the renewed awareness of the three-dimensional effects of drug action fueled by the exponential explosion of chiral technology emerged the new area "stereo-pharmacology". A more specific term is "chiral pharmacology", a phrase popularized by John
Caldwell. This
788:
Thalidomide is a classical example highlighting the alleged role of chirality in drug toxicity. Thalidomide was a racemic therapeutic and prescribed to pregnant women to control nausea and vomiting. The drug was withdrawn from world market when it became evident that the use in pregnancy causes
152:
notation (optical descriptor) described earlier. In this system, the enantiomers are named with reference to D- and L-glyceraldehyde which is taken as the standard for comparison. The structure of the chiral molecule should be represented in the
Fischer projection formula. If the hydroxyl group
941:
and enantiomerically pure drugs. Monochiral drugs has also been suggested as another synonym. Professor Eliel, Wilen, and Gal expressed their deep concern over the misuse of the term "homochiral" in articles to denote enantiomerically pure drugs, which is incorrect. Homochiral means objects or
99:
only used asymmetry arguments and talked about the asymmetry of the molecules as a whole instead of the asymmetry of each carbon atom. So, Le Bel's idea could be seen as the general theory of stereoisomerism, while van 't Hoff's could be seen as a special case (restricted to tetrahedral
39:
to drug molecules are stereogenic center. Stereogenic center can be due to the presence of tetrahedral tetra coordinate atoms (C,N,P) and pyramidal tricoordinate atoms (N,S). The word chiral describes the three-dimensional architecture of the molecule and does not reveal the stereochemical
116:
This is to give an overview of the evolving chirality nomenclature system commonly employed to distinguish enantiomers of a chiral drug. In the beginning, enantiomers were distinguished based on their ability to rotate the plane of plane-polarized light. The enantiomer that rotates the
728:
Ketamine is a widely used anaesthetic agent. It is a chiral molecule that is administered as a racemate. Studies show that (S)-(+)-ketamine is the active anaesthetic and the undesired side-effects (hallucination and agitation) reside in the distomer, (R)-(-)-ketamine.
649:
The toxicologic differences between enantiomers of have also been demonstrated. The following are examples of some of the chiral drugs where their toxic/undesirable side-effects dwell almost in the distomer. This would seem to be a clear cut case of going for a
762:
The antitubercular agent
Ethambutol contains two constitutionally symmetrical stereogenic centers in its structure and exists in three stereoisomeric forms. An enantiomeric pair (S,S)- and (R,R)-ethambutol, along with the achiral stereoisomer called
1594:
van't Hoff, J. H. (1874). "Proposal for the extension of current chemical structural formulas into space, together with related observation on the connection between optically active power and the chemical constitution of organic compounds".
156:
In the CIP or R/S convention, or sequence rule, the configuration, spatial arrangements of ligands/substituents around a chiral center, is labeled as either "R" or "S". This convention is now almost worldwide in use and become a part of the
60:
disposition emerged specialized field "chiral pharmacology". Simultaneously the chirality nomenclature system also evolved. A brief overview of chirality history and terminology/descriptors is given below. A detailed
767:-form, it holds a diastereomeric relationship with the optically active stereoisomers. The activity of the drug resides in the (S,S)-enantiomer which is 500 and 12 fold more potent than the (R,R)-ethambutol and the
942:
molecules of the same handedness. Hence should be used only for comparison of two or more objects of like "chirality". For instance, left hands of different individuals, or say R-naproxen and R-ibuprofen.
103:
Soon, scientists started to look into what chiral compounds meant for living things. In 1903, Cushny was the first person to show that enantiomers of a chiral molecule have different biological effects.
712:
is a chiral drug with one chiral center and exists as a pair of enantiomers. (S)-penicillamine is the eutomer with the desired antiarthritic activity while the (R)-penicillamine is extremely toxic.
40:
composition. Hence "chiral drug" does not say whether the drug is racemic (racemic drug), single enantiomer (chiral specific drug) or some other combination of stereoisomers. To resolve this issue
144:
Later, the
Fischer convention was introduced to specify the configuration of a stereogenic center and uses the symbols D and L. The use of capital letters is to differentiate from the
1685:
Kelvin, Lord (W. Thomson) (1904), Baltimore
Lectures on Molecular Dynamics and the Wave Theory of Light, C. J. Clay & Sons, London. The lectures were given in 1884 and 1893.
3077:
Trevor, Anthony; Marietta, Michael; Pudwill, Charles; Way, Walter (1977). "Metabolism and
Redistribution as Determinants of Duration of Effects of Ketamine in the Rat".
364:
In reality the drug-receptor interaction is not that simple, but this view of such complex phenomenon has provided major insights into the mechanism of action of drugs.
158:
2911:
3430:"Stereospecific determination, chiral inversion in vitro and pharmacokinetics in humans of the enantiomers of thalidomide: KINETICS OF THE ENANTIOMERS OF THALIDOMIDE"
789:
phocomelia (clinical conditions where babies are born with deformed hand and limbs). Later in late 1970s studies indicated that the (R)- enantiomer is an effective
937:
Unichiral indicates configurationally homogeneous substance (i.e. made up of chiral molecules of one and the same configuration). Other commonly used synonyms are
95:
came up with the idea of an asymmetric carbon atom. He said that all optically active carbon compounds have an asymmetric carbon atom. In the same year,
1317:
Gal, J (1998). "Problems of stereochemical nomenclature and terminology. 1. The homochiral controversy. Its nature, origins, and a proposed solution".
1610:
Le Bel, J. A. (1874). "On the relations which exist between the atomic formulas of organic compounds and the rotatory power of their solutions".
793:, the (S)-enantiomer harbors teratogenic effect and causes fetal abnormalities. Later studies established that under biological conditions the (
1569:"Mémoire sur la relation qui peut exister entre la forme crystalline et la composition chimique, et sur la cause de la polarisation rotatoire"
3620:
3390:
2560:
1488:
1401:
3577:
3851:
3228:
3094:
2825:
1301:
105:
815:. Hence the argument that the thalidomide tragedy could have been avoided by using a single enantiomer is ambiguous and pointless.
1161:
Assessing the purity of a unichiral drug or enantiopure drug is of great importance from a drug safety and efficacy perspective.
1339:
Jamali, F.; Mehvar, R.; Pasutto, F.M. (1989). "Enantioselective
Aspects of Drug Action and Disposition: Therapeutic Pitfalls".
92:
1234:
1571:[Note on the relationship of crystalline form to chemical composition, and on the cause of rotatory polarization]
51:
Unichiral indicates that the stereochemical composition of a chiral drug is homogenous consisting of a single enantiomer.
248:
Racemate or racemic mixture is an equimolar (1:1) mixture of enantiomers; corresponds to the enantiomeric excess of 0%.
72:
35:
drugs and these are obviously devoid of optical rotation. The most commonly encountered stereogenic unit, that confers
2927:"Methods for the analysis of enantiomers of racemic drugs application to pharmacological and pharmacokinetic studies"
1989:
Ariëns, E.J (1987). "Implications of the
Neglect of Stereochemistry in Pharmacokinetics and Clinical Pharmacology".
1568:
3897:
3414:
1789:
Cahn, B. S.; lngold, C. K; Prelog, V (1956). "The specification of asymmetric configuration in organic chemistry".
1292:
Gal, Joseph; Lindner, wolfgang (2006). "Chiral drugs from a historical point of view". In Francotte, Eric (ed.).
