275:
separation. However, the use of this small packing media causes the high back pressure and is why it is termed high pressure liquid chromatography. The LPLC columns are typically packed with silica of around 50 micrometres, thus reducing back pressure and resolution, but it also removes the need for expensive high pressure pumps. Manufacturers are now starting to move into higher pressure flash chromatography systems and have termed these as medium pressure liquid chromatography (MPLC) systems which operate above 1 MPa (150 psi).
20:
102:
159:
238:
290:
1118:
259:
85:. Fractions can be collected automatically by means of fraction collectors. The productivity of chromatography can be increased by running several columns at a time. In this case multi stream collectors are used. The composition of the eluent flow can be monitored and each fraction is analyzed for dissolved compounds, e.g. by analytical chromatography,
271:(HPLC) systems such as a gradient pump, sample injection ports, a UV detector and a fraction collector to collect the eluent. Typically these automated systems can separate samples from a few milligrams up to an industrial many kilogram scale and offer a much cheaper and quicker solution to doing multiple injections on prep-HPLC systems.
335:
A simplified method of calculating chromatogram resolution is to use the plate model. The plate model assumes that the column can be divided into a certain number of sections, or plates and the mass balance can be calculated for each individual plate. This approach approximates a typical chromatogram
245:
The particle size of the stationary phase is generally finer in flash column chromatography than in gravity column chromatography. For example, one of the most widely used silica gel grades in the former technique is mesh 230 – 400 (40 – 63 μm), while the latter technique typically requires mesh
80:
The individual components are retained by the stationary phase differently and separate from each other while they are running at different speeds through the column with the eluent. At the end of the column they elute one at a time. During the entire chromatography process the eluent is collected in
249:
A spreadsheet that assists in the successful development of flash columns has been developed. The spreadsheet estimates the retention volume and band volume of analytes, the fraction numbers expected to contain each analyte, and the resolution between adjacent peaks. This information allows users to
313:
For example, if you were to separate two different proteins with different binding capacities to the column from a solution sample, a good type of detector would be a spectrophotometer using a wavelength of 280 nm. The higher the concentration of protein that passes through the eluted solution
440:
For an adsorption column, the column resin (the stationary phase) is composed of microbeads. Even smaller particles such as proteins, carbohydrates, metal ions, or other chemical compounds are conjugated onto the microbeads. Each binding particle that is attached to the microbead can be assumed to
274:
The resolution (or the ability to separate a mixture) on an LPLC system will always be lower compared to HPLC, as the packing material in an HPLC column can be much smaller, typically only 5 micrometre thus increasing stationary phase surface area, increasing surface interactions and giving better
184:
value of the compound of interest is roughly around 0.2 - 0.3 in order to minimize the time and the amount of eluent to run the chromatography. The eluent has also been chosen so that the different compounds can be separated effectively. The eluent is optimized in small scale pretests, often using
63:
A column is prepared by packing a solid adsorbent into a cylindrical glass or plastic tube. The size will depend on the amount of compound being isolated. The base of the tube contains a filter, either a cotton or glass wool plug, or glass frit to hold the solid phase in place. A solvent reservoir
42:
from a mixture. Chromatography is able to separate substances based on differential adsorption of compounds to the adsorbent; compounds move through the column at different rates, allowing them to be separated into fractions. The technique is widely applicable, as many different adsorbents (normal
321:
The ultimate goal of chromatography is to separate different components from a solution mixture. The resolution expresses the extent of separation between the components from the mixture. The higher the resolution of the chromatogram, the better the extent of separation of the samples the column
301:
Typically, column chromatography is set up with peristaltic pumps, flowing buffers and the solution sample through the top of the column. The solutions and buffers pass through the column where a fraction collector at the end of the column setup collects the eluted samples. Prior to the fraction
67:
Two methods are generally used to prepare a column: the dry method and the wet method. For the dry method, the column is first filled with dry stationary phase powder, followed by the addition of mobile phase, which is flushed through the column until it is completely wet, and from this point is
266:
Column chromatography is an extremely time-consuming stage in any lab and can quickly become the bottleneck for any process lab. Many manufacturers like
Biotage, Buchi, Interchim and Teledyne Isco have developed automated flash chromatography systems (typically referred to as LPLC, low pressure
216:
for each particular separation. A faster flow rate of the eluent minimizes the time required to run a column and thereby minimizes diffusion, resulting in a better separation. However, the maximum flow rate is limited because a finite time is required for the analyte to equilibrate between the
209:. A common solvent system is a mixture of hexane and ethyl acetate, with proportions adjusted until the target compound has a retention factor of 0.2 - 0.3. Contrary to common misconception, methanol alone can be used as an eluent for highly polar compounds, and does not dissolve silica gel.
