808:, and facial and cervical swelling. Post-mortem examination in two of the affected lambs have revealed that all organs had a pale appearance, notably the liver, and that the lungs were heavier than usual and were slightly brownish. In four beavers exposed to 2.13 ± 0.4 mg/kg chlorophacinone, bleeding from the mouth, gasping for breath and convulsions were observed, and the beavers died within 15 days after exposure. Studies in rats have indicated that male rats experience more profound effects than female rats. Birds are not as sensitive to chlorophacinone as mammals, but they may still experience sublethal effects from it, such as external bleeding, internal hematoma and increased blood coagulation time. General toxic symptoms include dyspnea, lethargy, hemorrhage from the nose and urethral bleeding.
919:. The anticoagulant concentration is diluted ten-fold in secondary exposure, and even more when the predator also eats non-poisoned prey. Small, granivorous animals that share burrows with the target animal are mainly at risk to be exposed. In a study summarized by USEPA (2004), chlorophacinone baits were used to control California ground squirrels in rangelands, and nontarget deer mice and San Joaquin pocket mice were found dead with at least 86% of the mortalities likely due to bait exposure. The risk of chlorophacinone exposure to birds is minimal, and the aquatic and terrestrial plant exposure is considered negligible.
234:
139:
915:, chlorophacinone bait is distributed into burrow openings or on the ground just outside burrows. Although each placement is covered with grass or shingle to avoid exposing nontarget organisms and chlorophacinone is not likely to drain into soil, nontarget organisms could still be exposed to chlorophacinone by eating the bait. Predators could also eat animals poisoned with chlorophacinone, which is classified as secondary exposure, although multiple poisoned animals must be consumed to receive a
561:
760:. Hepatic metabolism is generally biphasic with a rapid initial phase and more prolonged terminal phase. However metabolite excretion pathways of chlorophacinone still remain poorly described. The major route of elimination of chlorophacinone is through the feces (~95%) however with minor excretion (<1%) through urine and respiration. 26% of chlorophacinone is excreted within eight hours post-exposure via the bile.
24:
642:
424:
developed during the 1940s to 1960s to control rodents in terrestrial environments. Its use began being replaced during the 1970s, along with the use of other rodenticides of its group, by the more potent second-generation anticoagulant rodenticides, when several studies provided information which depicted a developed resistance of rodents to
361:
428:(another first-generation anticoagulant rodenticide) in northern Europe and the United States along with a discovered cross-resistance to all first-generation anticoagulant rodenticides. This was found to be caused by a single, dominant and autosomal gene which raised the rodent's dietary requirement for
768:
Chlorophacinone is used as an anticoagulant rodenticide to control rodent populations in terrestrial environments. It has been proven to be very effective in efficacy studies in rats, mice and beavers. Out of the four toxicants strychnine, zinc phosphide, chlorophacinone and diphacinone, the efficacy
739:
in the liver. After 1–4 days of repeated exposure a steady-state phase is reached. The time it takes to reach a steady-state phase suggest rapid elimination of chlorophacinone from the body. Anticoagulants are rapidly and principally absorbed in the intestine. The rodenticide was found to metabolized
777:
Belonging to the group of first-generation anticoagulant rodenticides, chlorophacinone has similar symptoms on animals as the other chemicals in its category. Specifically, after being ingested several times by the target animal (most often a rodent), it interferes with the clotting of the blood and
423:
The French company
Liphatech (formerly known as Lipha), which had previous experience with creating anticoagulants for the treatment of heart patients, created chlorophacinone in 1961 and branded it “Rozol”. Chlorophacinone belongs to the first-generation anticoagulant rodenticide group, first being
886:
The SENSOR-pesticide database documented 12 human exposure cases involving chlorophacinone between 1998 and 2011. One was a moderate severity case, which involved an insulation worker being exposed to chlorophacinone dust by touching and/or inhaling it. The worker experienced shakiness, fever, and
799:
Chlorophacinone is classified as a highly toxic substance when administered orally, dermally, or through inhalation in mammals, falling under
Toxicity Category I. It is not a dermal or eye irritant, or a dermal sensitizer (Toxicity Category IV). Accidental exposure incidents involving lambs have
735:. When orally ingested absorption of chlorophacinone peaks between 4 and 6 hours after initial ingesting. The compound has a half-life of approximately 10 hours. Highest concentrations of chlorophacinone are found in the liver and kidneys. Repeated oral dosing in rats suggests
432:(the vitamin whose production anticoagulants primarily inhibited) to twenty times the normal amount. Even though its use has diminished, chlorophacinone can still be bought for rodenticide use, for situations in which conventional bait for rodenticidal purposes cannot be used.