3902:
3876:
165:= right). If the curve is counterclockwise, the chiral center is of S-absolute configuration, "S" (Latin,
1170:
96:
36:
20:
3607:, Topics in Current Chemistry, vol. 340, Cham: Springer International Publishing, pp. 1–20,
2205:
1755:
1826:"Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology"
1153:
1472:
Chapter 34. Significance of Drug Stereochemistry in Modern Pharmaceutical Research and Development
3892:
3504:
3408:
3018:
2905:
2878:
2783:
2728:
2673:
2618:
2482:
2435:
2400:"Pharmacodynamic and pharmacokinetic differences between drug enantiomers in humans: An overview"
2069:
2014:
1971:
1916:
1861:
1806:
1205:
81:
61:
172:
3651:"Racemic mixtures and single stereoisomers: Industrial concerns and issues in drug development"
930:
46:
3847:
3791:
3783:
3744:
3717:
3709:
3670:
3626:
3616:
3581:
3496:
3488:
3449:
3396:
3386:
3341:
3333:
3294:
3286:
3224:
3201:
3193:
3154:
3146:
3090:
3059:
3010:
2954:
2946:
2870:
2862:
2821:
2775:
2767:
2720:
2712:
2665:
2657:
2610:
2602:
2556:
2521:
2474:
2427:
2419:
2380:
2362:
2323:
2315:
2276:
2268:
2229:
2221:
2155:
2116:
2108:
2061:
2053:
2034:"Implications of the Neglect of Stereochemistry in Pharmacokinetics and Clinical Pharmacology"
2006:
1963:
1955:
1908:
1900:
1853:
1845:
1771:
1724:
1716:
1668:
1650:
1548:
Biot, J.B. (1812). "The rotation of the axes of polarization of light by certain substances".
1530:
1484:
1451:
1407:
1397:
1364:
1356:
1297:
1274:
1230:
3429:
3825:
3813:
3775:
3701:
3662:
3608:
3573:
3480:
3441:
3325:
3278:
3185:
3138:
3082:
3049:
3000:
2938:
2854:
2759:
2704:
2649:
2594:
2548:
2513:
2466:
2411:
2370:
2354:
2307:
2260:
2213:
2147:
2100:
2045:
1998:
1947:
1892:
1837:
1798:
1763:
1708:
1658:
1642:
1522:
1476:
1443:
1389:
1348:
1266:
1190:
1185:
938:
812:
808:
632:
378:
373:
310:
295:
Latin: sinister = left; absolute configuration as per Cahn-Ingold-Prelog rule/Sequence rule
1152:
Chiral purity is a measure of the purity of a chiral drug. Other synonyms employed include
284:
Latin: rectus = right; absolute configuration as per Cahn-Ingold-Prelog rule/sequence rule
273:
Relative configuration with respect to L-glyceraldehyde; referred to as Fischer convention
262:
Relative configuration with respect to D-glyceraldehyde; referred to as Fischer convention
3385:. S. K. Branch, Michel Eichelbaum, Bernard Testa, Andrew Somogyi. Berlin: Springer. 2003.
1200:
1180:
1157:
1077:
32:
2209:
1759:
780:
772:
side effects. Hence the use of S,S)-enantiomer greatly enhanced the risk/benefit ratio.
3086:
2552:
2375:
2104:
1663:
1012:
999:
754:
332:
328:
324:
3764:"The so-called ?interconversion? of stereoisomeric drugs: An attempt at clarification"
1480:
1447:
1393:
3886:
3705:
3561:
3054:
3037:
3005:
2988:
2942:
2858:
2708:
2598:
2540:
2517:
2264:
2151:
2088:
1175:
1087:
1054:
947:
709:
650:
85:
3022:
2882:
2787:
2732:
2677:
2622:
2486:
2439:
2343:"Studies on the relationship between chemical constitution and physiological action"
2073:
2018:
1975:
1920:
1865:
1810:
1630:
720:
356:
3808:
3508:
3428:
Eriksson, Tommy; Bjöurkman, Sven; Roth, Bodil; Fyge, Årsa; Höuglund, Peter (1995).
2842:
2692:
2582:
2311:
1646:
1475:, Annual Reports in Medicinal Chemistry, vol. 25, Elsevier, pp. 323–331,
1113:
1022:
970:
540:
412:
3468:
19:
Chemical compounds that come as mirror-image pairs are referred to by chemists as
3829:
141:. Now the d/l system of naming based on optical rotation is falling into disuse.
3600:
3282:
3189:
3142:
2217:
1470:
1431:
1383:
508:
476:
56:
3689:
2926:
2501:
2470:
2295:
2248:
2135:
2049:
2002:
737:
3266:
3173:
3126:
2193:
2173:
Caldwell, John (1995). "Chiral Pharmacology and the regulation of new drugs".
1064:
950:
approach. The table below list selected unichiral drugs used in drug therapy.
687:
666:
444:
117:
plane-polarized light to the right is named "dextro-rotatory", abbreviated as
24:
3787:
3713:
3674:
3585:
3492:
3337:
3290:
3197:
3150:
3063:
2950:
2866:
2771:
2716:
2661:
2606:
2525:
2478:
2454:
2423:
2366:
2319:
2272:
2225:
2159:
2112:
2057:
2033:
1959:
1904:
1849:
1775:
1720:
1654:
1455:
1360:
1278:
3400:
3359:
607:
3779:
3666:
3630:
3562:<428::aid-chir5>3.0.co;2-1 "Infelicitous stereochemical nomenclature"
3500:
3445:
3329:
3298:
3245:
3172:
Cotzias, George C.; Papavasiliou, Paul S.; Gellene, Rosemary (1969-02-13).
3125:
Cotzias, George C.; Van Woert, Melvin H.; Schiffer, Lewis M. (1967-02-16).
2763:
2653:
2384:
2233:
1951:
1896:
1728:
1712:
1672:
1526:
1352:
3871:
3795:
3748:
3721:
3690:"Importance of stereospecific bionalytical monitoring in drug development"
3484:
3453:
3345:
3205:
3158:
3014:
2958:
2874:
2779:
2724:
2669:
2614:
2431:
2415:
2327:
2280:
2120:
2065:
2010:
1967:
1912:
1857:
1534:
1411:
1368:
31:. Chiral drugs that are equimolar (1:1) mixture of enantiomers are called
3763:
3650:
3612:
3380:
3313:
2747:
2637:
1936:"Stereochemical implications of hybrid and pseudo-hybrid drugs. Part III"
1935:
1880:
1696:
1510:
1195:
1100:
790:
3578:
10.1002/(sici)1520-636x(1997)9:5/6<428::aid-chir5>3.0.co;2-1
2399:
1631:"Atropine and the hyoscyamines-a study of the action of optical isomers"
1270:
180:
An overview of the nomenclature system is presented in the table below.
2801:
Baldwin, J.J.; Abrams, W. B (1988). Wainer, I.W.; Drayer, D.E. (eds.).
1841:
1802:
2358:
1767:
1254:
1825:
1388:, Advances in Pharmacology, vol. 22, Elsevier, pp. 57–135,
3522:
Cornforth, R.H; Cornforth, J.W (1996). "How to be right and wrong".
2342:
1743:
2987:
White, Paul F.; Ham, Jay; Way, Walter L.; Trevor, Anthony (1980).
2247:
Ariëns, Everhardus J.; Wuis, Eveline W.; Veringa, Eric J. (1988).
779:
753:
736:
719:
355:
171:
3541:
Ernest, L Eliel; Samuel H, Wilen (1990). "Misuse of homochiral".
2194:"Optical Isomerism and Pharmacological Action, a Generalization"
1432:"The relevance of chirality to the study of biological activity"
3223:. New York: John Wiley & Sons, New York. pp. 235–261.
3174:"Modification of Parkinsonism — Chronic Treatment with L-Dopa"
2748:"Stereochemistry: A source of problems in medicinal chemistry"
3844:
Chromatographic enantioseparation: methods and applications
3762:
Testa, Bernard; Carrupt, Pierre-Alain; Gal, Joseph (1993).
228:
Optical rotation signs; does not reflect the configuration
211:
Optical rotation signs; does not reflect the configuration
3735:
Campbell, D.B. (1990). "The development of chiral drugs".