149:
are usually finely ground powders or gels and/or are microporous for an increased surface, though in EBA a fluidized bed is used. There is an important ratio between the stationary phase weight and the dry weight of the analyte mixture that can be applied onto the column. For silica column
317:
Because the column chromatography has a constant flow of eluted solution passing through the detector at varying concentrations, the detector must plot the concentration of the eluted sample over a course of time. This plot of sample concentration versus time is called a chromatogram.
225:
flow. The flow rate of such a column can be increased by extending the fresh eluent filled column above the top of the stationary phase or decreased by the tap controls. Faster flow rates can be achieved by using a pump or by using compressed gas (e.g. air,
76:
with the stationary phase powder and then carefully poured into the column. The top of the silica should be flat, and the top of the silica can be protected by a layer of sand. Eluent is slowly passed through the column to advance the organic material.
47:
used in the process. The latter prevents cross-contamination and stationary phase degradation due to recycling. Column chromatography can be done using gravity to move the solvent, or using compressed gas to push the solvent through the column.
43:
phase, reversed phase, or otherwise) can be used with a wide range of solvents. The technique can be used on scales from micrograms up to kilograms. The main advantage of column chromatography is the relatively low cost and disposability of the
521:
The
Freundlich isotherm is used when the column can bind to many different samples in the solution that needs to be purified. Because the many different samples have different binding constants to the beads, there are many different
340:
curve. By doing this, the curve width is estimated as 4 times the standard deviation of the curve, 4σ. The retention time is the time from the start of signal detection to the time of the peak height of the
Gaussian curve.
325:
The separate curves in the diagram represent different sample elution concentration profiles over time based on their affinity to the column resin. To calculate resolution, the retention time and curve width are required.
267:
liquid chromatography, around 350–525 kPa or 50.8–76.1 psi) that minimize human involvement in the purification process. Automated systems will include components normally found on more expensive
460:, and are the concentrations of the target molecule and the binding molecule on the column resin, respectively. is the concentration of the complex of the target molecule bound to the column resin.
55:
can show how a mixture of compounds will behave when purified by column chromatography. The separation is first optimised using thin-layer chromatography before performing column chromatography.
189:(TLC) with the same stationary phase, using solvents of different polarity until a suitable solvent system is found. Common mobile phase solvents, in order of increasing polarity, include
1035:
466:
The linear isotherm occurs when the solute concentration needed to be purified is very small relative to the binding molecule. Thus, the equilibrium can be defined as:
658:
322:
gives. This data is a good way of determining the column's separation properties of that particular sample. The resolution can be calculated from the chromatogram.
860:
329:
Retention time is the time from the start of signal detection by the detector to the peak height of the elution concentration profile of each different sample.
478:
For industrial scale uses, the total binding molecules on the column resin beads must be factored in because unoccupied sites must be taken into account. The
1030:
463:
Using this as a basis, three different isotherms can be used to describe the binding dynamics of a column chromatography: linear, Langmuir, and
Freundlich.
344:
From the variables in the figure above, the resolution, plate number, and plate height of the column plate model can be calculated using the equations:
1025:
893:
984:
979:
964:
541:
268:
722:
86:
642:
535:
833:
150:
chromatography, this ratio lies within 20:1 to 100:1, depending on how close to each other the analyte components are being eluted.