570:
Chlorophacinone can be synthesized through different mechanisms. A more recently studied mechanism will be discussed below (figure 1). In this synthesizes chlorophacinone is synthesized with less production of side products compared to the classic mechanisms.
769:
of chlorophacinone has been proven to be the highest in controlling mountain beaver populations. Chronic ingestion of smaller doses over time proves to be more toxic than acute ingestion of the same dose, a common trait among anticoagulant rodenticides.
782:
of the vitamin K(1)-2,3 epoxide reductase (VKOR) enzyme which is responsible for the synthesis of vitamin K and therefore the clotting factors II, VII, IX and X, factors critical to blood clotting, lack of which eventually causes mass
887:
vomiting, as well as respiratory, neurological, gastrointestinal, renal and cardiovascular symptoms. Another case involved a homeowner who experienced shortness of breath and coughing after accidentally inhaling chlorophacinone. No
714:
in the tissue will diminish, this in turn will decrease the carboxylation activity of γ-glutamyl carboxylase. Resulting in under-carboxylation of clotting factors, meaning they are no longer capable of binding to the
651:
Chlorophacinone is a first-generation anticoagulant rodenticide. The compound is an indandione derivate. It acts as a vitamin K antagonist and exerts its anticoagulatory effect by interfering with the
374:
412:
466:
constant of 5.12 x 10 atm-m3/mol suggests a low potential to volatilize from water or soil into the atmosphere. It is dissolves relatively good in organic solvents like
1211:
1076:
694:
cofactor is created within the vitamin K redox cycle. Chlorophacinone interferes with the vitamin K redox cycle by inhibiting vitamin K epoxide reductase (VKOR), an
787:
inside the animal. Although internal bleeding is the usual cause of death in this category of rodenticides, chlorophacinone has also been shown to cause additional
283:
955:
96:
1591:
408:
1250:
456:
are attached to the other side, one contains a chloride. Chlorophacinone contains one optically active carbon and therefore it occurs as two
702:(ER). The enzyme plays a vital role within this cycle. The catalytic activity of VKOR is required for the reduction of KO to vitamin K to KH
415:(42 U.S.C. 11002) and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
1512:
1383:"Developing a management strategy to reduce roof rat, Rattus rattus, impacts on open-cup nesting songbirds in California riparian forests"
2002:
1997:
248:
565:
Figure 1: the synthesis of
Chlorophacinone. a) SnCl4, 70°C, 8 h, 85% ; b) 25°C, 12 h, quant, ; c) AlCl3, 25°C, 12h, 60%
403:. The mechanism of action results in internal bleeding due to non-functional clotting factors. It was used as a toxin to control
1280:
656:
1765:
752:
step. Hydroxylation occurs on the phenyl and indandionyl rings, these metabolites can then further undergo conjugation with
1059:
381:
670:
by the enzyme γ-glutamyl carboxylase (GGCX). The γ-carboxyglutamate residues promote the binding of clotting factors to
191:
611:
949:"40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities"
212:
257:
InChI=1S/C23H15ClO3/c24-16-12-10-15(11-13-16)19(14-6-2-1-3-7-14)23(27)20-21(25)17-8-4-5-9-18(17)22(20)26/h1-13,19-20H
267:
InChI=1/C23H15ClO3/c24-16-12-10-15(11-13-16)19(14-6-2-1-3-7-14)23(27)20-21(25)17-8-4-5-9-18(17)22(20)26/h1-13,19-20H
1584:
1138:
1474:"Assessing the efficacy of registered underground baiting products for mountain beaver (Aplodontia rufa) control"
778:
leads to internal bleeding, eventually causing death within 5 to 7 days. This effect is due to the rodenticide's
757:
695:
1111:
1992:
655:
synthesis of vitamin K-dependent clotting factors. Synthesis of clotting factor II, VII, IX and X involves the
1946:
791:
or neurologic symptoms in laboratory rats, often leading to their death before significant bleeding occurs.
