371:
244:
65:
657:
35:
662:
652:
78:
683:
47:
2143:
1297:
the solid is practically zero, water evaporates out of each of the solid 6-, 2-, and 1- hydrates, leaving the next lower hydrate, at about 40°C, 89°C, and 125°C, respectively. If the partial pressure of the water vapor is in equilibrium with the solid, as in a confined but not pressurized contained, the decomposition occurs at about 115°C, 145°C, and 195°C, respectively.
1039:
1746:
688:
830:
924:= 1, 2, 6, and 9. Claims of the formation of tri- and tetrahydrates have not been confirmed. The anhydrous form is a blue crystalline solid; the dihydrate is purple and the hexahydrate is pink. Commercial samples are usually the hexahydrate, which is one of the most commonly used cobalt salts in the lab.
2167:
Cobalt is essential for most higher forms of life, but more than a few milligrams each day is harmful. Although poisonings have rarely resulted from cobalt compounds, their chronic ingestion has caused serious health problems at doses far less than the lethal dose. In 1966, the addition of cobalt
1296:
On rapid heating or in a closed container, each of the 6-, 2-, and 1- hydrates partially melts into a mixture of the next lower hydrate and a saturated solution—at 51.25 °C, 206 °C, and 335 °C, respectively. On slow heating in an open container, so that the water vapor pressure over
1289:
The solid dihydrate and hexahydrate can be obtained by evaporation. Cooling saturated aqueous solutions yields the dihydrate between 120.2 °C and 51.25 °C, and the hexahydrate below 51.25 °C. Water ice, rather than cobalt chloride, will crystallize from solutions with concentration
2150:
Cobalt chloride is a common visual moisture indicator due to its distinct colour change when hydrated. The colour change is from some shade of blue when dry, to a pink when hydrated, although the shade of colour depends on the substrate and concentration. It is impregnated into paper to make test
686:
2870:
Zug KA, Warshaw EM, Fowler JF Jr, Maibach HI, Belsito DL, Pratt MD, Sasseville D, Storrs FJ, Taylor JS, Mathias CG, Deleo VA, Rietschel RL, Marks J. Patch-test results of the North
American Contact Dermatitis Group 2005–2006. Dermatitis. 2009
2848:
11.1.5 The unusual type of myocardiopathy recognized in 1965 and 1966 in Quebec (Canada), Minneapolis (Minnesota), Leuven (Belgium), and Omaha (Nebraska) was associated with episodes of acute heart failure (e/g/, 50 deaths among 112 beer
632:
1390:
In the laboratory, cobalt(II) chloride serves as a common precursor to other cobalt compounds. Generally, diluted aqueous solutions of the salt behave like other cobalt(II) salts since these solutions consist of the
2233:. Cobalt supplementation is not banned and therefore would not be detected by current anti-doping testing. Cobalt chloride is a banned substance under the Australian Thoroughbred Racing Board.
2222:: when suspended in solution, cobalt(II) chloride can be made to appear invisible on a surface; when that same surface is subsequently exposed to significant heat (such as from a handheld
687:
843:
2695:
Erin K. Byrne; Klaus H. Theopold (1989). "Synthesis, characterization, and electron-transfer reactivity of norbornyl complexes of cobalt in unusually high oxidation states".
181:
689:
1290:
below 29%. The monohydrate and the anhydrous forms can be obtained by cooling solutions only under high pressure, above 206 °C and 335 °C, respectively.
2670:
Erin K. Byrne; Darrin S. Richeson & Klaus H. Theopold (1986). "Tetrakis(1-norbornyl)cobalt, a low spin tetrahedral complex of a first row transition metal".
420:
2956:
2196:
3013:
2986:
1926:
On the other hand, cobalt(III) chlorides can be obtained if the cobalt is bound also to other ligands of greater Lewis basicity than chloride, such as
2510:
John Dallas
Donaldson, Detmar Beyersmann, "Cobalt and Cobalt Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
711:
997:
in water is about 54% at the boiling point, 120.2 °C; 48% at 51.25 °C; 35% at 25 °C; 33% at 0 °C; and 29% at −27.8 °C.
2146:
The deep blue colour of this moisture indicating silica gel is due to cobalt chloride. When hydrated the colour changes to a light pink/purple.
2859:
2400:
2981:
2236:
Cobalt chloride is one method used to induce pulmonary arterial hypertension in animals for research and evaluation of treatment efficacy.
3585:
5295:
2614:
2544:
2461:
2334:
M. T. Saugier, M. Noailly, R. Cohen-Adad, F. Paulik, and J. Paulik (1977): "Equilibres solide ⇄ liquide ⇆ vapeur du systeme binaire
385:
2253:
2106:
in 1913 for his studies on a series of these cobalt(III) compounds, work that led to an understanding of the structures of such
5300:
3522:
3510:
2288:
Wojakowska, A.; Krzyżak, E.; Plińska, S. (2007). "Melting and high-temperature solid state transitions in cobalt(II) halides".
2131:
3006:
978:
957:
m) in which the cobalt(II) ions are octahedrally coordinated. At about 706 °C (20 degrees below the melting point), the
2134:— a rare example of a stable transition metal/saturated alkane compound, different products are obtained in other solvents.
2847:
5275:
1943:
850:
5280:
3036:
2672:
310:
2527:
Philip
Boudjouk; Jeung-Ho So (2007). "Solvated and Unsolvated Anhydrous Metal Chlorides from Metal Chloride Hydrates".
661:
656:
349:
697:
4943:
3930:
2885:
2595:
Gill, N. S. & Taylor, F. B. (1967). "Tetrahalo
Complexes of Dipositive Metals in the First Transition Series".
5224:
5200:
5109:
2999:
251:
3900:
3650:
5217:
5116:
5027:
5004:
4966:
4342:
4335:
3948:
2881:
1762:
1153:
1118:
2762:
Morin Y; Tětu A; Mercier G (1969). "Quebec beer-drinkers' cardiomyopathy: Clinical and hemodynamic aspects".
5192:
5176:
5161:
5153:
5073:
5020:
4958:
4927:
4497:
4479:
4393:
4386:
4371:
4356:
4328:
4291:
4198:
4113:
3578:
1301:
573:
4577:
2476:
Morosin, B.; Graeber, E. J. (1965). "Crystal structures of manganese(II) and iron(II) chloride dihydrate".
239:
5290:
5207:
5169:
5086:
5066:
4996:
4981:
4951:
4803:
4599:
4570:
4554:
4471:
4409:
4379:
4364:
4349:
4298:
4284:
4243:
4179:
4106:
4091:
4069:
4062:
4047:
4040:
3941:
3888:
3862:
3223:
3111:
1449:
2724:
2371:
Note: the lowest point of fig.6 is inconsistent with fig.7; probably should be at -27.8 C instead of 0 C.
1860:
5138:
5131:
5124:
5094:
5058:
5035:
5012:
4974:
4811:
4715:
4708:
4700:
4693:
4642:
4628:
4620:
4606:
4592:
4562:
4401:
4306:
4276:
4225:
4213:
4098:
4084:
4055:
4033:
3922:
3851:
3668:
3450:
2392:
962:
812:
808:
651:
644:
609:
94:
5184:
5145:
5101:
5051:
5043:
4935:
4765:
4761:
4753:
4727:
4686:
4664:
4649:
4635:
4613:
4584:
4490:
4464:
4441:
4313:
4251:
4191:
4143:
4076:
3978:
3855:
3828:
3806:
3739:
3720:
3563:
3500:
3458:
3442:
3410:
3382:
3244:
3200:
3187:
3163:
3071:
2771:
2485:
1893:
1821:
1750:
1173:
1169:
1149:
958:
4989:
4796:
4742:
4671:
4657:
4528:
4505:
4434:
4320:
4236:
4206:
4183:
4163:
4151:
4011:
3960:
3817:
3776:
3698:
3545:
3477:
3418:
3361:
3317:
3239:
3123:
3099:
2107:
2087:
1836:
785:
366:
122:
1030:
ions. Concentrated solutions are red at room temperature but become blue at higher temperatures.
5285:
4734:
4678:
4517:
4509:
4430:
4266:
4159:
4128:
4006:
3955:
3911:
3892:
3880:
3839:
3731:
3631:
3571:
3256:
3231:
3214:
3179:
3151:
3131:
3079:
3063:
3055:
2795:
1580:
982:
866:
789:
173:
64:
34:
4821:
4780:
4773:
4261:
4220:
4121:
4025:
3971:
3795:
3784:
3757:
3687:
3661:
3639:
3610:
3430:
3143:
2937:
2830:
2787:
2744:
2697:
2644:
2610:
2540:
2457:
2396:
2048:
1734:
1177:
793:
759:
591:
2450:
2415:
Yuzo Saeki, Ryoko
Matsuzaki, Naomi Aoyama (1977): "The vapor pressure of cobalt dichloride".