3601:"Molecular Chirality: Language, History, and Significance"
1296:. Germany: Wiley-VCH Verlag GmbH & Co. pp. 3–26.
3221:
The impact of stereochemistry on drug development and use
2898:
Drug stereochemistry. Analytical methods and pharmacology
2820:. Budapest: Akademiai Kiado, Budapest. pp. 181–204.
2803:
Drug Stereochemistry, Analytical Methods and Pharmacology
2693:"Importance of Drug Enantiomers in Clinical Pharmacology"
1881:"Stereochemistry in the analysis of drug-action. Part II"
1511:"The FDA perspective on the development of stereoisomers"
3467:
Eriksson, Tommy; Björkman, Sven; Höglund, Peter (2001).
2976:. Chichester: Ellis Horwood, Chichester. pp. 31–68.
1385:
Molecular Asymmetry and Its Pharmacological Consequences
84:
found out about a phenomenon called "optical activity."
3265:
Mellin, Gilbert W.; Katzenstein, Michael (1962-12-06).
3127:"Aromatic Amino Acids and Modification of Parkinsonism"
3079:
Sixth International Congress of Pharmacology: abstracts
2989:"Pharmacology of Ketamine Isomers in Surgical Patients"
822:
Chiral drugs: Adverse effects residing in the distomer
818:
The salient features are presented in the table below.
169:= left). Refer to figure, the Cahn-Ingold-Prelog rule.
2900:. New York: Marcel Dekker, New York. pp. 245–270.
2805:. New York: Marcel Dekker, New York. pp. 311–356.
3382:
Stereochemical aspects of drug action and disposition
80:
Chirality can be traced back to 1812, when physicist
3114:. New York: Academic Press, New York. pp. 1–28.
2931:
Journal of Pharmacological and Toxicological Methods
2341:
Easson, Leslie Hilton; Stedman, Edgar (1933-01-01).
1742:
Slocum, D. W.; Surgarman, D.; Tucker, S. P. (1971).
805:-thalidomide, evil partner and vice versa. It is a
108:used the word "chiral" for the first time in 1904.
27:. Drugs that exhibit handedness are referred to as
2541:"Racemic Therapeutics—Problems all Along the Line"
1550:Mém. Classe Sci. Math. Phys. Inst. Imperial France
2136:"Chirality in bioactive agents and its pitfalls"
2925:Wright, Matthew R.; Jamali, Fakhreddin (1993).
2583:"Drug Chirality and its Clinical Significance"
3846:. Chichester: Ellis Horwood. pp. 27–40.
8:
2910:: CS1 maint: multiple names: authors list (
2249:"Stereoselectivity of bioactive xenobiotics"
23:or handed molecules. Each twin is called an
3599:Gal, Joseph (2013), Schurig, Volker (ed.),
3036:Kohrs, Rainer; Durieux, Marcel E. (1998).
2300:American Journal of Health-System Pharmacy
2089:"Stereoselectivity in the Action of Drugs"
1695:Hartung, Walter H.; Andrako, John (1961).
3473:European Journal of Clinical Pharmacology
3053:
3004:
2374:
2038:Drug Intelligence & Clinical Pharmacy
1830:European Journal of Clinical Pharmacology
1662:
1248:
1246:
954:Unichiral drugs employed in drug therapy
797:-thalidomide, good partner, undergoes an
133:. A racemic mixture is denoted as "(±)",
2455:"Stereoisomers in Clinical Pharmacology"
2296:"Three-dimensional view of pharmacology"
1334:
1332:
1259:Journal of the American Chemical Society
1227:The Third Dimension in Organic Chemistry
952:
820:
383:
182:
76:Louis Pasteur - pioneering stereochemist
71:
55:pharmacodynamic profile. The tragedy of
2818:Problems and wonder of chiral molecules
2691:Williams, Kenneth; Lee, Edmund (1985).
1744:"The two faces of D and L nomenclature"
1217:
533:Antagonizes side effect of the eutomer
3469:"Clinical pharmacology of thalidomide"
3406:
3112:Chirality in Drug Design and Synthesis
2903:
2545:Chirality in Drug Design and Synthesis
2404:Clinical Pharmacology and Therapeutics
1255:"Stereoisomerism and local chirality"
746:observed with the single enantiomer.
469:Less active; no serious side-effects
7:
381:) are presented in the table below.
3087:10.1016/b978-0-08-021308-8.51428-9
2553:10.1016/b978-0-12-136670-4.50008-4
2506:Trends in Pharmacological Sciences
2140:Trends in Pharmacological Sciences
2105:10.1111/j.1600-0773.1989.tb00655.x
1701:Journal of Pharmaceutical Sciences
1341:Journal of Pharmaceutical Sciences
1253:Mislow, Kurt; Siegel, Jay (1984).
1229:. New York: John Wiley, New York.
1041:Rheumatology / Pain/ Inflammation
909:(R,R)-; and meso- form; Blindness
112:Chirality: terminology/descriptors
64:is not the focus of this article.
14:
2843:"Stereoisomers and Drug Toxicity"
2502:"Stereoisomerism and drug action"
254:Obsolete terms/falling in disuse
234:Obsolete terms/falling in disuse
217:Obsolete terms/falling in disuse
3870:
3605:Differentiation of Enantiomers I
3055:10.1213/00000539-199811000-00039
3006:10.1097/00000542-198003000-00008
2859:10.2165/00002018-199308020-00005
2709:10.2165/00003495-198530040-00003
2599:10.2165/00003495-199600525-00003
2581:Hutt, A. J.; Tan, S. C. (1996).
2294:Wainer, Irving W. (1992-09-01).
1629:Cushny, Arthur R. (1903-11-02).
686:
665:
580:Antitussive; (Levopropoxyphene)
339:Bio-environment and chiral twins
3543:Chemical & Engineering News
3271:New England Journal of Medicine
3178:New England Journal of Medicine
3131:New England Journal of Medicine
1118:Antianginal / Antihypertensive
962:Drug class/ Type of medication
2192:Pfeiffer, C. C. (1956-07-06).
1647:10.1113/jphysiol.1903.sp000988
573:Independent therapeutic value
368:Pharmacodynamic considerations
1:
3219:Blessington, Bernard (1997).
2746:Ariëns, Everardus J. (1986).
2093:Pharmacology & Toxicology
1879:Ariëns, Everardus J. (1987).
1748:Journal of Chemical Education
1597:Arch. Neerl. Sci. Exacts Nat.
1577:C. R. Hebd. Séances Acad. Sci
1481:10.1016/s0065-7743(08)61610-3
1448:10.1016/s0040-4020(01)80486-5
1394:10.1016/s1054-3589(08)60033-2
1382:Williams, Kenneth M. (1991),
548:Analgesic; (Dexpropoxyphene)
309:For many years scientists in
44:introduced a new term called
3830:10.1016/j.chroma.2009.12.071
3706:10.1016/0021-9673(95)00465-3
2943:10.1016/1056-8719(93)90044-f
2547:, Elsevier, pp. 29–43,
2518:10.1016/0165-6147(86)90353-6
2500:Lehmann, Pedro A.F. (1986).
2312:10.1093/ajhp/49.9_suppl_1.s4
2265:10.1016/0006-2952(88)90749-6
2152:10.1016/0165-6147(86)90313-5
1991:Br. J. Clin. Pharmacol. Ther
801:metabolic inversion to the (
93:Jacobus Henricus van 't Hoff
3842:Allenmark, Stig G. (1988).
3694:Journal of Chromatography A
3649:Cayen, Mitchell N. (1991).
3360:"Thalidomide – Chiralpedia"
3312:De Camp, Wilson H. (1989).