969:
444:
Binding between the target molecule to be separated and the binding molecule on the column beads can be modeled using a simple
1147:
1009:
672:
Still, WC; Kahn, M; Mitra, A (1978). "Rapid chromatographic technique for preparative separations with moderate resolution".
93:. Colored compounds (or fluorescent compounds with the aid of a UV lamp) can be seen through the glass wall as moving bands.
332:
Curve width is the width of the concentration profile curve of the different samples in the chromatogram in units of time.
999:
994:
564:
134:
746:
886:
674:
146:
1102:
1095:
949:
770:
284:
130:
101:
939:
1088:
1004:
918:
683:
218:
90:
52:
1142:
1121:
934:
913:
879:
825:
142:
138:
129:
powder has often been used in the past. A wide range of stationary phases are available in order to perform
441:
bind in a 1:1 ratio with the solute sample sent through the column that needs to be purified or separated.
1061:
768:
Fair, JD; Kormos, CM (2008). "Flash column chromatograms estimated from thin-layer chromatography data".
250:
select optimal parameters for preparative-scale separations before the flash column itself is attempted.
1051:
954:
337:
19:
1071:
989:
573:
457:
445:
310:
so that the concentration of the separated samples in the sample solution mixture can be determined.
213:
1056:
483:
117:
in column chromatography is a solid. The most common stationary phase for column chromatography is
158:
1066:
974:
959:
652:
180:
or a mixture of solvents used to move the compounds through the column. It is chosen so that the
39:
23:
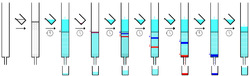
A chemist in the 1950s using column chromatography. The
Erlenmeyer receptacles are on the floor.
839:
829:
795:
787:
728:
718:
714:
638:
589:
479:
307:
303:
779:
687:
581:
181:
866:
633:
Furniss, Brian S.; Hannaford, Antony, J.; Smith, Peter W. G.; Tatchell, Austin S. (1989).
302:
collection, the samples that are eluted from the column pass through a detector such as a
237:
194:
122:
577:
902:
608:"How to set-up a flash chromatography silica column and actually succeed at separation"
186:
44:
35:
289:
1136:
707:
562:
Shusterman, AJ; McDougal, PG; Glasfeld, A (1997). "Dry-Column Flash
Chromatography".
198:
82:
526:
s. Therefore, the
Langmuir isotherm is not a good model for binding in this case.
783:
607:
732:
486:
are useful in describing this equilibrium. The
Langmuir isotherm is given by:
294:
118:
843:
791:
593:
126:
31:
799:
227:
206:
691:
258:
234:) to push the solvent through the column (flash column chromatography).
585:
222:
202:
177:
105:
Automated fraction collector and sampler for chromatography techniques
190:
172:
73:
69:
747:"Material Harvest Silica Gel for Normal Phase Column Chromatography"
314:
through the column, the higher the absorbance of that wavelength.
231:
871:
875:
538:(FPLC) – separation of proteins using column chromatography
709:
Experimental organic chemistry: Principles and
Practice
820:
Harrison RG, Todd PW, Rudge SR, Petrides DP (2003).
713:(Illustrated ed.). London: Blackwell. pp.
162:
Column chromatography proceeds by a series of steps.
1080:
1044:
1018:
927:
637:. Longman Scientific & Technical. p. 203.
706:
544:(HPLC) – column chromatography using high pressure
241:Photographic sequence of a column chromatography
68:never allowed to run dry. For the wet method, a
635:Vogel's Textbook of Practical Organic Chemistry
1036:Pyrolysis–gas chromatography–mass spectrometry
887:
8:
657:: CS1 maint: multiple names: authors list (
506:is the total binding molecules on the beads.
894:
880:
872:
279:Column chromatogram resolution calculation
64:may be attached at the top of the column.
16:Method to isolate a compound in a mixture
288:
257:
246:70 – 230 (63 – 200 μm) silica gel.
236:
157:
100:
18:
1031:Liquid chromatography–mass spectrometry
554:
262:An automated ion chromatography system.