4788:
4723:
4538:
4521:
4456:
4449:
4422:
4015:
3985:
3799:
3753:
3727:
3709:
3626:
3366:
3195:
3022:
2927:
2919:
2822:
2779:
2736:
2705:
2677:
2652:
2602:
2577:
2532:
2511:
2493:
2420:
2384:
2364:
2297:
1799:
1409:
938:
597:
530:
448:
326:
319:
221:
139:
4826:
4543:
4417:
3875:
3679:
3405:
1798:. This 19-electron species is a good reducing agent, being readily oxidised to the yellow
147:
132:
2976:
765:
2775:
2489:
370:
243:
201:
3765:
3746:
3284:
3264:
3208:
2932:
2907:
2783:
2740:
2230:
2173:
2083:, induce the formation of Co(III) derivatives. Simple carboxylates and halides do not.
1402:
961:
is believed to change to tetrahedral. The vapor pressure has been reported as 7.6
821:
2642:
Barton K. Bower & Howard G. Tennent (1972). "Transition metal bicyclohept-1-yls".
2261:
5269:
4845:
4171:
2597:
2424:
2229:
Cobalt chloride is an established chemical inducer of hypoxia-like responses such as
2219:
2192:
2099:
2076:
519:
503:
232:
283:
2799:
1133:
2515:
77:
46:
5240:
3846:
2908:"Cobalt chloride administration in athletes: a new perspective in blood doping?"
2159:
can incorporate cobalt chloride to indicate when it is "spent" (i.e. hydrated).
2103:
1766:
1445:
1157:
1129:
747:
723:
2606:
2536:
1401:
ion regardless of the anion. For example, such solutions give a precipitate of
4861:
4837:
2301:
2207:
2188:
2156:
2142:
1441:
1433:
974:
619:
547:
463:
212:
2923:
2748:
2560:
Long, Gary J.; Clarke, Peter J. (1978). "Crystal and
Molecular Structures of
2169:
2152:
2072:
1754:
1745:
338:
2941:
2834:
2314:
16:"CoCl₂" redirects here. For the compound with molecular formula COCl₂, see
2826:
2791:
2071:
substituted for atmospheric oxygen. Other highly basic ligands, including
1733:
The tetrachlorocobaltate ion is the blue ion that forms upon addition of
5248:
3993:
3595:
2681:
2223:
2203:
1920:
1907:
is not stable in normal conditions, and would decompose immediately into
1027:
874:
710:
703:
696:
669:
565:
561:
17:
2709:
2656:
2581:
2151:
strips for detecting moisture in solutions, or more slowly, in air/gas.
5232:
4873:
4853:
2368:
2080:
1931:
1122:
1001:
890:
557:
489:
270:
252:
2564:-Tetrakis(pyridine)dichloroiron(II), -Nickel(II), and -Cobalt(II) and
2497:
1038:
2184:
2091:
2044:
1939:
1437:
870:
820:
Except where otherwise noted, data are given for materials in their
2991:
2957:"Cobalt crisis turns the eyes of the world onto Australian racing"
2141:
2040:
1935:
1927:
1744:
1737:
to aqueous solutions of hydrated cobalt chloride, which are pink.
1037:
294:
192:
180:
172:
161:
1382:
The anhydrous compound can be purified by sublimation in vacuum.
704:
2725:"The use of cobalt salts as indicators of humidity and moisture"
1293:
The anhydrous compound can be prepared by heating the hydrates.
3567:
2995:
2813:
Barceloux, Donald G. & Barceloux, Donald (1999). "Cobalt".
802:
779:
2438:, 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960.
1820:
Compounds of cobalt in the +3 oxidation state exist, such as
354:
45:
33:
1156:. The octahedron is completed by a pair of mutually trans
2202:
In 2005–06, cobalt chloride was the eighth-most-prevalent
2043:. These reactions are often performed in the presence of
2183:
Furthermore, cobalt(II) chloride is suspected of causing
1168:
Cobalt chloride can be prepared in aqueous solution from
681:
937:
At room temperature, anhydrous cobalt chloride has the
838:
508:
726 °C (1,339 °F; 999 K) ±2 (anhydrous)
2632:, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
2130:·THF in pentane produces the brown, thermally stable
3538:
3493:
3470:
3398:
3375:
3354:
3048:
3029:
1711:can be prepared using tetraethylammonium chloride:
2568:-Tetrakis(pyridine)dichloroiron(II) Monohydrate".
2449:
2172:in Canada led to a peculiar form of toxin-induced
2982:National Pollutant Inventory – Cobalt fact sheet
2906:Lippi G, Franchini M, Guidi GC (November 2005).
1432:The hexahydrate and the anhydrous salt are weak
1152:. Each Co center is coordinated to four doubly
282:
2226:or lighter) the ink reversibly changes to blue.
685:
146:
138:
131:
2090:, Co(III) complexes are very slow to exchange
1121:. This species dissolves readily in water and
3579:
3007:
2315:Santa Cruz Biotechnology: Cobalt(II) chloride
8:
2987:IARC Monograph "Cobalt and Cobalt Compounds"
524:1,049 °C (1,920 °F; 1,322 K)
2290:Journal of Thermal Analysis and Calorimetry
2197:International Agency for Research on Cancer
977:in water. Under atmospheric pressure, the
3586:
3572:
3564:
3014:
3000:
2992:
2764:Annals of the New York Academy of Sciences
1058:The crystal unit of the solid hexahydrate
369:
242:
220:
22:
2931:
2456:(5th ed.), Oxford: Clarendon Press,
2379:
2377:
2330:
2328:
2326:
2324:
2322:
325:
318:
5210:
3503:
2118:Reaction of 1-norbornyllithium with the
1761:Reaction of the anhydrous compound with
472:237.93 g/mol (hexahydrate)
2977:International Chemical Safety Card 0783
2283:
2281:
2279:
2254:"Cobalt muriate, CAS Number: 7646-79-9"
2245:
425:
390:
365:
3616:
3594:Salts and covalent derivatives of the
1300:Dehydration can also be effected with
233:
4916:
2419:, volume 55, issue 2, pages 289-291.
397:Key: GVPFVAHMJGGAJG-UHFFFAOYSA-L
200:
7:
3606:
2955:Bartley, Patrick (6 February 2015).
2363:, volume 11, issue 1, pages 87–100.
2039:Similar reactions occur with other
1930:. For example, in the presence of
407:Key: GVPFVAHMJGGAJG-NUQVWONBAU
273:
2784:10.1111/j.1749-6632.1969.tb16751.x
2741:10.1111/j.1744-7348.1945.tb06763.x
2531:. Vol. 29. pp. 108–111.
1613:), a tetrahedral complex results:
433:hexahydrate: Cl(Cl)()()().O.O
14:
2630:Handbook of Chemistry and Physics
2601:. Vol. 9. pp. 136–142.
2417:Journal of the Less Common Metals
1934:, cobalt(II) chloride is readily
1707:Salts of the anionic complex CoCl
394:InChI=1S/2ClH.Co/h2*1H;/q;;+2/p-2
2723:Solomon, M. E. (February 1945).
1450:octahedral complex with pyridine
828:
660:
655:
650:
484:rose red crystals (hexahydrate)
404:InChI=1/2ClH.Co/h2*1H;/q;;+2/p-2
76:
63:
2132:tetrakis(1-norbornyl)cobalt(IV)
824:(at 25 °C , 100 kPa).
70:Structure of anhydrous compound
2452:Structural Inorganic Chemistry
1749:The structure of a cobalt(IV)
1083:contains the neutral molecule
1:
2516:10.1002/14356007.a07_281.pub2
2178:beer drinker's cardiomyopathy
1944:hexamminecobalt(III) chloride
889:. The compound forms several
2673:J. Chem. Soc., Chem. Commun.
2425:10.1016/0022-5088(77)90204-1
2176:, which came to be known as
2478:Journal of Chemical Physics
2361:Journal of Thermal Analysis
736:or concentration (LD, LC):
29:
5317:
4918:
2886:Royal Society of Chemistry
2607:10.1002/9780470132401.ch37
2537:10.1002/9780470132609.ch26
973:Cobalt chloride is fairly
480:blue crystals (anhydrous)
468:129.839 g/mol (anhydrous)
15:
5296:IARC Group 2B carcinogens
3618:
3603:
2961:The Sydney Morning Herald
2729:Annals of Applied Biology
2389:Chemistry of the Elements
2387:; Earnshaw, Alan (1997).