3283:10.1056/nejm196212062672305
3190:10.1056/nejm196902132800701
3143:10.1056/nejm196702162760703
2218:10.1126/science.124.3210.29
176:The Cahn-Ingold-Prelog rule
68:Chirality: history overview
3919:
3246:"Ethambutol – Chiralpedia"
3042:Anesthesia & Analgesia
2974:Chiral Separations by HPLC
2752:Medicinal Research Reviews
2642:Medicinal Research Reviews
2471:10.1177/009286159002400123
2050:10.1177/106002808702101013
2003:10.1177/106002808702101013
1940:Medicinal Research Reviews
1885:Medicinal Research Reviews
1294:Chirality in drug research
3560:Eliel, Ernest L. (1997).
3267:"The Saga of Thalidomide"
3081:. Elsevier. p. 583.
2841:Scott, Andrew K. (1993).
2453:Scott, Andrew K. (1990).
2398:Drayer, Dennis E (1986).
2032:Ariëns, Everd J. (1987).
1635:The Journal of Physiology
1120:
1071:
1040:
1006:
977:
836:
575:
572:
544:
539:
2636:Simonyi, Miklós (1984).
2459:Drug Information Journal
2253:Biochemical Pharmacology
1430:Crossley, Roger (1992).
906:(S,S)-; Tuberculostatic
3688:Caldwell, John (1996).
3244:Kannappan, Valliappan.
2816:Williams, K. M (1990).
2638:"On chiral drug action"
1469:Gross, Michael (1990),
895:(R)-; Granulocytopenia
784:Thalidomide enantiomers
501:Inactive; half placebo
125:and the counterpart as
3780:10.1002/chir.530050302
3667:10.1002/chir.530030203
3446:10.1002/chir.530070109
3413:: CS1 maint: others (
3330:10.1002/chir.530010202
3314:"Letter to the editor"
2896:Powell, J, R. (1988).
2764:10.1002/med.2610060404
2654:10.1002/med.2610040304
2593:(Supplement 5): 1–12.
2175:Chemistry and Industry
2087:Ariëns, E. J. (1989).
1952:10.1002/med.2610080206
1934:Ariëns, E. J. (1988).
1897:10.1002/med.2610070305
1824:Ariens, E. J. (1984).
1713:10.1002/jps.2600501002
1527:10.1002/chir.530010103
1353:10.1002/jps.2600780902
1225:Bassindale, A (1984).
994:Proton pump inhibitor
986:Proton pump inhibitor
975:Proton pump inhibitor
785:
759:
758:Ethambutol enantiomers
742:
725:
372:Racemic drugs are not
361:
177:
77:
3485:10.1007/s002280100320
2539:ARIENS, E.J. (1990),
2416:10.1038/clpt.1986.150
2134:Ariëns, E.J. (1986).
1171:Chirality (chemistry)
1038:Rheumatoid Arthritis
783:
757:
740:
723:
359:
348:Chiral discrimination
175:
97:Joseph Achille Le Bel
75:
3879:at Wikimedia Commons
3613:10.1007/128_2013_435
2972:Ariens, E.J (1989).
1567:Pasteur, L. (1848).
1092:Anti-hypothyroidism
1030:Infectious diseases
892:(S)-; Antiparkinson
864:(S)-; Antiarthritic
724:Ketamine enantiomers
360:Easson-Stedman model
186:Chirality descriptor
2347:Biochemical Journal
2210:1956Sci...124...29P
1760:1971JChEd..48..597S
1612:Bull. Soc. Chim. Fr
1271:10.1021/ja00323a043
1154:enantiomeric excess
955:
881:(R)-; Hallucinogen
823:
395:Therapeutic action
318:Chiral pharmacology
3110:Hyneck, M (1990).
2306:(9_Suppl): S4–S8.
1842:10.1007/bf00541922
1803:10.1007/BF02157171
1509:Decamp, W (1989).
1206:Chirality timeline
953:
923:(S)-; Teratogenic
852:Distomer; Activity
848:Eutomer; Activity
821:
786:
760:
743:
726:
635:(unidirectional;
615:Anti-inflammatory
362:
178:
82:Jean-Baptiste Biot
78:
62:chirality timeline
3898:Enantiopure drugs
3875:Media related to
3622:978-3-319-03238-2
3524:Croat. Chem. Acta
3392:978-3-540-41593-0
3277:(23): 1184–1193.
2562:978-0-12-136670-4
2359:10.1042/bj0271257
1768:10.1021/ed048p597
1697:"Stereochemistry"
1490:978-0-12-040525-1
1442:(38): 8155–8178.
1403:978-0-12-032922-9
1265:(11): 3319–3328.
1141:
1140:
1137:Antihypertensive
1129:Antihypertensive
1082:Local anesthetic
978:Gastroenterology
965:Therapeutic area
939:enantiopure drugs
927:
926:
878:(S)-; Anesthetic
695:
674:
641:
640:
484:Antihypertensive
374:drug combinations
302:
301:
3910:
3874:
3858:
3857:
3839:
3833:
3814:J. Chromatogr. A
3806:
3800:
3799:
3759:
3753:
3752:
3737:Acta Pharm. Nord
3732:
3726:
3725:
3685:
3679:
3678:
3646:
3640:
3639:
3638:
3637:
3596:
3590:
3589:
3572:(5–6): 428–430.
3557:
3551:
3550:
3538:
3532:
3531:
3519:
3513:
3512:
3464:
3458:
3457:
3425:
3419:
3418:
3412:
3404:
3377:
3371:
3370:
3368:
3367:
3362:. 20 August 2022
3356:
3350:
3349:
3309:
3303:
3302:
3262:
3256:
3255:
3253:
3252:
3241:
3235:
3234:
3216:
3210:
3209:
3169:
3163:
3162:
3122:
3116:
3115:
3107:
3101:
3100:
3074:
3068:
3067:
3057:
3048:(5): 1186–1193.
3033:
3027:
3026:
3008:
2984:
2978:
2977:
2969:
2963:
2962:
2922:
2916:
2915:
2909:
2901:
2893:
2887:
2886:
2838:
2832:
2831:
2813:
2807:
2806:
2798:
2792:
2791:
2743:
2737:
2736:
2688:
2682:
2681:
2633:
2627:
2626:
2578:
2572:
2571:
2570:
2569:
2536:
2530:
2529:
2497:
2491:
2490:
2450:
2444:
2443:
2395:
2389:
2388:
2378:
2353:(4): 1257–1266.
2338:
2332:
2331:
2291:
2285:
2284:
2244:
2238:
2237:
2189:
2183:
2182:
2170:
2164:
2163:
2131:
2125:
2124:
2084:
2078:
2077:
2029:
2023:
2022:
1986:
1980:
1979:
1931:
1925:
1924:
1876:
1870:
1869:
1821:
1815:
1814:
1786:
1780:
1779:
1739:
1733:
1732:
1692:
1686:
1683:
1677:
1676:
1666:
1626:
1620:
1619:
1607:
1601:
1600:
1591:
1585:
1584:
1574:
1564:
1558:
1557:
1545:
1539:
1538:
1506:
1500:
1499:
1498:
1497:
1466:
1460:
1459:
1427:
1421:
1420:
1419:
1418:
1379:
1373:
1372:
1336:
1327:
1326:
1314:
1308:
1307:
1289:
1283:
1282:
1250:
1241:
1240:
1222:
1191:Chiral inversion
1186:Enantiopure drug
1108:Neuropsychiatry
1035:S-penicillamine
959:Unichiral drugs
956:
838:Clinical effects
824:
741:Dopa enantiomers
693:
690:
672:
669:
633:Chiral inversion
404:Distomer action
384:
379:chiral inversion
311:drug development
194:
183:
3918:
3917:
3913:
3912:
3911:
3909:
3908:
3907:
3903:Stereochemistry
3883:
3882:
3867:
3862:
3861:
3854:
3841:
3840:
3836:
3807:
3803:
3761:
3760:
3756:
3734:
3733:
3729:
3687:
3686:
3682:
3648:
3647:
3643:
3635:
3633:
3623:
3598:
3597:
3593:
3559:
3558:
3554:
3540:
3539:
3535:
3521:
3520:
3516:
3466:
3465:
3461:
3427:
3426:
3422:
3405:
3393:
3379:
3378:
3374:
3365:
3363:
3358:
3357:
3353:
3311:
3310:
3306:
3264:
3263:
3259:
3250:
3248:
3243:
3242:
3238:
3231:
3218:
3217:
3213:
3171:
3170:
3166:
3124:
3123:
3119:
3109:
3108:
3104:
3097:
3076:
3075:
3071:
3035:
3034:
3030:
2986:
2985:
2981:
2971:
2970:
2966:
2924:
2923:
2919:
2902:
2895:
2894:
2890:
2840:
2839:
2835:
2828:
2815:
2814:
2810:
2800:
2799:
2795:
2745:
2744:
2740:
2690:
2689:
2685:
2635:
2634:
2630:
2580:
2579:
2575:
2567:
2565:
2563:
2538:
2537:
2533:
2499:
2498:
2494:
2452:
2451:
2447:
2397:
2396:
2392:
2340:
2339:
2335:
2293:
2292:
2288:
2246:
2245:
2241:
2204:(3210): 29–31.