683:
134:
1926:
728:
1770:
1577:
970:
779:
699:
1325:
1966:
1617:
1485:
1425:
948:
805:
667:
36:
1153:
1987:
1841:
1816:
229:
62:
891:
assessments have been conducted on chlorophacinone since chronic exposure is not likely to occur.
1808:
1631:
1226:
1205:
997:
732:
1058:
Lemay A, McCaskill M, Warren J, Hall, T.C, Paz L, Deliberto S, Ruell E, Wimberly (March 2023).
1831:
1555:
1547:
1449:
1441:
1394:
1363:
1355:
1300:
1296:
1256:
1246:
1175:
1070:
749:
596:
1527:
706:. The inhibition of VKOR by chlorophacinone prevents the recycling of vitamin K from KO to KH
1879:
1826:
1539:
1493:
1433:
1292:
1238:
1165:
589:
441:
306:
581:
as starting product as it is a cheap and commercially available. Mandelic acid reacts with
560:
200:
116:
1936:
1793:
1755:
1414:"Efficacy of rodenticide baits for the control of three invasive rodent species in Hawaii"
1139:"Evaluation of active substances; Renewal of approval; Assessment Report; Chlorophacinone"
983:
912:
908:
888:
788:
753:
741:
736:
603:
72:
1489:
1429:
233:
138:
1856:
1846:
1798:
1710:
928:
619:
463:
449:
352:
1242:
1981:
1667:
1651:
1612:
745:
663:
659:
582:
575:
444:
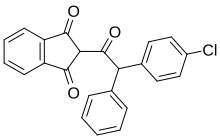
with the following systematic name: (2-indan-1,3-dione. The structure consists of an
397:
127:
690:
is converted to vitamin K 2,3 epoxide (KO) during the carboxylation reaction. The KH
1961:
1941:
1691:
1681:
1600:
1473:
671:
630:
453:
445:
1528:"Chlorophacinone Exposure Causing an Epizootic of Acute Fatal Hemorrhage in Lambs"
1095:"Forty Five Years of Anticoagulant Rodenticides - Past, Present and Future Trends"
646:
Figure 2: The vitamin K redox cycle and the inhibition of chlorophacinone of VKOR.
180:
1497:
1343:
474:(786 mg/L at 25 °C), compared to water (3.43 mg/L at 25 °C).
1931:
1728:
1696:
1676:
1604:
1381:
Whisson, Desley A.; Quinn, Jessica H.; Collins, Kellie; Engilis, Andrew (2004).
916:
900:
716:
675:
610:. No purification is needed to start the last step of the synthesis, which is a
400:
1543:
1094:
1951:
1887:
1864:
1760:
1742:
1686:
1437:
784:
457:
337:
107:
23:
1569:
1551:
1445:
1398:
1359:
1179:
1956:
1897:
1785:
1750:
1413:
1382:
801:
641:
429:
1559:
1453:
1304:
1260:
1367:
1170:
1905:
1646:
1626:
471:
425:
1412:
Pitt, William C.; Driscoll, Laura C.; Sugihara, Robert T. (April 2011).
1836:
1821:
1723:
1656:
167:
1910:
1869:
904:
467:
404:
351:
Except where otherwise noted, data are given for materials in their
606:
at room temperature to obtain 6-chloro-2,2-diphenylacetyl chloride
155:
652:
95:
85:
1017:
146:
1573:
1775:
1326:"Anticoagulant rodenticides, evironmental health criteria 175"
998:"Rodenticides: Background & Hazards | Safe Rodent Control"
719:
surface of blood vessels, and thus are biologically inactive.
1152:
Csuk, René; Barthel, Alexander; Ströhl, Dieter (2011-01-01).
1344:"Metabolism and disposition of diphacinone in rats and mice"
1281:"Vitamin K-Dependent Biosynthesis of γ-Carboxyglutamic Acid"
1196:
Van den Brink NW, Elliott JE, Shore RF, Rattner BA. (2017).
625:. This reaction provided the final product, chlorophacinone
217:
1279:
Furie, Bruce; Bouchard, Beth A.; Furie, Barbara C. (1999).
756:
prior to entering the systemic circulation, with potential
516:
H410: Very toxic to aquatic life with long lasting effects
411:
in the United States as defined in
Section 302 of the U.S.
1060:"The use of chlorophacinone in Wildlife damage management"
1342:
Yu, C. C.; Atallah, Y. H.; Whitacre, D. M. (1982-11-01).