2302:10.1007/s10973-006-8000-9
2094:, so they are said to be
1154:bridging chloride ligands
818:
771:
732:
631:
626:
584:
552:38.5 g/100 mL (methanol)
498:1.924 g/cm (hexahydrate)
470:165.87 g/mol (dihydrate)
441:
416:
381:
115:
103:
93:
88:
75:
62:
28:
2924:10.1136/bjsm.2005.019232
1816:Oxidation to cobalt(III)
1763:sodium cyclopentadienide
1119:water of crystallization
482:violet-blue (dihydrate)
83:Structure of hexahydrate
2168:compounds to stabilize
2114:Oxidation to cobalt(IV)
2047:as a catalyst, or with
1302:trimethylsilyl chloride
1132:and the hexahydrate is
617:monoclinic (dihydrate)
574:Magnetic susceptibility
554:8.6 g/100 mL (acetone)
496:2.477 g/cm (dihydrate)
494:3.356 g/cm (anhydrous)
5301:Deliquescent materials
2882:"Making invisible ink"
2147:
2108:coordination compounds
1758:
1128:The anhydrous salt is
1055:
965:at the melting point.
692:
615:hexagonal (anhydrous)
540:52.9 g/100 mL (20 °C)
50:
38:
2871:May–Jun;20(3):149-60.
2827:10.1081/CLT-100102420
2448:Wells, A. F. (1984),
2393:Butterworth-Heinemann
2385:Greenwood, Norman N.
2145:
2098:. The German chemist
1802:cobaltocenium cation
1748:
1136:. The dihydrate, CoCl
1117:and two molecules of
1041:
813:Iridium(III) chloride
809:Rhodium(III) chloride
754:80 mg/kg (rat, oral)
691:
610:Coordination geometry
542:105 g/100 mL (96 °C)
536:43.6 g/100 mL (0 °C)
510:140 °C (monohydrate)
49:
37:
5276:Cobalt(II) compounds
2682:10.1039/C39860001491
2258:www.chemindustry.com
1894:cobalt(III) chloride
1822:cobalt(III) fluoride
1751:coordination complex
1408:upon treatment with
1174:cobalt(II) carbonate
1170:cobalt(II) hydroxide
1150:coordination polymer
1016:contain the species
674:(fire diamond)
514:86 °C (hexahydrate)
428:anhydrous: ClCl
24:Cobalt(II) chloride
5281:Inorganic compounds
2815:Clinical Toxicology
2776:1969NYASA.156..566M
2710:10.1021/ja00193a021
2657:10.1021/ja00762a056
2598:Inorganic Syntheses
2582:10.1021/ic50184a002
2570:Inorganic Chemistry
2529:Inorganic Syntheses
2490:1965JChPh..42..898M
2199:(IARC) Monographs.
2138:Moisture indication
1440:are usually either
1428:Complexed chlorides
877:, with the formula
863:Cobalt(II) chloride
786:Cobalt(II) fluoride
531:Solubility in water
512:100 °C (dihydrate)
330: (hexahydrate)
151: (hexahydrate)
98:Cobalt(II) chloride
25:
2369:10.1007/BF02104087
2148:
1759:
1581:triphenylphosphine
1056:
983:saturated solution
979:mass concentration
867:inorganic compound
851:Infobox references
790:Cobalt(II) bromide
772:Related compounds
693:
580:+12,660·10 cm/mol
538:45 g/100 mL (7 °C)
179:hexahydrate:
106:Cobaltous chloride
51:
39:
23:
5263:
5262:
5257:
5256:
3561:
3560:
2704:(11): 3887–3896.
2698:J. Am. Chem. Soc.
2676:(19): 1491–1492.
2645:J. Am. Chem. Soc.
2498:10.1063/1.1696078
2402:978-0-08-037941-8
2096:kinetically inert
2049:hydrogen peroxide
1735:hydrochloric acid
1178:hydrochloric acid
859:Chemical compound
857:
856:
794:Cobalt(II) iodide
760:Safety data sheet
592:Crystal structure
350:CompTox Dashboard
182:Interactive image
174:Interactive image
143: (dihydrate)
110:Muriate of cobalt
108:Cobalt dichloride
58:
57:
5308:
5213:
3607:
3588:
3581:
3574:
3565:
3506:
3346:
3345:
3344:
3336:
3335:
3327:
3326:
3313:
3312:
3311:
3303:
3302:
3294:
3293:
3023:Cobalt compounds
3016:
3009:
3002:
2993:
2965:
2964:
2952:
2946:
2945:
2935:
2903:
2897:
2896:
2894:
2892:
2878:
2872:
2868:
2862:
2857:
2851:
2845:
2839:
2838:
2810:
2804:
2803:
2759:
2753:
2752:
2720:
2714:
2713:
2692:
2686:
2685:
2667:
2661:
2660:
2651:(7): 2512–2514.
2639:
2633:
2627:
2621:
2620:
2592:
2586:
2585:
2576:(6): 1394–1401.
2557:
2551:
2550:
2524:
2518:
2508:
2502:
2501:
2473:
2467:
2466:
2455:
2445:
2439:
2433:
2427:
2413:
2407:
2406:
2391:(2nd ed.).
2381:
2372:
2358:
2356:
2355:
2345:
2344:
2343:
2332:
2317:
2312:
2306:
2305:
2285:
2274:
2273:
2271:
2269:
2260:. Archived from
2250:
2187:(i.e., possibly
2129:
2128:
2127:
2102:was awarded the
2070:
2069:
2068:
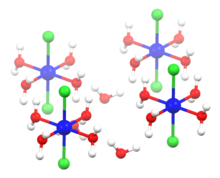
2060:
2059:
2035:
2033:
2032:
2022:
2021:
2020:
2010:
2009:
2008:
1998:
1997:
1996:
1986:
1985:
1984:
1974:
1972:
1971:
1961:
1960:
1959:
1918:
1917:
1916:
1906:
1905:
1904:
1891:
1890:
1889:
1881:
1880:
1872:
1871:
1858:
1857:
1856:
1848:
1847:
1834:
1833:
1832:
1811:
1810:
1809:
1797:
1796:
1795:
1787:
1786:
1778:
1777:
1725:
1724:
1723:
1703:
1701:
1700:
1690:
1689:
1688:
1681:
1680:
1670:
1669:
1668:
1660:
1659:
1651:
1650:
1640:
1638:
1637:
1627:
1626:
1625:
1612:
1611:
1610:
1602:
1601:
1593:
1592:
1575:
1573:
1572:
1562:
1561:
1560:
1552:
1551:
1543:
1542:
1534:
1533:
1523:
1521:
1520:
1512:
1511:
1501:
1499:
1498:
1488:
1487:
1486:
1473:
1471:
1470:
1462:
1461:
1423:
1421:
1420:
1410:hydrogen sulfide
1407:
1400:
1399:
1398:
1377:
1375:
1374:
1365:
1364:
1363:
1353:
1351:
1350:
1342:
1341:
1331:
1329:
1328:
1318:
1317:
1316:
1285:
1283:
1282:
1269:
1268:
1267:
1253:
1252:
1251:
1238:
1236:
1235:
1225:
1224:
1223:
1210:
1209:
1208:
1194:
1193:
1192:
1116:
1115:
1114:
1106:
1105:
1097:
1096:
1082:
1080:
1079:
1069:
1068:
1067:
1025:
1024:
1023:
1015:
1014:
1013:
996:
995:
994:
956:
952:
951:
950:
939:cadmium chloride
919:
917:
916:
903:
902:
901:
888:
887:
886:
841:
835:
832:
831:
713:
706:
699:
684:
664:
659:
654:
449:Chemical formula
374:
373:
358:
356:
329:
322:
286:
275:
254:
246:
235:
224:
204:
184:
176:
171:anhydrous:
150:
142:
135:
80:
67:
30:
26:
5316:
5315:
5311:
5310:
5309:
5307:
5306:
5305:
5266:
5265:
5264:
5259:
5258:
5252:
5244:
5236:
5228:
5223:
5221:
5212:
5208:
5206:
5204:
5196:
5188:
5180:
5175:
5173:
5165:
5157:
5149:
5144:
5142:
5137:
5135:
5130:
5128:
5120:
5115:
5113:
5105:
5100:
5098:
5090:
5077:
5072:
5070:
5062:
5057:
5055:
5047:
5039:
5031:
5026:
5024:
5016:
5008:
5000:
4995:
4993:
4985:
4980:
4978:
4970:
4962:
4957:
4955:
4947:
4939:
4931:
4877:
4869:
4865:
4857:
4849:
4841:
4830:
4807:
4802:
4800:
4792:
4784:
4779:
4777:
4769:
4764:
4757:
4752:
4750:
4746:
4738:
4733:
4731:
4726:
4719:
4714:
4712:
4704:
4699:
4697:
4692:
4690:
4682:
4677:
4675:
4670:
4668:
4663:
4661:
4653:
4648:
4646:
4641:
4639:
4634:
4632:
4624:
4619:
4617:
4612:
4610:
4605:
4603:
4598:
4596:
4588:
4583:
4581:
4576:
4574:
4566:
4558:
4547:
4532:
4527:
4525:
4520:
4513:
4508:
4501:
4496:
4494:
4489:
4487:
4483:
4475:
4470:
4468:
4460:
4455:
4453:
4445:
4440:
4438:
4433:
4426:
4413:
4405:
4397:
4392:
4390:
4385:
4383:
4375:
4370:
4368:
4360:
4355:
4353:
4348:
4346:
4341:
4339:
4334:
4332:
4324:
4319:
4317:
4312:
4310:
4302:
4297:
4295:
4290:
4288:
4280:
4270:
4247:
4242:
4240:
4235:
4233:
4229:
4219:
4217:
4212:
4210:
4202:
4197:
4195:
4187:
4182:
4175:
4167:
4162:
4155:
4147:
4142:
4140:
4132:
4127:
4125:
4117:
4112:
4110:
4102:
4097:
4095:
4090:
4088:
4080:
4075:
4073:
4068:
4066:
4061:
4059:
4051:
4046:
4044:
4039:
4037:
4029:
4019:
4014:
3997:
3989:
3984:
3982:
3977:
3975:
3970:
3968:
3964:
3954:
3952:
3947:
3945:
3940:
3938:
3934:
3926:
3921:
3919:
3915:
3910:
3908:
3904:
3896:
3891:
3884:
3866:
3861:
3859:
3854:
3845:
3843:
3838:
3836:
3832:
3827:
3825:
3821:
3816:
3814:
3810:
3805:
3803:
3798:
3794:
3792:
3788:
3783:
3780:
3775:
3773:
3769:
3761:
3756:
3752:
3750:
3745:
3743:
3735:
3730:
3726:
3724:
3719:
3717:
3713:
3708:
3706:
3702:
3697:
3695:
3691:
3683:
3678:
3676:
3672:
3667:
3665:
3660:
3658:
3654:
3649:
3647:
3643:
3635:
3599:
3592:
3562:
3557:
3553:
3549:
3534:
3530:
3526:
3518:
3514:
3505:
3501:
3489:
3485:
3481:
3466:
3462:
3454:
3446:
3438:
3434:
3426:
3422:
3414:
3394:
3390:
3386:
3376:Cobalt(II, III)
3371:
3350:
3343:
3340:
3339:
3338:
3334:
3331:
3330:
3329:
3325:
3322:
3321:
3320:
3318:
3310:
3307:
3306:
3305:
3301:
3298:
3297:
3296:
3292:
3289:
3288:
3287:
3285:
3280:
3276:
3272:
3268:
3260:
3252:
3248:
3235:
3227:
3218:
3204:
3191:
3183:
3175:
3171:
3167:
3159:
3155:
3147:
3139:
3135:
3127:
3119:
3115:
3107:
3103:
3095:
3087:
3083:
3075:
3067:
3059:
3044:
3040:
3025:
3020:
2973:
2968:
2954:
2953:
2949:
2918:(11): 872–873.
2912:Br J Sports Med
2905:
2904:
2900:
2890:
2888:
2880:
2879:
2875:
2869:
2865:
2858:
2854:
2846:
2842:
2812:
2811:
2807:
2761:
2760:
2756:
2722:
2721:
2717:
2694:
2693:
2689:
2669:
2668:
2664:
2641:
2640:
2636:
2628:
2624:
2617:
2594:
2593:
2589:
2559:
2558:
2554:
2547:
2526:
2525:
2521:
2509:
2505:
2475:
2474:
2470:
2464:
2447:
2446:
2442:
2436:The Merck Index
2434:
2430:
2414:
2410:
2403:
2383:
2382:
2375:
2354:
2351:
2350:
2349:
2347:
2342:
2339:
2338:
2337:
2335:
2333:
2320:
2313:
2309:
2287:
2286:
2277:
2267:
2265:
2252:
2251:
2247:
2243:
2216:
2165:
2140:
2126:
2123:
2122:
2121:
2119:
2116:
2077:acetylacetonate
2067:
2064:
2063:
2062:
2058:
2055:
2054:
2053:
2051:
2031:
2028:
2027:
2026:
2024:
2019:
2016:
2015:
2014:
2012:
2007:
2004:
2003:
2002:
2000:
1995:
1992:
1991:
1990:
1988:
1983:
1980:
1979:
1978:
1976:
1970:
1967:
1966:
1965:
1963:
1958:
1955:
1954:
1953:
1951:
1938:by atmospheric
1915:
1912:
1911:
1910:
1908:
1903:
1900:
1899:
1898:
1896:
1888:
1885:
1884:
1883:
1879:
1876:
1875:
1874:
1870:
1867:
1866:
1865:
1863:
1855:
1852:
1851:
1850:
1846:
1843:
1842:
1841:
1839:
1831:
1828:
1827:
1826:
1824:
1818:
1808:
1806:
1805:
1804:
1803:
1794:
1791:
1790:
1789:
1785:
1782:
1781:
1780:
1776:
1773:
1772:
1771:
1769:
1743:
1729:
1722:
1719:
1718:
1717:
1715:
1710:
1699:
1696:
1695:
1694:
1692:
1687:
1684:
1683:
1682:
1679:
1676:
1675:
1674:
1672:
1667:
1664:
1663:
1662:
1658:
1655:
1654:
1653:
1649:
1646:
1645:
1644:
1642:
1636:
1633:
1632:
1631:
1629:
1624:
1621:
1620:
1619:
1617:
1609:
1606:
1605:
1604:
1600:
1597:
1596:
1595:
1591:
1588:
1587:
1586:
1584:
1571:
1568:
1567:
1566:
1564:
1559:
1556:
1555:
1554:
1550:
1547:
1546:
1545:
1541:
1538:
1537:
1536:
1532:
1529:
1528:
1527:
1525:
1519:
1516:
1515:
1514:
1510:
1507:
1506:
1505:
1503:
1497:
1494:
1493:
1492:
1490:
1485:
1482:
1481:
1480:
1478:
1469:
1466:
1465:
1464:
1460:
1457:
1456:
1455:
1453:
1430:
1419:
1416:
1415:
1414:
1412:
1405:
1397:
1395:
1394:
1393:
1392:
1388:
1373:
1370:
1369:
1368:
1367:
1362:
1359:
1358:
1357:
1355:
1349:
1346:
1345:
1344:
1340:
1337:
1336:
1335:
1333:
1327:
1324:
1323:
1322:
1320:
1315:
1312:
1311:
1310:
1308:
1281:
1278:
1277:
1276:
1274:
1272:
1266:
1263:
1262:
1261:
1259:
1257:
1250:
1247:
1246:
1245:
1243:
1234:
1231:
1230:
1229:
1227:
1222:
1219:
1218:
1217:
1215:
1213:
1207:
1204:
1203:
1202:
1200:
1198:
1191:
1188:
1187:
1186:
1184:
1166:
1147:
1143:
1139:
1113:
1110:
1109:
1108:
1104:
1101:
1100:
1099:
1095:
1092:
1091:
1090:
1088:
1078:
1075:
1074:
1073:
1071:
1066:
1063:
1062:
1061:
1059:
1053:
1049:
1045:
1042:Subunit of CoCl
1036:
1022:
1020:
1019:
1018:
1017:
1012:
1009:
1008:
1007:
1005:
993:
990:
989:
988:
986:
971:
954:
949:
946:
945:
944:
942:
935:
930:
915:
912:
911:
910:
908:
900:
897:
896:
895:
893:
885:
882:
881:
880:
878:
860:
853:
848:
847:
846: ?)