2191:
2190:
2186:
2172:
2171:
2167:
2133:
2132:
2128:
2086:
2085:
2081:
2044:(10): 827–829.
2031:
2030:
2026:
1997:(10): 827–829.
1988:
1987:
1983:
1933:
1932:
1928:
1878:
1877:
1873:
1823:
1822:
1818:
1788:
1787:
1783:
1741:
1740:
1736:
1707:(10): 805–818.
1694:
1693:
1689:
1684:
1680:
1628:
1627:
1623:
1609:
1608:
1604:
1593:
1592:
1588:
1572:
1566:
1565:
1561:
1547:
1546:
1542:
1508:
1507:
1503:
1495:
1493:
1491:
1468:
1467:
1463:
1429:
1428:
1424:
1416:
1414:
1404:
1381:
1380:
1376:
1338:
1337:
1330:
1316:
1315:
1311:
1304:
1291:
1290:
1286:
1252:
1251:
1244:
1237:
1224:
1223:
1219:
1214:
1201:Stereochemistry
1181:Chiral analysis
1167:
1158:chiral analysis
1150:
1105:Anti-Parkinson
1078:Levobupivacaine
1072:Anesthesiology
1017:Antihistaminic
1004:Bronchodilator
991:Dexrabiprazole
983:S-pantoprazole
935:
920:(R)-; Sedative
778:
752:
735:
718:
707:
706:
705:
704:
703:
701:
700:)-Penicillamine
691:
683:
682:
681:(antiarthritic)
680:
679:)-Penicillamine
670:
659:
646:
452:Bronchodilator
420:Antihistaminic
391:
370:
350:
341:
320:
307:
188:
187:
114:
70:
17:
12:
11:
5:
3916:
3914:
3906:
3905:
3900:
3895:
3885:
3884:
3881:
3880:
3866:
3865:External links
3863:
3860:
3859:
3852:
3834:
3824:, 1395–1398. (
3801:
3774:(3): 105–111.
3754:
3743:(3): 217–226.
3727:
3680:
3641:
3621:
3591:
3552:
3533:
3514:
3479:(5): 365–376.
3459:
3420:
3391:
3372:
3351:
3304:
3257:
3236:
3229:
3211:
3184:(7): 337–345.
3164:
3137:(7): 374–379.
3117:
3102:
3095:
3069:
3028:
2999:(3): 231–239.
2993:Anesthesiology
2979:
2964:
2917:
2888:
2853:(2): 149–159.
2833:
2826:
2808:
2793:
2758:(4): 451–466.
2738:
2703:(4): 333–354.
2683:
2648:(3): 359–413.
2628:
2573:
2561:
2531:
2492:
2465:(1): 121–123.
2445:
2410:(2): 125–133.
2390:
2333:
2286:
2239:
2184:
2165:
2126:
2099:(4): 319–320.
2079:
2024:
1981:
1946:(2): 309–320.
1926:
1891:(3): 367–387.
1871:
1836:(6): 663–668.
1816:
1781:
1734:
1687:
1678:
1641:(2): 176–194.
1621:
1602:
1586:
1559:
1540:
1501:
1489:
1461:
1422:
1402:
1374:
1347:(9): 695–715.
1328:
1309:
1302:
1284:
1242:
1235:
1216:
1215:
1213:
1210:
1209:
1208:
1203:
1198:
1193:
1188:
1183:
1178:
1173:
1166:
1163:
1149:
1146:
1139:
1138:
1135:
1131:
1130:
1127:
1123:
1122:
1119:
1116:
1110:
1109:
1106:
1103:
1097:
1096:
1095:Endocrinology
1093:
1090:
1084:
1083:
1080:
1074:
1073:
1070:
1067:
1061:
1060:
1057:
1051:
1050:
1047:
1043:
1042:
1039:
1036:
1032:
1031:
1028:
1027:Antibacterial
1025:
1019:
1018:
1015:
1009:
1008:
1005:
1002:
1000:Levosalbutamol
996:
995:
992:
988:
987:
984:
980:
979:
976:
973:
967:
966:
963:
960:
934:
928:
925:
924:
921:
918:
915:
911:
910:
907:
904:
901:
897:
896:
893:
890:
887:
883:
882:
879:
876:
873:
869:
868:
867:(R)-; Mutagen
865:
862:
859:
858:Penicillamine
855:
854:
849:
846:
844:
841:
840:
835:
833:Chiral centers
830:
807:bidirectional
777:
774:
751:
748:
734:
731:
717:
714:
692:
685:
684:
671:
664:
663:
662:
661:
660:
658:
655:
645:
642:
639:
638:
636:
630:
623:
616:
613:
610:
604:
603:
592:
581:
577:
576:
574:
571:
560:
549:
546:
543:
537:
536:
534:
531:
524:
517:
514:
511:
505:
504:
502:
499:
492:
485:
482:
479:
473:
472:
470:
467:
460:
453:
450:
447:
441:
440:
438:
435:
432:
421:
418:
415:
409:
408:
405:
402:
399:
396:
393:
388:
369:
366:
349:
346:
340:
337:
333:eudysmic ratio
319:
316:
306:
303:
300:
299:
296:
293:
289:
288:
285:
282:
278:
277:
274:
271:
267:
266:
263:
260:
256:
255:
249:
246:
236:
235:
229:
226:
219:
218:
212:
209:
202:
201:
198:
195:
191:used as prefix
113:
110:
69:
66:
16:Class of drugs
15:
13:
10:
9:
6:
4:
3:
2:
3915:
3904:
3901:
3899:
3896:
3894:
3891:
3890:
3888:
3878:
3873:
3869:
3868:
3864:
3855:
3853:9780131329454
3849:
3845:
3838:
3835:
3831:
3827:
3823:
3819:
3816:
3815:
3811:; Tanwar, S.