686:
is needed for the carboxylation reaction to occur. The KH
1526:
Piero, Fabio Del; Poppenga, Robert H. (September 2006).
1227:"Structure and Function of Vitamin K Epoxide Reductase"
1154:"An Alternative and Efficient Route to Chlorophacinone"
369:
1513:"Chlorophacinone - an overview | ScienceDirect Topics"
1418:
899:
In order to control the population of animals such as
510:
H372: Causes damage to the blood through prolonged or
602:. Thereafter, the phenylacetic acid is treated with
1919:
1896:
1878:
1855:
1807:
1784:
1741:
1709:
1665:
1639:
1625:
1611:
413:Emergency Planning and Community Right-to-Know Act
179:
291:Clc1ccc(cc1)C(c2ccccc2)C(=O)C4C(=O)c3ccccc3C4=O
71:
1532:Journal of Veterinary Diagnostic Investigation
731:and may also be absorbed through the skin and
1585:
1387:Proceedings of the Vertebrate Pest Conference
8:
1210:: CS1 maint: multiple names: authors list (
1075:: CS1 maint: multiple names: authors list (
727:The chlorophacinone is absorbed through the
674:of the blood vessels, thereby accelerating
1636:
1622:
1592:
1578:
1570:
1478:USDA Wildlife Services: Staff Publications
1225:Tie, Jian-Ke; Stafford, Darrel W. (2008),
232:
137:
115:
15:
1297:10.1182/blood.v93.6.1798.406k22_1798_1808
1169:
199:
817:
740:in the liver. Metabolism is mediated by
640:
559:
476:
1472:Arjo, Wendy; Nolte, Dale (2004-05-09).
1198:Anticoagulant Rodenticides and Wildlife
940:
710:(figure 2). Therefore, the supply of KH
678:. However, a vitamin K hydroquinone (KH
288:
253:
228:
1203:
1068:
979:
968:
128:
1467:
1465:
1463:
260:Key: UDHXJZHVNHGCEC-UHFFFAOYSA-N
7:
1191:
1189:
1133:
1131:
1088:
1086:
1053:
1051:
1049:
1047:
1045:
1043:
1041:
1039:
1037:
614:of the previously obtained compound
407:populations. It is classified as an
499:H360D: May damage the unborn child
270:Key: UDHXJZHVNHGCEC-UHFFFAOYAM
170:
154:
470:(854 mg/L at 25 °C) and
14:
1947:1,3-Difluoro-2-propanol (Gliftor)
504:H310: Fatal in contact with skin
436:Structure and physical properties
1158:Zeitschrift für Naturforschung B
748:also appears to be an important
629:, with no significant amount of
359:
324:
318:
22:
1348:Drug Metabolism and Disposition
355:(at 25 °C , 100 kPa).
1766:Tetramethylenedisulfotetramine
815:values for different species:
327:
312:
1:
1243:10.1016/s0083-6729(07)00006-4
549:~260 nm and 315 nm
409:extremely hazardous substance
1498:10.1016/j.cropro.2003.09.011
1331:. World Health organization.
1200:. Springer. pp. 87–108.
1093:Hadler M, Buckle A (1992).
758:enterohepatic recirculation
533:4.76 x 10 Pa at 23 °C
448:, connected on one side to
2019:
2003:Anticoagulant rodenticides
1544:10.1177/104063870601800512
956:Government Printing Office
1438:10.1007/s00244-010-9554-x
954:(July 1, 2008 ed.).
861:Black-tailed prairie dog
800:shown symptoms including
696:integral membrane protein
501:H300: Fatal if swallowed
349:
299:
279:
244:
55:
35:
30:
21:
1998:4-Chlorophenyl compounds
633:or other side products.
1231:Vitamins & Hormones
1112:"Rozol Tracking Powder"
612:Friedel-Crafts reaction
507:H330: Fatal if inhaled
978:Cite journal requires
729:gastrointestinal tract
648:
567:
440:Chlorophacinone is an
396:is a first-generation
1771:Chlorophenylsilatrane
1618:Vitamin K antagonists
1237:, Elsevier: 103–130,
1171:10.1515/znb-2011-0116
700:endoplasmic reticulum
644:
595:to afford 85% of the
563:
541:5.12 x 10 atm-m3/mol
538:Henry's law constant
1967:Sodium fluoroacetate
1511:Pelfrene AF (2001).