837:
833:
829:
825:
811:
805:
792:
788:
782:
751:
745:
718:
717:
716:
715:
708:
701:
694:
690:
682:
647:
618:
616:
612:
601:
594:
577:
555:
553:
541:
539:
537:
533:
513:
511:
509:
497:
495:
483:
481:
471:
469:
457:
451:
437:
434:
429:
424:
423:
412:
409:
408:
405:
399:
398:
395:
389:
388:
377:
359:
352:
333:
305:
289:
276:
264:
227:
207:
187:
165:
154:
125:
111:
109:
107:
99:
84:
81:
71:
68:
54:
42:
21:
12:
11:
5:
5314:
5312:
5304:
5303:
5298:
5293:
5288:
5283:
5278:
5268:
5267:
5261:
5260:
5255:
5254:
5250:
5246:
5242:
5238:
5234:
5230:
5226:
5219:
5215:
5202:
5198:
5194:
5190:
5186:
5182:
5178:
5171:
5167:
5163:
5159:
5155:
5151:
5147:
5140:
5133:
5126:
5122:
5118:
5111:
5107:
5103:
5096:
5092:
5088:
5084:
5080:
5079:
5075:
5068:
5064:
5060:
5053:
5049:
5045:
5041:
5037:
5033:
5029:
5022:
5018:
5014:
5010:
5006:
5002:
4998:
4991:
4987:
4983:
4976:
4972:
4968:
4964:
4960:
4953:
4949:
4945:
4941:
4937:
4933:
4929:
4925:
4921:
4920:
4917:
4914:
4913:
4910:
4907:
4904:
4901:
4898:
4895:
4892:
4889:
4886:
4883:
4880:
4875:
4871:
4867:
4863:
4859:
4855:
4851:
4847:
4843:
4839:
4835:
4832:
4828:
4824:
4818:
4817:
4814:
4809:
4805:
4798:
4794:
4790:
4786:
4782:
4775:
4771:
4767:
4759:
4755:
4748:
4744:
4740:
4736:
4729:
4721:
4717:
4710:
4706:
4702:
4695:
4688:
4684:
4680:
4673:
4666:
4659:
4655:
4651:
4644:
4637:
4630:
4626:
4622:
4615:
4608:
4601:
4594:
4590:
4586:
4579:
4572:
4568:
4564:
4560:
4556:
4552:
4549:
4545:
4541:
4535:
4534:
4530:
4523:
4515:
4511:
4503:
4499:
4492:
4485:
4481:
4477:
4473:
4466:
4462:
4458:
4451:
4447:
4443:
4436:
4428:
4424:
4420:
4415:
4411:
4407:
4403:
4399:
4395:
4388:
4381:
4377:
4373:
4366:
4362:
4358:
4351:
4344:
4337:
4330:
4326:
4322:
4315:
4308:
4304:
4300:
4293:
4286:
4282:
4278:
4274:
4272:
4268:
4264:
4258:
4257:
4254:
4249:
4245:
4238:
4231:
4227:
4223:
4215:
4208:
4204:
4200:
4193:
4189:
4185:
4177:
4173:
4169:
4165:
4157:
4153:
4149:
4145:
4138:
4134:
4130:
4123:
4119:
4115:
4108:
4104:
4100:
4093:
4086:
4082:
4078:
4071:
4064:
4057:
4053:
4049:
4042:
4035:
4031:
4027:
4023:
4021:
4017:
4009:
4003:
4002:
3999:
3995:
3991:
3987:
3980:
3973:
3966:
3962:
3958:
3950:
3943:
3936:
3932:
3928:
3924:
3917:
3913:
3906:
3902:
3898:
3894:
3886:
3882:
3878:
3872:
3871:
3868:
3864:
3857:
3849:
3841:
3834:
3830:
3823:
3819:
3812:
3808:
3801:
3790:
3786:
3778:
3771:
3767:
3763:
3759:
3748:
3741:
3737:
3733:
3722:
3715:
3711:
3704:
3700:
3693:
3689:
3685:
3681:
3674:
3670:
3663:
3656:
3652:
3645:
3641:
3637:
3633:
3629:
3623:
3622:
3619:
3617:
3615:
3613:
3605:
3604:
3601:
3600:
3593:
3591:
3590:
3583:
3576:
3568:
3559:
3558:
3556:
3555:
3551:
3547:
3542:
3540:
3536:
3535:
3533:
3532:
3528:
3524:
3520:
3516:
3512:
3508:
3497:
3495:
3491:
3490:
3488:
3487:
3483:
3479:
3474:
3472:
3471:Cobalt(III,IV)
3468:
3467:
3465:
3464:
3460:
3456:
3452:
3448:
3444:
3440:
3436:
3432:
3428:
3424:
3420:
3416:
3412:
3408:
3402:
3400:
3396:
3395:
3393:
3392:
3388:
3384:
3379:
3377:
3373:
3372:
3370:
3369:
3364:
3358:
3356:
3355:Cobalt(0, III)
3352:
3351:
3349:
3348:
3341:
3332:
3323:
3315:
3308:
3299:
3290:
3282:
3278:
3274:
3270:
3266:
3262:
3258:
3254:
3250:
3246:
3242:
3237:
3233:
3229:
3225:
3221:
3216:
3211:
3206:
3202:
3198:
3193:
3189:
3185:
3181:
3177:
3173:
3169:
3165:
3161:
3157:
3153:
3149:
3145:
3141:
3137:
3133:
3129:
3125:
3121:
3117:
3113:
3109:
3105:
3101:
3097:
3093:
3089:
3085:
3081:
3077:
3073:
3069:
3065:
3061:
3057:
3052:
3050:
3046:
3045:
3043:
3042:
3038:
3033:
3031:
3027:
3026:
3021:
3019:
3018:
3011:
3004:
2996:
2990:
2989:
2984:
2979:
2972:
2971:External links
2969:
2967:
2966:
2947:
2898:
2873:
2863:
2852:
2840:
2821:(2): 201–216.
2805:
2770:(1): 566–576.
2754:
2715:
2687:
2662:
2634:
2622:
2615:
2587:
2552:
2545:
2519:
2503:
2484:(3): 898–901.
2468:
2462:
2440:
2428:
2408:
2401:
2373:
2352:
2340:
2318:
2307:
2296:(2): 525–530.
2275:
2264:on 28 May 2019
2244:
2242:
2239:
2238:
2237:
2234:
2231:erythropoiesis
2227:
2215:
2212:
2174:cardiomyopathy
2164:
2161:
2139:
2136:
2124:
2115:
2112:
2086:Unlike Co(II)
2065:
2056:
2037:
2036:
2029:
2017:
2005:
1993:
1981:
1968:
1956:
1913:
1901:
1886:
1877:
1868:
1853:
1844:
1829:
1817:
1814:
1807:
1792:
1783:
1774:
1742:
1739:
1731:
1730:
1727:
1720:
1708:
1705:
1704:
1697:
1685:
1677:
1665:
1656:
1647:
1634:
1622:
1607:
1598:
1589:
1577:
1576:
1569:
1557:
1548:
1539:
1530:
1517:
1508:
1495:
1483:
1467:
1458:
1448:. It forms an
1429:
1426:
1417:
1403:cobalt sulfide
1396:
1387:
1384:
1380:
1379:
1371:
1360:
1347:
1338:
1325:
1313:
1287:
1286:
1279:
1270:
1264:
1255:
1248:
1240:
1239:
1232:
1220:
1211:
1205:
1196:
1189:
1165:
1162:
1145:
1141:
1137:
1111:
1102:
1093:
1076:
1064:
1051:
1047:
1043:
1035:
1032:
1021:
1010:
991:
970:
967:
947:
934:
931:
929:
926:
913:
898:
883:
858:
855:
854:
849:
827:
826:
822:standard state
819:
816:
815:
806:
800:
797:
796:
783:
777:
774:
773:
769:
768:
763:
756:
755:
752:
743:
741:
738:
737:
730:
729:
728:Non-flammable
726:
720:
719:
709:
702:
695:
680:
679:
678:
677:
675:
666:
665:
648:
643:
640:
639:
629:
628:
624:
623:
622:(hexahydrate)
613:
608:
605:
604:
599:
595:
590:
587:
586:
582:
581:
578:
572:
569:
568:
550:
544:
543:
534:
529:
526:
525:
522:
516:
515:
506:
500:
499:
492:
486:
485:
478:
474:
473:
466:
460:
459:
455:
452:
447:
444:
443:
439:
438:
436:
435:
432:
430:
427:
419:
418:
417:
414:
413:
411:
410:
406:
403:
402:
400:
396:
393:
392:
384:
383:
382:
379:
378:
376:
375:
362:
360:
348:
345:
344:
341:
335:
334:
332:
331:
323:
315:
313:
307:
306:
304:
303:
299:
297:
291:
290:
288:
287:
279:
277:
269:
266:
265:
263:
262:
258:
256:
248:
247:
237:
229:
228:
226:
225:
217:
215:
209:
208:
206:
205:
197:
195:
189:
188:
186:
185:
177:
168:
166:
159:
156:
155:
153:
152:
144:
136:
128:
126:
121:
118:
117:
113:
112:
105:
101:
100:
97:
91:
90:
86:
85:
82:
73:
72:
69:
60:
59:
56:
55:
52:
43:
40:
13:
10:
9:
6:
4:
3:
2:
5313:
5302:
5299:
5297:
5294:
5292:
5291:Metal halides
5289:
5287:
5284:
5282:
5279:
5277:
5274:
5273:
5271:
5253:
5247:
5245:
5239:
5237:
5231:
5229:
5222:
5216:
5214:
5205:
5199:
5197:
5191:
5189:
5183:
5181:
5174:
5168:
5166:
5160:
5158:
5152:
5150:
5143:
5136:
5129:
5123:
5121:
5114:
5108:
5106:
5099:
5093:
5091:
5085:
5082:
5081:
5078:
5071:
5065:
5063:
5056:
5050:
5048:
5042:
5040:
5034:
5032:
5025:
5019:
5017:
5011:
5009:
5003:
5001:
4994:
4988:
4986:
4979:
4973:
4971:
4965:
4963:
4956:
4950:
4948:
4942:
4940:
4934:
4932:
4926:
4923:
4922:
4915:
4911:
4908:
4905:
4902:
4899:
4896:
4893:
4890:
4887:
4884:
4881:
4879:
4872:
4870:
4860:
4858:
4852:
4850:
4844:
4842:
4836:
4833:
4831:
4825:
4823:
4820:
4819:
4815:
4813:
4810:
4808:
4801:
4795:
4793:
4787:
4785:
4778:
4772:
4770:
4763:
4760:
4758:
4751:
4741:
4739:
4732:
4725:
4722:
4720:
4713:
4707:
4705:
4698:
4691:
4685:
4683:
4676:
4669:
4662:
4656:
4654:
4647:
4640:
4633:
4627:
4625:
4618:
4611:
4604:
4597:
4591:
4589:
4582:
4575:
4569:
4567:
4561:
4559:
4553:
4550:
4548:
4542:
4540:
4537:
4536:
4533:
4526:
4519:
4516:
4514:
4507:
4504:
4502:
4495:
4488:
4478:
4476:
4469:
4463:
4461:
4454:
4448:
4446:
4439:
4432:
4429:
4427:
4421:
4419:
4416:
4414:
4408:
4406:
4400:
4398:
4391:
4384:
4378:
4376:
4369:
4363:
4361:
4354:
4347:
4340:
4333:
4327:
4325:
4318:
4311:
4305:
4303:
4296:
4289:
4283:
4281:
4275:
4273:
4271:
4265:
4263:
4260:
4259:
4255:
4253:
4250:
4248:
4241:
4234:
4224:
4222:
4218:
4211:
4205:
4203:
4196:
4190:
4188:
4181:
4178:
4176:
4170:
4168:
4161:
4158:
4156:
4150:
4148:
4141:
4135:
4133:
4126:
4120:
4118:
4111:
4105:
4103:
4096:
4089:
4083:
4081:
4074:
4067:
4060:
4054:
4052:
4045:
4038:
4032:
4030:
4024:
4022:
4020:
4013:
4010:
4008:
4005:
4004:
4000:
3998:
3992:
3990:
3983:
3976:
3969:
3959:
3957:
3953:
3946:
3939:
3929:
3927:
3920:
3909:
3899:
3897:
3890:
3887:
3885:
3879:
3877:
3874:
3873:
3869:
3867:
3860:
3853:
3850:
3848:
3844:
3837:
3826:
3815:
3804:
3797:
3793:
3782:
3774:
3764:
3762:
3755:
3751:
3744:
3738:
3736:
3729:
3725:
3718:
3707:
3696:
3686:
3684:
3677:
3666:
3659:
3648:
3638:
3636:
3630:
3628:
3625:
3624:
3620:
3614:
3612:
3609:
3608:
3602:
3597:
3589:
3584:
3582:
3577:
3575:
3570:
3569:
3566:
3554:
3544:
3543:
3541:
3537:
3531:
3521:
3519:
3509:
3507:
3499:
3498:
3496:
3492:
3486:
3476:
3475:
3473:
3469:
3463:
3457:
3455:
3449:
3447:
3441:
3439:
3429:
3427:
3417:
3415:
3409:
3407:
3404:
3403:
3401:
3397:
3391:
3381:
3380:
3378:
3374:
3368:
3365:
3363:
3360:
3359:
3357:
3353:
3347:
3316:
3314:
3283:
3281:
3263:
3261:
3255:
3253:
3243:
3241:
3238:
3236:
3230:
3228:
3222:
3220:
3219:
3212:
3210:
3207:
3205:
3199:
3197:
3194:
3192:
3186:
3184:
3178:
3176:
3162:
3160:
3150:
3148:
3142:
3140:
3130:
3128:
3122:
3120:
3110:
3108:
3098:
3096:
3090:
3088:
3078:
3076:
3070:
3068:
3062:
3060:
3054:
3053:
3051:
3047:
3041:
3035:
3034:
3032:
3028:
3024:
3017:
3012:
3010:
3005:
3003:
2998:
2997:
2994:
2988:
2985:
2983:
2980:
2978:
2975:
2974:
2970:
2962:
2958:
2951:
2948:
2943:
2939:
2934:
2929:
2925:
2921:
2917:
2913:
2909:
2902:
2899:
2887:
2883:
2877:
2874:
2867:
2864:
2861:
2856:
2853:
2850:
2844:
2841:
2836:
2832:
2828:
2824:
2820:
2816:
2809:
2806:
2801:
2797:
2793:
2789:
2785:
2781:
2777:
2773:
2769:
2765:
2758:
2755:
2750:
2746:
2742:
2738:
2734:
2730:
2726:
2719:
2716:
2711:
2707:
2703:
2700:
2699:
2691:
2688:
2683:
2679:
2675:
2674:
2666:
2663:
2658:
2654:
2650:
2647:
2646:
2638:
2635:
2631:
2626:
2623:
2618:
2616:9780470132401
2612:
2608:
2604:
2600:
2599:
2591:
2588:
2583:
2579:
2575:
2571:
2567:
2563:
2556:
2553:
2548:
2546:9780470132609
2542:
2538:
2534:
2530:
2523:
2520:
2517:
2513:
2507:
2504:
2499:
2495:
2491:
2487:
2483:
2479:
2472:
2469:
2465:
2463:0-19-855370-6
2459:
2454:
2453:
2444:
2441:
2437:
2432:
2429:
2426:
2422:
2418:
2412:
2409:
2404:
2398:
2394:
2390:
2386:
2380:
2378:
2374:
2370:
2366:
2362:
2331:
2329:
2327:
2325:
2323:
2319:
2316:
2311:
2308:
2303:
2299:
2295:
2291:
2284:
2282:
2280:
2276:
2263:
2259:
2255:
2249:
2246:
2240:
2235:
2232:
2228:
2225:
2221:
2220:Invisible ink
2218:
2217:
2213:
2211:
2209:
2205:
2200:
2198:
2195:) as per the
2194:
2193:IARC Group 2B
2190:
2186:
2181:
2179:
2175:
2171:
2163:Health issues
2162:
2160:
2158:
2154:
2144:
2137:
2135:
2133:
2113:
2111:
2109:
2105:
2101:
2100:Alfred Werner
2097:
2093:
2089:
2084:
2082:
2078:
2074:
2050:
2046:
2042:
1949:
1948:
1947:
1945:
1941:
1937:
1933:
1929:
1924:
1922:
1895:
1862:
1838:
1823:
1815:
1813:
1801:
1768:
1764:
1756:
1752:
1747:
1740:
1738:
1736:
1714:
1713:
1712:
1616:
1615:
1614:
1582:
1477:
1476:
1475:
1451:
1447:
1443:
1439:
1435:
1427:
1425:
1411:
1404:
1385:
1383:
1307:
1306:
1305:
1303:
1298:
1294:
1291:
1242:
1241:
1183:
1182:
1181:
1179:
1175:
1171:
1163:
1161:
1159:
1155:
1151:
1135:
1131:
1126:
1124:
1120:
1086:
1040:
1033:
1031:
1029:
1004:solutions of
1003:
998:
984:
980:
976:
968:
966:
964:
960:
940:
932:
927:
925:
923:
907:
892:
876:
872:
868:
864:
852:
845:
840:
823:
817:
814:
810:
807:
804:
799:
798:
795:
791:
787:
784:
781:
776:
775:
770:
767:
764:
761:
758:
757:
753:
749:
740:
739:
735:
731:
727:
725:
722:
721:
714:
707:
700:
676:
673:
672:
668:
667:
663:
658:
653:
649:
646:
642:
641:
637:
635:
630:
625:
621:
614:
611:
607:
606:
603:
596:
593:
589:
588:
583:
579:
575:
571:
570:
567:
563:
559:
551:
549:
546:
545:
535:
532:
528:
527:
523:
521:
520:Boiling point
518:
517:
507:
505:
504:Melting point
502:
501:
493:
491:
488:
487:
479:
476:
475:
467:
465:
462:
461:
453:
450:
446:
445:
440:
431:
426:
422:
415:
401:
391:
387:
380:
372:
368:
367:DTXSID9040180
364:
363:
361:
351:
347:
346:
342:
340:
337:
336:
328:
324:
321:
317:
316:
314:
312:
309:
308:
301:
300:
298:
296:
293:
292:
285:
281:
280:
278:
272:
268:
267:
260:
259:
257:
255:
250:
249:
245:
241:
238:
236:
234:ECHA InfoCard
231:
230:
223:
219:
218:
216:
214:
211:
210:
203:
199:
198:
196:
194:
191:
190:
183:
178:
175:
170:
169:
167:
163:
158:
157:
149:
145:
141:
137:
134:
130:
129:
127:
124:
120:
119:
114:
102:
96:
92:
87:
79:
74:
66:
61:
48:
44:
36:
32:
31:
27:
19:
4136:
3213:
3091:
2960:
2950:
2915:
2911:
2901:
2889:. Retrieved
2876:
2866:
2855:
2843:
2818:
2814:
2808:
2767:
2763:
2757:
2735:(1): 75–85.