3810:
3805:
3802:
3797:
3793:
3789:
3785:
3781:
3777:
3773:
3769:
3765:
3758:
3755:
3750:
3746:
3742:
3738:
3731:
3728:
3723:
3719:
3715:
3711:
3707:
3703:
3699:
3695:
3691:
3684:
3681:
3676:
3672:
3668:
3664:
3660:
3656:
3652:
3645:
3642:
3632:
3628:
3624:
3618:
3614:
3610:
3606:
3602:
3595:
3592:
3587:
3583:
3579:
3575:
3571:
3567:
3563:
3556:
3553:
3548:
3544:
3537:
3534:
3529:
3525:
3518:
3515:
3510:
3506:
3502:
3498:
3494:
3490:
3486:
3482:
3478:
3474:
3470:
3463:
3460:
3455:
3451:
3447:
3443:
3439:
3435:
3431:
3424:
3421:
3416:
3410:
3402:
3398:
3394:
3388:
3384:
3383:
3376:
3373:
3361:
3355:
3352:
3347:
3343:
3339:
3335:
3331:
3327:
3323:
3319:
3315:
3308:
3305:
3300:
3296:
3292:
3288:
3284:
3280:
3276:
3272:
3268:
3261:
3258:
3247:
3240:
3237:
3232:
3230:0-471-59644-2
3226:
3222:
3215:
3212:
3207:
3203:
3199:
3195:
3191:
3187:
3183:
3179:
3175:
3168:
3165:
3160:
3156:
3152:
3148:
3144:
3140:
3136:
3132:
3128:
3121:
3118:
3113:
3106:
3103:
3098:
3096:9780080213088
3092:
3088:
3084:
3080:
3073:
3070:
3065:
3061:
3056:
3051:
3047:
3043:
3039:
3032:
3029:
3024:
3020:
3016:
3012:
3007:
3002:
2998:
2994:
2990:
2983:
2980:
2975:
2968:
2965:
2960:
2956:
2952:
2948:
2944:
2940:
2936:
2932:
2928:
2921:
2918:
2913:
2907:
2899:
2892:
2889:
2884:
2880:
2876:
2872:
2868:
2864:
2860:
2856:
2852:
2848:
2844:
2837:
2834:
2829:
2827:963-05-5881-5
2823:
2819:
2812:
2809:
2804:
2797:
2794:
2789:
2785:
2781:
2777:
2773:
2769:
2765:
2761:
2757:
2753:
2749:
2742:
2739:
2734:
2730:
2726:
2722:
2718:
2714:
2710:
2706:
2702:
2698:
2694:
2687:
2684:
2679:
2675:
2671:
2667:
2663:
2659:
2655:
2651:
2647:
2643:
2639:
2632:
2629:
2624:
2620:
2616:
2612:
2608:
2604:
2600:
2596:
2592:
2588:
2584:
2577:
2574:
2564:
2558:
2554:
2550:
2546:
2542:
2535:
2532:
2527:
2523:
2519:
2515:
2511:
2507:
2503:
2496:
2493:
2488:
2484:
2480:
2476:
2472:
2468:
2464:
2460:
2456:
2449:
2446:
2441:
2437:
2433:
2429:
2425:
2421:
2417:
2413:
2409:
2405:
2401:
2394:
2391:
2386:
2382:
2377:
2372:
2368:
2364:
2360:
2356:
2352:
2348:
2344:
2337:
2334:
2329:
2325:
2321:
2317:
2313:
2309:
2305:
2301:
2297:
2290:
2287:
2282:
2278:
2274:
2270:
2266:
2262:
2258:
2254:
2250:
2243:
2240:
2235:
2231:
2227:
2223:
2219:
2215:
2211:
2207:
2203:
2199:
2195:
2188:
2185:
2180:
2176:
2169:
2166:
2161:
2157:
2153:
2149:
2145:
2141:
2137:
2130:
2127:
2122:
2118:
2114:
2110:
2106:
2102:
2098:
2094:
2090:
2083:
2080:
2075:
2071:
2067:
2063:
2059:
2055:
2051:
2047:
2043:
2039:
2035:
2028:
2025:
2020:
2016:
2012:
2008:
2004:
2000:
1996:
1992:
1985:
1982:
1977:
1973:
1969:
1965:
1961:
1957:
1953:
1949:
1945:
1941:
1937:
1930:
1927:
1922:
1918:
1914:
1910:
1906:
1902:
1898:
1894:
1890:
1886:
1882:
1875:
1872:
1867:
1863:
1859:
1855:
1851:
1847:
1843:
1839:
1835:
1831:
1827:
1820:
1817:
1812:
1808:
1804:
1800:
1796:
1792:
1785:
1782:
1777:
1773:
1769:
1765:
1761:
1757:
1753:
1749:
1745:
1738:
1735:
1730:
1726:
1722:
1718:
1714:
1710:
1706:
1702:
1698:
1691:
1688:
1682:
1679:
1674:
1670:
1665:
1660:
1656:
1652:
1648:
1644:
1640:
1636:
1632:
1625:
1622:
1617:
1613:
1606:
1603:
1599:(9): 445–454.
1598:
1590:
1587:
1582:
1578:
1570:
1563:
1560:
1555:
1551:
1544:
1541:
1536:
1532:
1528:
1524:
1520:
1516:
1512:
1505:
1502:
1492:
1486:
1482:
1478:
1474:
1473:
1465:
1462:
1457:
1453:
1449:
1445:
1441:
1437:
1433:
1426:
1423:
1413:
1409:
1405:
1399:
1395:
1391:
1387:
1386:
1378:
1375:
1370:
1366:
1362:
1358:
1354:
1350:
1346:
1342:
1335:
1333:
1329:
1324:
1320:
1313:
1310:
1305:
1303:3-527-31076-2
1299:
1295:
1288:
1285:
1280:
1276:
1272:
1268:
1264:
1260:
1256:
1249:
1247:
1243:
1238:
1232:
1228:
1221:
1218:
1211:
1207:
1204:
1202:
1199:
1197:
1194:
1192:
1189:
1187:
1184:
1182:
1179:
1177:
1176:Chiral switch
1174:
1172:
1169:
1168:
1164:
1162:
1159:
1155:
1148:Chiral purity
1147:
1145:
1136:
1133:
1132:
1128:
1126:S-metoprolol
1125:
1124:
1117:
1115:
1112:
1111:
1107:
1104:
1102:
1099:
1098:
1094:
1091:
1089:
1088:Levothyroxine
1086:
1085:
1081:
1079:
1076:
1075:
1068:
1066:
1063:
1062:
1058:
1056:
1055:Dexketoprofen
1053:
1052:
1048:
1045:
1044:
1037:
1034:
1033:
1029:
1026:
1024:
1021:
1020:
1016:
1014:
1013:Levocetrizine
1011:
1010:
1003:
1001:
998:
997:
993:
990:
989:
985:
982:
981:
974:
972:
969:
968:
964:
961:
958:
957:
951:
949:
948:chiral switch
943:
940:
932:
929:
922:
919:
916:
913:
912:
908:
905:
902:
899:
898:
894:
891:
888:
885:
884:
880:
877:
874:
871:
870:
866:
863:
860:
857:
856:
853:
850:
847:
845:
843:
842:
839:
834:
831:
829:
826:
825:
819:
816:
814:
811:
810:
804:
800:
796:
792:
782:
775:
773:
770:
766:
756:
749:
747:
739:
732:
730:
722:
715:
713:
711:
710:Penicillamine
699:
689:
678:
668:
657:Penicillamine
656:
654:
652:
651:chiral switch
644:Drug toxicity
643:
637:
634:
631:
628:
624:
621:
617:
614:
611:
609:
606:
605:
601:
597:
593:
590:
586:
582:
579:
578:
569:
565:
561:
558:
554:
550:
547:
542:
538:
535:
532:
529:
525:
522:
518:
515:
512:
510:
507:
506:
503:
500:
497:
493:
490:
486:
483:
480:
478:
475:
474:
471:
468:
465:
461:
458:
454:
451:
448:
446:
443:
442:
439:
436:
433:
430:
426:
422:
419:
416:
414:
411:
410:
406:
403:
400:
397:
394:
389:
386:
385:
382:
380:
375:
367:
365:
358:
354:
347:
345:
338:
336:
334:
330:
326:
317:
315:
312:
305:Racemic drugs
304:
297:
294:
291:
290:
286:
283:
280:
279:
275:
272:
269:
268:
264:
261:
258:
257:
253:
250:
247:
245:
242:
238:
237:
233:
230:
227:
225:
221:
220:
216:
213:
210:
208:
204:
203:
199:
196:
192:
185:
184:
181:
174:
170:
168:
164:
160:
154:
151:
147:
142:
140:
136:
132:
128:
124:
120:
111:
109:
107:
101:
98:
94:
89:
87:
86:Louis Pasteur
83:
74:
67:
65:
63:
58:
52:
50:
48:
43:
38:
34:
30:
26:
22:
3877:Chiral drugs
3843:
3837:
3821:
3817:
3812:
3804:
3771:
3767:
3757:
3740:
3736:
3730:
3697:
3693:
3683:
3661:(2): 94–98.