806:respiratory distress
37:Preferred IUPAC name
1817:Aluminium phosphide
1809:Inorganic compounds
1490:2004CrPro..23..425A
1430:2011ArECT..60..533P
1324:Tasheva M. (1995).
637:Mechanism of action
574:The synthesis uses
483:Pale-yellow powder
345: g·mol
18:
1927:α-Naphthylthiourea
1632:4-Hydroxycoumarins
895:Environmental risk
869:Northern bobwhite
773:Effects on animals
744:isozymes and ring
733:respiratory system
668:γ-carboxyglutamate
649:
568:
546:UV/Vis absorption
513:repeated exposure
496:Hazard statements
382:Infobox references
16:
1975:
1974:
1832:Calcium phosphide
1737:
1736:
1705:
1704:
1252:978-0-12-374113-4
884:
883:
750:biotransformation
657:posttranslational
597:phenylacetic acid
553:
552:
522:Relative density
390:Chemical compound
388:
387:
213:CompTox Dashboard
97:Interactive image
2010:
1880:Organophosphorus
1842:Thallium sulfate
1827:Barium carbonate
1666:2nd generation (
1637:
1623:
1594:
1587:
1580:
1571:
1564:
1563:
1523:
1517:
1516:
1508:
1502:
1501:
1469:
1458:
1457:
1409:
1403:
1402:
1378:
1372:
1371:
1339:
1333:
1332:
1330:
1321:
1315:
1314:
1312:
1311:
1291:(6): 1798–1808.
1276:
1270:
1269:
1268:
1267:
1222:
1216:
1215:
1209:
1201:
1193:
1184:
1183:
1173:
1149:
1143:
1142:
1135:
1126:
1125:
1123:
1122:
1108:
1102:
1101:
1099:
1090:
1081:
1080:
1074:
1066:
1064:
1055:
1032:
1031:
1029:
1028:
1014:
1008:
1007:
1005:
1004:
994:
988:
987:
981:
976:
974:
966:
964:
962:
953:
945:
913:ground squirrels
909:mountain beavers
818:
620:1,3- indanedione
530:Vapour pressure
477:
442:organic compound
372:
366:
363:
362:
344:
329:
326:
320:
314:
307:Chemical formula
237:
236:
221:
219:
203:
183:
172:
158:
141:
130:
119:
99:
75:
26:
19:
17:Chlorophacinone
2018:
2017:
2013:
2012:
2011:
2009:
2008:
2007:
1993:1,3-Indandiones
1978:
1977:
1976:
1971:
1937:Fluoroacetamide
1915:
1892:
1874:
1851:
1803:
1794:Cholecalciferol
1780:
1756:Phenylsilatrane
1733:
1719:Chlorophacinone
1711:1,3-Indandiones
1701:
1661:
1630:
1616:
1607:
1598:
1568:
1567:
1525:
1524:
1520:
1510:
1509:
1505:
1471:
1470:
1461:
1411:
1410:
1406:
1380:
1379:
1375:
1341:
1340:
1336:
1328:
1323:
1322:
1318:
1309:
1307:
1278:
1277:
1273:
1265:
1263:
1253:
1224:
1223:
1219:
1202:
1195:
1194:
1187:
1151:
1150:
1146:
1137:
1136:
1129:
1120:
1118:
1110:
1109:
1105:
1097:
1092:
1091:
1084:
1067:
1062:
1057:
1056:
1035:
1026:
1024:
1016:
1015:
1011:
1002:
1000:
996:
995:
991:
977:
967:
960:
958:
951:
947:
946:
942:
937:
925:
897:
889:carcinogenicity
830:
814:
797:
789:cardiopulmonary
775:
766:
754:glucuronic acid
742:cytochrome P450
737:bioaccumulation
725:
713:
709:
705:
698:present in the
693:
689:
681:
639:
604:oxalyl chloride
593:
588:in presence of
558:
438:
421:
394:Chlorophacinone
391:
384:
379:
378:
377: ?)