217:stationary phase and mobile phase, see
980:Micellar electrokinetic chromatography
965:High-performance liquid chromatography
822:Bioseparations science and engineering
650:
542:High-performance liquid chromatography
269:high performance liquid chromatography
510:The Freundlich isotherm is given by:
432:where L is the length of the column.
221:. A simple laboratory column runs by
7:
1026:Gas chromatography–mass spectrometry
815:
813:
811:
809:
705:Harwood LM, Moody CJ (13 Jun 1989).
536:Fast protein liquid chromatography
408:= Gaussian curve width of solute A
401:= Gaussian curve width of solute B
14:
861:Flash Column Chromatography Guide
1117:
1116:
38:method used to isolate a single
970:Capillary electrochromatography
1010:Two-dimensional chromatography
824:(2nd ed.). New York, NY:
1:
1000:Size-exclusion chromatography
995:Reversed-phase chromatography
436:Column adsorption equilibrium
135:reversed-phase chromatography
121:, the next most common being
784:10.1016/j.chroma.2008.09.085
394:= retention time of solute A
387:= retention time of solute B
1103:Journal of Chromatography B
1096:Journal of Chromatography A
985:Normal-phase chromatography
950:Displacement chromatography
285:Resolution (chromatography)
131:ion exchange chromatography
1164:
940:Argentation chromatography
867:Radial Flow Chromatography
282:
1112:
1089:Biomedical Chromatography
1005:Thin-layer chromatography
909:
297:for column chromatography
187:thin layer chromatography
749:. Material Harvest. 2008
53:thin-layer chromatograph
935:Affinity chromatography
826:Oxford University Press
143:expanded bed adsorption
139:affinity chromatography
1081:Prominent publications
1062:Kovats retention index
298:
263:
242:
219:Van Deemter's equation
163:
106:
24:
1148:Laboratory techniques
1052:Distribution constant
955:Electrochromatography
945:Column chromatography
338:Gaussian distribution
292:
261:
240:
161:
154:Mobile phase (eluent)
104:
87:UV absorption spectra
28:Column chromatography
22:
1072:Van Deemter equation
990:Paper chromatography
614:. REACH Devices, LLC
458:equilibrium constant
446:equilibrium reaction
212:There is an optimum
1057:Freundlich equation
692:10.1021/jo00408a041
578:1997JChEd..74.1222S
484:Freundlich isotherm
72:is prepared of the
1019:Hyphenated methods
975:Ion chromatography
960:Gas chromatography
586:10.1021/ed074p1222
424:Plate Height (H):
412:Plate Number (N):
299:
264:
243:
164:
107:
59:Column preparation
25:
1130:
1129:
724:978-0-632-02017-1
480:Langmuir isotherm
308:mass spectrometer
304:spectrophotometer
254:Automated systems
147:stationary phases
40:chemical compound
1155:
1120:
1119:
1067:Retention factor
896:
889:
882:
873:
848:
847:
817:
804:
803:
765:
759:
758:
756:
754:
743:
737:
736:
712:
702:
696:
695:
669:
663:
662:
656:
648:
630:
624:
623:
621:
619:
612:reachdevices.com
604:
598:
597:
559:
182:retention factor
111:stationary phase
97:Stationary phase
45:stationary phase
1163:
1162:
1158:
1157:
1156:
1154:
1153:
1152:
1133:
1132:
1131:
1126:
1108:
1076:
1040:
1014:
923:
905:
900:
857:
852:
851:
836:
819:
818:
807:
767:
766:
762:
752:
750:
745:
744:
740:
725:
704:
703:
699:
671:
670:
666:
649:
645:
632:
631:
627:
617:
615:
606:
605:
601:
561:
560:
556:
551:
532:
525:
517:
505:
501:
497:
493:
473:
455:
451:
438:
419:
407:
400:
393:
386:
374:
370:
366:
362:
358:
350:
287:
281:
256:
195:dichloromethane
156:
99:
61:
17:
12:
11:
5:
1161:
1159:
1151:
1150:
1145:
1143:Chromatography
1135:
1134:
1128:
1127:
1125:
1124:
1113:
1110:
1109:
1107:
1106:
1099:
1092:
1084:
1082:
1078:
1077:
1075:
1074:
1069:
1064:
1059:
1054:
1048:
1046:
1042:
1041:
1039:
1038:
1033:
1028:
1022:
1020:
1016:
1015:
1013:
1012:
1007:
1002:
997:
992:
987:
982:
977:
972:
967:
962:
957:
952:
947:
942:
937:
931:
929:
925:
924:
922:
921:
916:
910:
907:
906:
903:Chromatography
901:
899:
898:
891:
884:
876:
870:
869:
864:
856:
855:External links
853:
850:
849:
834:
805:
778:(1–2): 49–54.