3658:
3654:
3644:
3634:, retrieved
3604:
3594:
3569:
3565:
3555:
3546:
3542:
3536:
3527:
3523:
3517:
3476:
3472:
3462:
3440:(1): 44–52.
3437:
3433:
3423:
3381:
3375:
3364:. Retrieved
3354:
3324:(2): 97–98.
3321:
3317:
3307:
3274:
3270:
3260:
3249:. Retrieved
3239:
3220:
3214:
3181:
3177:
3167:
3134:
3130:
3120:
3111:
3105:
3078:
3072:
3045:
3041:
3031:
2996:
2992:
2982:
2973:
2967:
2934:
2930:
2920:
2897:
2891:
2850:
2846:
2836:
2817:
2811:
2802:
2796:
2755:
2751:
2741:
2700:
2696:
2686:
2645:
2641:
2631:
2590:
2586:
2576:
2566:, retrieved
2544:
2534:
2509:
2505:
2495:
2462:
2458:
2448:
2407:
2403:
2393:
2350:
2346:
2336:
2303:
2299:
2289:
2256:
2252:
2242:
2201:
2197:
2187:
2178:
2174:
2168:
2143:
2139:
2129:
2096:
2092:
2082:
2041:
2037:
2027:
1994:
1990:
1984:
1943:
1939:
1929:
1888:
1884:
1874:
1833:
1829:
1819:
1797:(3): 81–88.
1794:
1790:
1784:
1751:
1747:
1737:
1704:
1700:
1690:
1681:
1638:
1634:
1624:
1615:
1611:
1605:
1596:
1589:
1580:
1576:
1562:
1553:
1549:
1543:
1518:
1514:
1504:
1494:, retrieved
1471:
1464:
1439:
1435:
1425:
1415:, retrieved
1384:
1377:
1344:
1340:
1322:
1318:
1312:
1293:
1287:
1262:
1258:
1226:
1220:
1151:
1142:
1114:S-amlodipine
1023:Levofloxacin
1007:Pulmonology
971:Esomeprazole
944:
936:
914:Thalidomide
851:
837:
832:
827:
817:
806:
802:
798:
794:
787:
768:
764:
761:
744:
727:
708:
697:
676:
647:
626:
619:
599:
595:
588:
584:
567:
563:
556:
552:
541:Propoxyphene
527:
520:
495:
488:
463:
456:
428:
424:
413:promethazine
387:Chiral drug
371:
363:
351:
342:
321:
308:
251:
243:
240:
231:
223:
214:
206:
197:Description
190:
179:
166:
162:
155:
149:
145:
143:
138:
134:
130:
126:
122:
118:
115:
102:
90:
79:
53:
45:
41:
29:chiral drugs
28:
18:
3809:Bhushan, R.
3700:(1): 3–13.
2847:Drug Safety
2512:: 281–285.
2259:(1): 9–18.
2146:: 200–205.
1791:Experientia
1436:Tetrahedron
1134:S-atenolol
1121:Cardiology
1069:Anesthetic
1046:S-etodolac
900:Ethambutol
828:Chiral drug
776:Thalidomide
509:Indacrinone
477:Propranolol
437:Equipotent
390:Stereogenic
207:dextro-/ d-
106:Lord Kelvin
57:thalidomide
3887:Categories
3636:2021-07-04
3530:: 427–433.
3366:2022-08-27
3251:2022-08-27
3038:"Ketamine"
2937:(1): 1–9.
2568:2021-06-03
2181:: 176–179.
1754:(9): 597.
1618:: 337–347.
1583:: 535–538.
1521:(1): 2–6.
1496:2021-06-03
1417:2021-06-03
1325:: 263–273.
1319:Enantiomer
1236:047190189X
1212:References
1065:S-ketamine
750:Ethambutol
445:Salbutamol
407:Reference
392:center(s)
42:Joseph Gal
25:enantiomer
3893:Chirality
3788:0899-0042
3768:Chirality
3714:0021-9673
3675:0899-0042
3655:Chirality
3586:0899-0042
3566:Chirality
3493:0031-6970
3434:Chirality
3409:cite book
3338:0899-0042
3318:Chirality
3291:0028-4793
3198:0028-4793
3151:0028-4793
3064:0003-2999
2951:1056-8719
2906:cite book
2867:0114-5916
2772:0198-6325
2717:0012-6667
2662:0198-6325
2607:0012-6667
2526:0165-6147
2479:0092-8615
2424:0009-9236
2367:0306-3283
2320:1079-2082
2273:0006-2952
2226:0036-8075
2160:0165-6147
2113:0901-9928
2058:0012-6578
1960:0198-6325
1905:0198-6325
1850:0031-6970
1776:0021-9584
1721:0022-3549
1655:0022-3751
1556:: 41–136.
1515:Chirality
1456:0040-4020
1361:0022-3549
1279:0002-7863
931:Unichiral
872:Ketamine
813:inversion
608:Ibuprofen
516:Diuretic
401:Distomer
200:Comments
100:carbon).
91:In 1874,
47:unichiral
37:chirality
3631:23666078
3501:11599654
3401:52515592
3299:13934699
3023:38711416
2883:20426452
2788:36115871
2733:23999344
2678:38829275
2623:41802235
2487:72095771
2440:33537650
2385:16745220
2234:13337345
2074:23007083
2019:23007083
1976:33013615
1921:31403941
1866:30916093
1811:43026989
1729:14036070
1673:16992694
1196:Racemate
1165:See also
1101:Levodopa
791:sedative
716:Ketamine
398:Eutomer
329:distomer
167:sinister
119:"dextro"
3796:8338720
3749:2200432
3722:8589835
3509:7417671
3454:7702998
3346:2642047
3206:4178641
3159:5334614
3015:6989292
2959:8481555
2875:8452656
2780:3534485
2725:3905334
2670:6087043
2615:8922553
2432:3731675
2376:1253018
2328:1530004
2281:3276322
2206:Bibcode
2198:Science
2121:2748538
2066:3322758
2011:3322758
1968:3288823
1913:3041134
1858:6092093
1756:Bibcode
1664:1540678
1535:2642032
1412:1958505
1369:2685226
799:in vivo
702:(toxic)
325:eutomer
239:(±)- /
222:(-)- /
205:(+)- /
33:racemic
3850:
3794:
3786:
3747:
3720:
3712:
3673:
3629:
3619:
3584:
3507:
3499:
3491:
3452:
3399:
3389:
3344:
3336:
3297:
3289:
3227:
3204:
3196:
3157:
3149:
3093:
3062:
3021:
3013:
2957:
2949:
2881:
2873:
2865:
2824:
2786:
2778:
2770:
2731:
2723:
2715:
2676:
2668:
2660:
2621:
2613:
2605:
2559:
2524:
2485:
2477:
2438:
2430:
2422:
2383:
2373:
2365:
2326:
2318:
2279:
2271:
2232:
2224:
2158:
2119:
2111:
2072:
2064:
2056:
2017:
2009:
1974:
1966:
1958:
1919:
1911:
1903:
1864:
1856:
1848:
1809:
1774:
1727:
1719:
1671:
1661:
1653:
1533:
1487:
1454:
1410:
1400:
1367:
1359:
1300:
1277:
1233:
1059:NSAID
1049:NSAID
809:chiral
696:-(+)-(
675:-(–)-(
241:rac- /
163:rectus
127:"levo"
21:chiral
3505:S2CID
3019:S2CID
2879:S2CID
2784:S2CID
2729:S2CID
2697:Drugs
2674:S2CID
2619:S2CID
2587:Drugs
2483:S2CID
2436:S2CID
2070:S2CID
2015:S2CID
1972:S2CID
1917:S2CID
1862:S2CID
1807:S2CID
1573:(PDF)
933:drugs
886:Dopa
769:meso-
224:levo-
159:IUPAC
137:, or
135:"rac"
3848:ISBN
3822:1217
3818:2010
3792:PMID
3784:ISSN
3745:PMID
3718:PMID
3710:ISSN
3671:ISSN
3627:PMID
3617:ISBN
3582:ISSN
3549:: 2.