368:
364:
360:
356:
342:
332:
323:
317:
309:
295:
292:
287:
286:
275:
272:
271:
268:
262:
261:
258:
252:
251:
240:
222:
215:
206:
186:
173:
161:
122:
102:
89:
78:
65:
51:
50:
12:
11:
5:
2016:
2014:
2006:
2005:
2000:
1995:
1990:
1980:
1979:
1973:
1972:
1970:
1969:
1964:
1959:
1954:
1949:
1944:
1939:
1934:
1929:
1923:
1921:
1917:
1916:
1914:
1913:
1908:
1902:
1900:
1894:
1893:
1891:
1890:
1884:
1882:
1876:
1875:
1873:
1872:
1867:
1861:
1859:
1857:Organochlorine
1853:
1852:
1850:
1849:
1847:Zinc phosphide
1844:
1839:
1834:
1829:
1824:
1819:
1813:
1811:
1805:
1804:
1802:
1801:
1799:Ergocalciferol
1796:
1790:
1788:
1782:
1781:
1779:
1778:
1773:
1768:
1763:
1758:
1753:
1747:
1745:
1739:
1738:
1735:
1734:
1732:
1731:
1726:
1721:
1715:
1713:
1707:
1706:
1703:
1702:
1700:
1699:
1694:
1689:
1684:
1679:
1673:
1671:
1668:Superwarfarins
1663:
1662:
1660:
1659:
1654:
1649:
1643:
1641:
1640:1st generation
1634:
1620:
1613:Anticoagulants
1609:
1608:
1599:
1597:
1596:
1589:
1582:
1574:
1566:
1565:
1538:(5): 483–485.
1518:
1503:
1459:
1424:(3): 533–542.
1404:
1373:
1354:(6): 645–648.
1334:
1316:
1271:
1251:
1217:
1185:
1144:
1127:
1103:
1082:
1033:
1009:
989:
980:|journal=
939:
938:
936:
933:
932:
931:
929:1,3-Indandione
924:
921:
905:pocket gophers
896:
893:
882:
881:
878:
874:
873:
870:
866:
865:
862:
858:
857:
854:
850:
849:
846:
842:
841:
838:
834:
833:
828:
824:
812:
796:
793:
774:
771:
765:
762:
724:
721:
711:
707:
703:
691:
687:
679:
638:
635:
591:
557:
554:
551:
550:
547:
543:
542:
539:
535:
534:
531:
527:
526:
523:
519:
518:
497:
493:
492:
491:143.0 °C
489:
488:Melting point
485:
484:
481:
437:
434:
420:
417:
389:
386:
385:
380:
358:
357:
353:standard state
350:
347:
346:
340:
334:
333:
330:
321:
315:
310:
305:
302:
301:
297:
296:
294:
293:
290:
282:
281:
280:
277:
276:
274:
273:
269:
266:
265:
263:
259:
256:
255:
247:
246:
245:
242:
241:
239:
238:
225:
223:
211:
208:
207:
205:
204:
196:
194:
188:
187:
185:
184:
176:
174:
166:
163:
162:
160:
159:
151:
149:
143:
142:
132:
124:
123:
121:
120:
112:
110:
104:
103:
101:
100:
92:
90:
83:
80:
79:
77:
76:
68:
66:
61:
58:
57:
53:
52:
40:
39:
33:
32:
28:
27:
13:
10:
9:
6:
4:
3:
2:
2015:
2004:
2001:
1999:
1996:
1994:
1991:
1989:
1986:
1985:
1983:
1968:
1965:
1963:
1960:
1958:
1955:
1953:
1950:
1948:
1945:
1943:
1940:
1938:
1935:
1933:
1930:
1928:
1925:
1924:
1922:
1918:
1912:
1909:
1907:
1904:
1903:
1901:
1899:
1895:
1889:
1886:
1885:
1883:
1881:
1877:
1871:
1868:
1866:
1863:
1862:
1860:
1858:
1854:
1848:
1845:
1843:
1840:
1838:
1835:
1833:
1830:
1828:
1825:
1823:
1820:
1818:
1815:
1814:
1812:
1810:
1806:
1800:
1797:
1795:
1792:
1791:
1789:
1787:
1783:
1777:
1774:
1772:
1769:
1767:
1764:
1762:
1759:
1757:
1754:
1752:
1749:
1748:
1746:
1744:
1740:
1730:
1727:
1725:
1722:
1720:
1717:
1716:
1714:
1712:
1708:
1698:
1695:
1693:
1690:
1688:
1685:
1683:
1680:
1678:
1675:
1674:
1672:
1669:
1664:
1658:
1655:
1653:
1652:Coumatetralyl
1650:
1648:
1645:
1644:
1642:
1638:
1635:
1633:
1628:
1624:
1621:
1619:
1614:
1610:
1606:
1602:
1595:
1590:
1588:
1583:
1581:
1576:
1575:
1572:
1561:
1557:
1553:
1549:
1545:
1541:
1537:
1533:
1529:
1522:
1519:
1514:
1507:
1504:
1499:
1495:
1491:
1487:
1483:
1479:
1475:
1468:
1466:
1464:
1460:
1455:
1451:
1447:
1443:
1439:
1435:
1431:
1427:
1423:
1419:
1415:
1408:
1405:
1400:
1396:
1392:
1388:
1384:
1377:
1374:
1369:
1365:
1361:
1357:
1353:
1349:
1345:
1338:
1335:
1327:
1320:
1317:
1306:
1302:
1298:
1294:
1290:
1286:
1282:
1275:
1272:
1262:
1258:
1254:
1248:
1244:
1240:
1236:
1232:
1228:
1221:
1218:
1213:
1207:
1199:
1192:
1190:
1186:
1181:
1177:
1172:
1167:
1163:
1159:
1155:
1148:
1145:
1140:
1134:
1132:
1128:
1117:
1116:liphatech.com
1113:
1107:
1104:
1100:. p. 36.