2732:
2728:
2718:
2701:
2696:
2690:
2671:
2665:
2648:
2643:
2637:
2629:
2625:
2596:
2590:
2573:
2569:
2565:
2561:
2555:
2528:
2522:
2506:
2481:
2477:
2471:
2451:
2443:
2435:
2431:
2416:
2411:
2388:
2360:
2310:
2293:
2289:
2266:. Retrieved
2262:the original
2257:
2248:
2201:
2189:carcinogenic
2182:
2177:
2166:
2149:
2117:
2095:
2085:
2038:
1925:
1819:
1760:
1732:
1706:
1578:
1431:
1389:
1381:
1299:
1295:
1292:
1288:
1167:
1158:aquo ligands
1134:deliquescent
1127:
1084:
1057:
999:
972:
959:coordination
936:
921:
905:
869:, a salt of
862:
861:
733:
670:
633:
295:RTECS number
116:Identifiers
104:Other names
3399:Cobalt(III)
2208:patch tests
2104:Nobel prize
1892:; however,
1800:18-electron
1767:cobaltocene
1446:tetrahedral
1434:Lewis acids
1164:Preparation
1130:hygroscopic
941:structure (
748:median dose
734:Lethal dose
724:Flash point
556:soluble in
477:Appearance
442:Properties
240:100.028.718
202:CHEBI:35696
53:Hexahydrate
5270:Categories
3494:Cobalt(IV)
3049:Cobalt(II)
2849:drinkers).
2241:References
2214:Other uses
2157:silica gel
2153:Desiccants
1442:octahedral
1026:, besides
928:Properties
645:Pictograms
620:Octahedral
585:Structure
548:Solubility
464:Molar mass
327:17AVG63ZBC
320:EVS87XF13W
213:ChemSpider
160:3D model (
140:16544-92-6
123:CAS Number
95:IUPAC name
5286:Chlorides
3539:Cobalt(V)
3030:Cobalt(I)
2749:0003-4746
2170:beer foam
2088:complexes
2073:carbonate
1755:norbornyl
1753:with the
1741:Reduction
1726:+ 2 Cl →
1386:Reactions
969:Solutions
933:Anhydrous
766:ICSC 0783
636:labelling
602:structure
339:UN number
302:GF9800000
261:231-589-4
253:EC Number
148:7791-13-1
133:7646-79-9
41:Anhydrous
3596:chloride
2942:16244201
2835:10382556
2268:19 April
2224:heat gun
2210:(8.4%).
2204:allergen
2155:such as
2045:charcoal
1987:Cl + 20
1936:oxidised
1921:chlorine
1378:+ 12 HCl
1054:lattice.
1034:Hydrates
1028:chloride
1000:Diluted
891:hydrates
875:chlorine
671:NFPA 704
627:Hazards
576:(χ)
566:glycerol
562:pyridine
18:Phosgene
4919:
3224:Co(SCN)
3215:Co(OCN)
3180:Co(OAc)
3037:HCo(CO)
2933:1725077
2891:7 April
2800:7422045
2792:5291148
2772:Bibcode
2486:Bibcode
2092:ligands
2081:oxalate
1932:ammonia
1861:sulfate
1837:nitrate
1438:adducts
1436:. The
1254:+ 2 HCl
1195:+ 2 HCl
1148:, is a
1123:alcohol
1002:aqueous
975:soluble
844:what is
842: (
803:cations
558:ethanol
490:Density
458:
284:3032536
271:PubChem
3451:Co(OH)
3201:Co(OH)
3132:Co(HCO
3112:Co(ClO
3100:Co(ClO
3064:Co(CN)
2940:
2930:
2833:
2798:
2790:
2747:
2613:
2543:
2460:
2399:
2185:cancer
2079:, and
2041:amines
1940:oxygen
1928:amines
1859:, and
1765:gives
1244:Co(OH)
920:, for
871:cobalt
865:is an
839:verify
836:
801:Other
780:anions
778:Other
762:(SDS)
421:SMILES
89:Names
3459:LiCoO
3419:Co(NO
3188:CoGeO
3152:Co(NO
2796:S2CID
2566:trans
2562:trans
2023:+ 26
1840:Co(NO
1757:anion
1579:With
1332:+ 12
1085:trans
981:of a
386:InChI
343:3288
222:22708
193:ChEBI
162:JSmol
5249:NoCl
5241:MdCl
5233:FmCl
5225:EsCl
5218:EsCl
5209:CfCl
5201:CfCl
5193:BkCl
5185:CmCl
5177:AmCl
5170:AmCl
5162:PuCl
5154:NpCl
5117:PaCl
5110:PaCl
5102:ThCl
5095:ThCl
5087:AcCl
5074:YbCl
5067:YbCl
5059:TmCl
5052:TmCl
5044:ErCl
5036:HoCl
5028:DyCl
5021:DyCl
5013:TbCl
5005:GdCl
4997:EuCl
4990:EuCl
4982:SmCl
4975:SmCl
4967:PmCl
4959:NdCl
4952:NdCl
4944:PrCl
4936:CeCl
4928:LaCl
4854:DbCl
4846:RfCl
4838:LrCl
4827:RaCl
4822:FrCl
4812:AtCl
4804:PoCl
4797:PoCl
4789:BiCl
4781:PbCl
4774:PbCl
4766:TlCl
4762:TlCl
4754:HgCl
4735:AuCl
4728:(Au)
4724:AuCl
4716:PtCl
4709:PtCl
4701:IrCl
4694:IrCl
4687:IrCl
4679:OsCl
4672:OsCl
4665:OsCl
4658:OsCl
4650:ReCl
4643:ReCl
4636:ReCl
4629:ReCl
4585:TaCl
4578:TaCl
4571:TaCl
4563:HfCl
4555:LuCl
4544:BaCl
4539:CsCl
4529:XeCl
4522:XeCl
4518:XeCl
4498:TeCl
4491:TeCl
4472:SbCl
4465:SbCl
4457:SnCl
4450:SnCl
4442:InCl
4435:InCl
4431:InCl
4423:CdCl
4418:AgCl
4410:PdCl
4402:RhCl
4394:RuCl
4387:RuCl
4380:RuCl
4372:TcCl
4365:TcCl
4357:MoCl
4350:MoCl
4343:MoCl
4336:MoCl
4329:MoCl
4321:NbCl
4314:NbCl
4307:NbCl
4299:ZrCl
4292:ZrCl
4285:ZrCl
4267:SrCl
4262:RbCl
4252:BrCl
4244:SeCl
4237:SeCl
4214:AsCl
4207:AsCl
4199:GeCl
4192:GeCl
4184:GaCl
4180:GaCl
4172:ZnCl
4164:CuCl
4160:CuCl
4152:NiCl
4144:CoCl
4137:CoCl
4129:FeCl
4122:FeCl
4114:MnCl
4107:MnCl
4099:CrCl
4092:CrCl
4085:CrCl
4048:TiCl
4041:TiCl
4034:TiCl
4026:ScCl
4016:CaCl
4012:CaCl
3923:SiCl
3893:AlCl
3889:AlCl
3881:MgCl
3876:NaCl
3632:BeCl
3627:LiCl
3411:CoCl
3406:CoAs
3367:CoGe
3362:CoSi
3265:Co(C
3240:CoSe
3232:CoSO
3092:CoCl
3072:CoCO
3056:CoBr
2938:PMID
2893:2023
2860:[PDF
2831:PMID
2788:PMID
2745:ISSN
2611:ISBN
2541:ISBN
2458:ISBN
2397:ISBN
2336:CoCl
2270:2018
2120:CoCl
2011:→ 4
1975:+ 4
1952:CoCl
1919:and
1909:CoCl
1897:CoCl
1770:Co(C
1716:CoCl
1691:+ 6
1673:CoCl
1641:+ 2
1618:CoCl
1563:+ 6
1526:CoCl
1502:+ 4
1479:CoCl
1356:CoCl
1352:SiCl
1309:CoCl
1273:+ 2
1271:(aq)
1260:CoCl
1256:(aq)
1212:(aq)
1201:CoCl
1197:(aq)
1185:CoCO
1176:and
1089:CoCl
1060:CoCl
1006:CoCl
987:CoCl
963:mmHg
953:) (R
943:CdCl
894:CoCl
879:CoCl
873:and
598:CdCl
454:CoCl
311:UNII
5146:UCl
5139:UCl
5132:UCl
5125:UCl
5083:**
4912:Og
4909:Ts
4906:Lv
4903:Mc
4900:Fl
4897:Nh
4894:Cn
4891:Rg
4888:Ds
4885:Mt
4882:Hs
4874:BhO
4862:SgO
4834:**
4816:Rn
4621:WCl
4614:WCl
4607:WCl
4600:WCl
4593:WCl
4510:ICl
4506:ICl
4277:YCl
4256:Kr
4221:+As
4077:VCl
4070:VCl
4063:VCl
4056:VCl
4007:KCl
4001:Ar
3986:+SO
3979:SCl
3972:SCl
3949:PCl
3942:PCl
3870:Ne
3863:ClF
3856:ClF
3852:ClF
3840:ClO
3800:ClO
3796:ClO
3758:+NO
3747:ClN
3740:NCl
3732:+CO
3721:CCl
3680:+BO
3662:BCl
3621:He
3611:HCl
3598:ion
3550:CoO
3523:CoC
3515:CoF
3502:CoF
3482:CoO
3443:CoF
3337:CoO
3304:CoO
3257:CoH
3209:CoS
3196:CoO
3168:(PO
3144:CoI
3124:CoF
3080:CoC
2928:PMC
2920:doi
2823:doi
2780:doi
2768:156
2737:doi
2706:doi
2702:111
2678:doi
2653:doi
2603:doi
2578:doi
2533:doi
2512:doi
2494:doi
2421:doi
2365:doi
2298:doi
2206:in
1942:to
1873:(SO
1825:CoF
1643:P(C
1585:P(C
1524:→
1474:):
1444:or
1406:CoS
1366:+ 6
1334:(CH
1226:+
1214:+
1172:or
985:of
634:GHS
355:EPA
274:CID
5272::
4924:*
4878:Cl
4866:Cl
4747:Cl
4743:Hg
4551:*
4484:Cl
4480:Te
4230:Cl
4226:Se
3994:Cl
3965:Cl
3956:+P
3935:Cl
3916:Cl
3912:Si
3907:12
3905:Cl
3901:Si
3847:+O
3829:Cl
3818:Cl
3807:Cl
3785:Cl
3777:Cl
3766:Cl
3754:+N
3728:+C
3714:Cl
3703:Cl
3692:Cl
3673:Cl
3657:12
3655:Cl
3653:12
3644:Cl
3546:Na
3529:44
3525:28
3511:Cs
3478:Na
3431:Co
3383:Co
3333:70
3324:36
3300:48
3291:24
3245:Co
3164:Co
2959:.