3497:PMID
3489:ISSN
3450:PMID
3415:link
3397:OCLC
3387:ISBN
3342:PMID
3334:ISSN
3295:PMID
3287:ISSN
3225:ISBN
3202:PMID
3194:ISSN
3155:PMID
3147:ISSN
3091:ISBN
3060:ISSN
3011:PMID
2955:PMID
2947:ISSN
2912:link
2871:PMID
2863:ISSN
2822:ISBN
2776:PMID
2768:ISSN
2721:PMID
2713:ISSN
2666:PMID
2658:ISSN
2611:PMID
2603:ISSN
2557:ISBN
2522:ISSN
2475:ISSN
2428:PMID
2420:ISSN
2381:PMID
2363:ISSN
2324:PMID
2316:ISSN
2277:PMID
2269:ISSN
2230:PMID
2222:ISSN
2156:ISSN
2117:PMID
2109:ISSN
2062:PMID
2054:ISSN
2007:PMID
1964:PMID
1956:ISSN
1909:PMID
1901:ISSN
1854:PMID
1846:ISSN
1772:ISSN
1725:PMID
1717:ISSN
1669:PMID
1651:ISSN
1531:PMID
1485:ISBN
1452:ISSN
1408:PMID
1398:ISBN
1365:PMID
1357:ISSN
1298:ISBN
1275:ISSN
1231:ISBN
765:meso
733:Dopa
598:),(3
587:),(3
566:),(3
555:),(3
427:)-/(
327:and
139:"dl"
3826:doi
3776:doi
3702:doi
3698:719
3663:doi
3609:doi
3574:doi
3481:doi
3442:doi
3326:doi
3279:doi
3275:267
3186:doi
3182:280
3139:doi
3135:276
3083:doi
3050:doi
3001:doi
2939:doi
2855:doi
2760:doi
2705:doi
2650:doi
2595:doi
2549:doi
2514:doi
2467:doi
2412:doi
2371:PMC
2355:doi
2308:doi
2261:doi
2214:doi
2202:124
2148:doi
2101:doi
2046:doi
1999:doi
1948:doi
1893:doi
1838:doi
1799:doi
1764:doi
1709:doi
1659:PMC
1643:doi
1523:doi
1477:doi
1444:doi
1390:doi
1349:doi
1267:doi
1263:106
946:of
629:)-
622:)-
602:)-
591:)-
570:)-
559:)-
530:)-
523:)-
498:)-
491:)-
466:)-
459:)-
431:)-
292:S-
281:R-
270:L-
259:D-
252:dl;
244:dl-
150:"l"
146:"d"
131:"l"
129:or
123:"d"
121:or
3889::
3820:,
3790:.
3782:.
3770:.
3766:.
3739:.
3716:.
3708:.
3696:.
3692:.
3669:.
3657:.
3653:.
3625:,
3615:,
3603:,
3580:.
3568:.
3564:.
3547:10
3545:.
3528:69
3526:.
3503:.
3495:.
3487:.
3477:57
3475:.
3471:.
3448:.
3436:.
3432:.
3411:}}
3407:{{
3395:.
3340:.
3332:.
3320:.
3316:.
3293:.
3285:.
3273:.
3269:.
3200:.
3192:.
3180:.
3176:.
3153:.
3145:.
3133:.
3129:.
3089:.
3058:.
3046:87
3044:.
3040:.
3017:.
3009:.
2997:52
2995:.
2991:.
2953:.
2945:.
2935:29
2933:.
2929:.
2908:}}
2904:{{
2877:.
2869:.
2861:.
2849:.
2845:.
2782:.
2774:.
2766:.
2754:.
2750:.
2727:.
2719:.
2711:.
2701:30
2699:.
2695:.
2672:.
2664:.
2656:.
2644:.
2640:.
2617:.
2609:.
2601:.
2591:52
2589:.
2585:.
2555:,
2543:,
2520:.
2508:.
2504:.
2481:.
2473:.
2463:24
2461:.
2457:.
2434:.
2426:.
2418:.
2408:40
2406:.
2402:.
2379:.
2369:.
2361:.
2351:27
2349:.
2345:.
2322:.
2314:.
2304:49
2302:.
2298:.
2275:.
2267:.
2257:37
2255:.
2251:.
2228:.
2220:.
2212:.
2200:.
2196:.
2177:.
2154:.
2142:.
2138:.
2115:.
2107:.
2097:64
2095:.
2091:.
2068:.
2060:.
2052:.
2042:21
2040:.
2036:.
2013:.
2005:.
1995:21
1993:.
1970:.
1962:.
1954:.
1942:.
1938:.
1915:.
1907:.
1899:.
1887:.
1883:.
1860:.
1852:.
1844:.
1834:26
1832:.
1828:.
1805:.
1795:12
1793:.
1770:.
1762:.
1752:48
1750:.
1746:.
1723:.
1715:.
1705:50
1703:.
1699:.
1667:.
1657:.
1649:.
1639:30
1637:.
1633:.
1616:22
1614:.
1581:26
1579:.
1575:.
1554:II
1552:.
1529:.
1517:.
1513:.
1483:,
1450:.
1440:48
1438:.
1434:.
1406:,
1396:,
1363:.
1355:.
1345:78
1343:.
1331:^
1321:.
1273:.
1261:.
1257:.
1245:^
917:1
903:2
889:1
875:1
861:1
803:S)
795:R)
653:.
612:1
594:(2
583:(2
562:(2
551:(2
545:2
513:1
481:1
449:1
434:-
417:1
335:.
298:-
287:-
276:-
265:-
232:l;
215:d;
148:/
3856:.
3832:)
3828::
3798:.
3778::
3772:5
3751:.
3741:2
3724:.
3704::
3677:.
3665::
3659:3
3611::
3588:.
3576::
3570:9
3511:.
3483::
3456:.
3444::
3438:7
3417:)
3403:.
3369:.
3348:.
3328::
3322:1
3301:.
3281::
3254:.
3233:.
3208:.
3188::
3161:.
3141::
3099:.
3085::
3066:.
3052::
3025:.
3003::
2961:.
2941::
2914:)
2885:.
2857::
2851:8
2830:.
2790:.
2762::
2756:6
2735:.
2707::
2680:.
2652::
2646:4
2625:.
2597::
2551::
2528:.
2516::
2510:7
2489:.
2469::
2442:.
2414::
2387:.
2357::
2330:.
2310::
2283:.
2263::
2236:.
2216::
2208::
2179:6
2162:.
2150::
2144:7
2123:.
2103::
2076:.
2048::
2021:.
2001::
1978:.
1950::
1944:8
1923:.
1895::
1889:7
1868:.
1840::
1813:.
1801::
1778:.
1766::
1758::
1731:.
1711::
1675:.
1645::
1537:.
1525::
1519:1
1479::
1458:.
1446::
1392::
1371:.
1351::
1323:3
1306:.
1281:.
1269::
1239:.
698:R
694:L
677:S
673:D
627:R
625:(
620:S
618:(
600:S
596:R
589:R
585:S
568:R
564:S
557:S
553:R
528:S
526:(
521:R
519:(
496:R
494:(
489:S
487:(
464:S
462:(
457:R
455:(
429:S
425:R
423:(
193:)
189:(
49:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.