1096:
1089:
1087:
1083:
1078:
1072:
1061:
1054:
1052:
1050:
1048:
1046:
1044:
1042:
1040:
1038:
1034:
1023:
1022:liphatech.com
1019:
1013:
1010:
999:
993:
990:
985:
972:
957:
950:
944:
941:
934:
930:
927:
926:
922:
920:
918:
914:
910:
906:
902:
894:
892:
890:
879:
876:
875:
871:
868:
867:
863:
860:
859:
855:
852:
851:
847:
844:
843:
839:
836:
835:
832:
831:value (mg/kg)
825:
823:
820:
819:
816:
809:
807:
803:
794:
792:
790:
786:
781:
772:
770:
763:
761:
759:
755:
751:
747:
746:hydroxylation
743:
738:
734:
730:
722:
720:
718:
701:
697:
685:
677:
673:
672:phospholipids
669:
665:
661:
660:carboxylation
658:
654:
647:
643:
636:
634:
632:
628:
624:
621:
617:
613:
609:
605:
601:
598:
594:
587:
584:
583:chlorobenzene
580:
577:
576:mandelic acid
572:
566:
562:
555:
548:
545:
544:
540:
537:
536:
532:
529:
528:
524:
521:
520:
517:
514:
511:
508:
505:
502:
498:
495:
494:
490:
487:
486:
482:
479:
478:
475:
473:
469:
465:
461:
459:
455:
454:phenyl groups
451:
447:
443:
435:
433:
431:
427:
418:
416:
414:
410:
406:
402:
399:
398:anticoagulant
395:
383:
376:
371:
354:
348:
341:
339:
336:
335:
311:
308:
304:
303:
298:
289:
285:
278:
264:
254:
250:
243:
235:
231:
230:DTXSID2032348
227:
226:
224:
214:
210:
209:
202:
198:
197:
195:
193:
190:
189:
182:
178:
177:
175:
169:
165:
164:
157:
153:
152:
150:
148:
145:
144:
140:
136:
133:
131:
129:ECHA InfoCard
126:
125:
118:
114:
113:
111:
109:
106:
105:
98:
94:
93:
91:
87:
82:
81:
74:
70:
69:
67:
64:
60:
59:
54:
48:
45:-indene-1,3(2
44:
38:
34:
29:
25:
20:
1962:Scilliroside
1942:Flupropadine
1718:
1692:Difethialone
1682:Bromadiolone
1605:Rodenticides
1601:Pest control
1535:
1531:
1521:
1506:
1481:
1477:
1421:
1417:
1407:
1390:
1386:
1376:
1351:
1347:
1337:
1319:
1308:. Retrieved
1288:
1284:
1274:
1264:, retrieved
1234:
1230:
1220:
1197:
1164:(1): 95–97.
1161:
1157:
1147:
1141:. July 2016.