2936:.
2926:.
2916:39
2914:.
2910:.
2884:.
2829:.
2819:37
2817:.
2794:.
2786:.
2778:.
2766:.
2743:.
2733:32
2731:.
2727:.
2649:94
2609:.
2574:17
2572:.
2539:.
2492:.
2482:42
2480:.
2395:.
2376:^
2359:"
2321:^
2294:88
2292:.
2278:^
2256:.
2191:,
2180:.
2110:.
2075:,
2013:Cl
1999:+
1989:NH
1977:NH
1962:·6
1950:4
1946::
1923:.
1864:Co
1835:,
1812:.
1671:→
1628:·6
1553:N)
1535:(C
1489:·6
1424:.
1354:→
1319:•6
1304::
1258:→
1216:CO
1199:→
1180::
1160:.
1144:O)
1140:(H
1125:.
1107:O)
1098:(H
1070:•6
1050:O)
1046:(H
744:50
742:LD
638::
564:,
560:,
5251:2
5243:2
5235:2
5227:3
5220:2
5211:2
5203:3
5195:3
5187:3
5179:3
5172:2
5164:3
5156:3
5148:6
5141:5
5134:4
5127:3
5119:5
5112:4
5104:4
5097:3
5089:3
5076:3
5069:2
5061:3
5054:2
5046:3
5038:3
5030:3
5023:2
5015:3
5007:3
4999:3
4992:2
4984:3
4977:2
4969:3
4961:3
4954:2
4946:3
4938:3
4930:3
4876:3
4868:2
4864:2
4856:5
4848:4
4840:3
4829:2
4806:4
4799:2
4791:3
4783:4
4776:2
4768:3
4756:2
4749:2
4745:2
4737:3
4730:2
4718:4
4711:2
4703:4
4696:3
4689:2
4681:5
4674:4
4667:3
4660:2
4652:6
4645:5
4638:4
4631:3
4623:6
4616:5
4609:4
4602:3
4595:2
4587:5
4580:4
4573:3
4565:4
4557:3
4546:2
4531:4
4524:2
4512:3
4500:4
4493:2
4486:2
4482:3
4474:5
4467:3
4459:4
4452:2
4444:3
4437:2
4425:2
4412:2
4404:3
4396:4
4389:3
4382:2
4374:4
4367:3
4359:6
4352:5
4345:4
4338:3
4331:2
4323:5
4316:4
4309:3
4301:4
4294:3
4287:2
4279:3
4269:2
4246:4
4239:2
4232:2
4228:2
4216:5
4209:3
4201:4
4194:2
4186:3
4174:2
4166:2
4154:2
4146:3
4139:2
4131:3
4124:2
4116:3
4109:2
4101:4
4094:3
4087:2
4079:5
4072:4
4065:3
4058:2
4050:4
4043:3
4036:2
4028:3
4018:2
3996:2
3988:4
3981:4
3974:2
3967:2
3963:2
3961:S
3951:5
3944:3
3937:4
3933:2
3931:P
3925:4
3918:6
3914:2
3903:5
3895:3
3883:2
3865:5
3858:3
3842:4
3835:7
3833:O
3831:2
3824:6
3822:O
3820:2
3813:4
3811:O
3809:2
3802:2
3791:2
3789:O
3787:2
3781:O
3779:2
3772:y
3770:O
3768:x
3760:3
3749:3
3742:3
3734:3
3723:4
3716:6
3712:2
3710:C
3705:4
3701:2
3699:C
3694:2
3690:2
3688:C
3682:3
3675:4
3671:2
3669:B
3664:3
3651:B
3646:4
3642:4
3640:B
3634:2
3587:e
3580:t
3573:v
3552:4
3548:3
3527:H
3517:6
3513:2
3504:4
3484:2
3480:x
3461:2
3453:3
3445:3
3437:3
3435:O
3433:2
3425:3
3423:)
3421:3
3413:3
3389:4
3387:O
3385:3
3342:4
3328:H
3319:C
3309:4
3295:H
3286:C
3279:2
3277:)
3275:3
3273:O
3271:6
3269:H
3267:3
3259:2
3251:2
3249:P
3247:3
3234:4
3226:2
3217:2
3203:2
3190:3
3182:2
3174:2
3172:)
3170:4
3166:3
3158:2
3156:)
3154:3
3146:2
3138:2
3136:)
3134:2
3126:2
3118:2
3116:)
3114:4
3106:2
3104:)
3102:3
3094:2
3086:4
3084:O
3082:2
3074:3
3066:2
3058:2
3039:4
3015:e
3008:t
3001:v
2963:.
2944:.
2922::
2895:.
2837:.
2825::
2802:.
2782::
2774::
2751:.
2739::
2712:.
2708::
2684:.
2680::
2659:.
2655::
2619:.
2605::
2584:.
2580::
2549:.
2535::
2514::
2500:.
2496::
2488::
2423::
2405:.
2367::
2357:O
2353:2
2348:H
2346:-
2341:2
2304:.
2300::
2272:.
2125:2
2066:2
2061:O
2057:2
2052:H
2034:O
2030:2
2025:H
2018:3
2006:2
2001:O
1994:3
1982:4
1973:O
1969:2
1964:H
1957:2
1914:2
1902:3
1887:3
1882:)
1878:4
1869:2
1854:3
1849:)
1845:3
1830:3
1793:2
1788:)
1784:5
1779:H
1775:5
1728:2
1721:2
1709:4
1702:O
1698:2
1693:H
1686:2
1678:2
1666:3
1661:)
1657:5
1652:H
1648:6
1639:O
1635:2
1630:H
1623:2
1608:3
1603:)
1599:5
1594:H
1590:6
1583:(
1574:O
1570:2
1565:H
1558:4
1549:5
1544:H
1540:5
1531:2
1522:N
1518:5
1513:H
1509:5
1504:C
1500:O
1496:2
1491:H
1484:2
1472:N
1468:5
1463:H
1459:5
1454:C
1452:(
1422:S
1418:2
1413:H
1376:O
1372:2
1361:2
1348:3
1343:)
1339:3
1330:O
1326:2
1321:H
1314:2
1284:O
1280:2
1275:H
1265:2
1249:2
1237:O
1233:2
1228:H
1221:2
1206:2
1190:3
1146:2
1142:2
1138:2
1112:4
1103:2
1094:2
1087:-
1081:O
1077:2
1072:H
1065:2
1052:2
1048:2
1044:2
1011:2
992:2
955:3
948:2
922:n
918:O
914:2
909:H
906:n
904:·
899:2
884:2
834:N
750:)
746:(
712:0
705:0
698:3
600:2
456:2
357:)
353:(
164:)
20:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.