1119:. Retrieved
1115:
1106:
1025:. Retrieved
1021:
1012:
1001:. Retrieved
992:
971:cite journal
959:. Retrieved
943:
901:prairie dogs
898:
885:
826:
821:
810:
798:
776:
767:
726:
650:
645:
626:
622:
615:
607:
599:
585:
578:
573:
569:
564:
525:1.4301 g/mL
515:
512:
509:
506:
503:
500:
462:
446:acetyl group
439:
422:
393:
392:
56:Identifiers
46:
42:
1932:Bromethalin
1786:Calciferols
1743:Convulsants
1729:Diphacinone
1697:Flocoumafen
1677:Brodifacoum
961:October 29,
917:lethal dose
845:Female rat
717:endothelial
676:coagulation
631:diphacinone
480:Appearance
464:Henry's law
458:enantiomers
450:indanedione
401:rodenticide
300:Properties
135:100.020.912
1988:Pesticides
1982:Categories
1952:Norbormide
1898:Carbamates
1888:Phosacetim
1865:Chloralose
1761:Strychnine
1687:Difenacoum
1484:(5): 425.
1310:2024-03-14
1266:2024-03-14
1121:2024-03-14
1027:2024-03-14
1003:2024-03-14
935:References
785:hemorrhage
780:inhibition
723:Metabolism
452:ring. Two
338:Molar mass
201:34Y6E0063Y
108:ChemSpider
84:3D model (
63:CAS Number
1957:Pyrinuron
1751:Crimidine
1627:Coumarins
1552:1040-6387
1446:1432-0703
1399:0507-6773
1360:0090-9556
1206:cite book
1180:1865-7117
1018:"History"
837:Male rat
802:epistaxis
664:glutamate
556:Synthesis
430:vitamin K
73:3691-35-8
1906:Aldicarb
1647:Warfarin
1560:17037620
1454:20552335
1305:10068650
1261:18374192
1071:cite web
923:See also
880:>300
877:Redworm
795:Toxicity
764:Efficacy
684:cofactor
472:methanol
426:Warfarin
1837:Cyanide
1822:Arsenic
1724:Pindone
1657:Fumarin
1486:Bibcode
1426:Bibcode
1368:6130915
853:Rabbit
822:Species
653:hepatic
419:History
375:what is
373: (
168:PubChem
49:)-dione
1920:Others
1911:T-1152
1870:Endrin
1558:
1550:
1452:
1444:
1397:
1393:(21).
1366:
1358:
1303:
1259:
1249:
1178:
856:0.329
848:10.95
811:The LD
468:hexane
405:rodent
370:verify
367:
343:374.82
284:SMILES
156:C18514
31:Names
1329:(PDF)
1285:Blood
1098:(PDF)
1063:(PDF)
952:(PDF)
864:1.94
840:3.15
618:with
249:InChI
181:19402
117:18286
86:JSmol
1556:PMID
1548:ISSN
1450:PMID
1442:ISSN
1395:ISSN
1364:PMID
1356:ISSN
1301:PMID
1257:PMID
1247:ISBN
1212:link
1176:ISSN
1077:link
984:help
963:2011
911:and
872:258
590:SnCl
192:UNII
147:KEGG
41:2--1
1776:RDX
1540:doi
1494:doi
1434:doi
1293:doi
1239:doi
1166:doi
666:to
662:of
218:EPA
171:CID
1984::
1603::
1554:.
1546:.
1536:18
1534:.
1530:.
1492:.
1482:23
1480:.
1476:.
1462:^
1448:.
1440:.
1432:.
1422:60
1420:.
1416:.
1391:21
1389:.
1385:.
1362:.
1352:10
1350:.
1346:.
1299:.
1289:93
1287:.
1283:.
1255:,
1245:,
1235:78
1233:,
1229:,
1208:}}
1204:{{
1188:^
1174:.
1162:66
1160:.
1156:.
1130:^
1114:.
1085:^
1073:}}
1069:{{
1036:^
1020:.
975::
973:}}
969:{{
907:,
903:,
829:50
827:LD
813:50
804:,
682:)
460:.
325:Cl
322:15
316:23
1670:)
1629:/
1615:/
1593:e
1586:t
1579:v
1562:.
1542::
1515:.
1500:.
1496::
1488::
1456:.
1436::
1428::
1401:.
1370:.
1313:.
1295::
1241::
1214:)
1182:.
1168::
1124:.
1079:)
1065:.
1030:.
1006:.
986:)
982:(
965:.
712:2
708:2
704:2
692:2
688:2
680:2
627:6
623:5
616:4
608:4
600:3
592:4
586:2
579:1
365:N
331:3
328:O
319:H
313:C
220:)
216:(
88:)
47:H
43:H
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.