771:J Chromatogr A
760:
738:
723:
697:
664:
644:978-0582462366
643:
625:
599:
553:
552:
550:
547:
546:
545:
539:
531:
528:
523:
519:
518:
515:
508:
507:
503:
499:
495:
491:
476:
475:
471:
453:
449:
437:
434:
430:
429:
422:
421:
417:
410:
409:
405:
402:
398:
395:
391:
388:
384:
377:
376:
372:
368:
364:
360:
356:
348:
283:Main article:
280:
277:
255:
252:
155:
152:
98:
95:
60:
57:
36:chromatography
15:
13:
10:
9:
6:
4:
3:
2:
1160:
1149:
1146:
1144:
1141:
1140:
1138:
1123:
1115:
1114:
1111:
1105:
1104:
1100:
1098:
1097:
1093:
1091:
1090:
1086:
1085:
1083:
1079:
1073:
1070:
1068:
1065:
1063:
1060:
1058:
1055:
1053:
1050:
1049:
1047:
1043:
1037:
1034:
1032:
1029:
1027:
1024:
1023:
1021:
1017:
1011:
1008:
1006:
1003:
1001:
998:
996:
993:
991:
988:
986:
983:
981:
978:
976:
973:
971:
968:
966:
963:
961:
958:
956:
953:
951:
948:
946:
943:
941:
938:
936:
933:
932:
930:
926:
920:
917:
915:
912:
911:
908:
904:
897:
892:
890:
885:
883:
878:
877:
874:
868:
865:
862:
859:
858:
854:
845:
841:
837:
835:9780190213732
831:
827:
823:
816:
814:
812:
810:
806:
801:
797:
793:
789:
785:
781:
777:
773:
772:
764:
761:
748:
742:
739:
734:
730:
726:
720:
716:
711:
710:
701:
698:
693:
689:
686:: 2923–2925.
685:
681:
677:
676:
668:
665:
660:
654:
646:
640:
636:
629:
626:
613:
609:
603:
600:
595:
591:
587:
583:
579:
575:
571:
567:
566:
558:
555:
548:
543:
540:
537:
534:
533:
529:
527:
513:
512:
511:
489:
488:
487:
485:
481:
469:
468:
467:
464:
461:
459:
452:= /() where K
447:
442:
435:
433:
427:
426:
425:
415:
414:
413:
403:
396:
389:
382:
381:
380:
354:
353:
352:
347:Resolution (R
345:
342:
339:
333:
330:
327:
323:
319:
315:
311:
309:
305:
296:
291:
286:
278:
276:
272:
270:
260:
253:
251:
247:
239:
235:
233:
229:
224:
220:
215:
210:
208:
204:
200:
199:ethyl acetate
196:
192:
188:
183:
179:
175:
174:
169:
160:
153:
151:
148:
144:
140:
136:
132:
128:
124:
120:
116:
112:
103:
96:
94:
92:
88:
84:
78:
75:
71:
65:
58:
56:
54:
49:
46:
41:
37:
33:
29:
21:
1101:
1094:
1087:
944:
821:
775:
769:
763:
751:. Retrieved
741:
708:
700:
679:
673:
667:
634:
628:
616:. Retrieved
611:
602:
572:(10): 1222.
569:
563:
557:
520:
509:
477:
465:
462:
443:
439:
431:
423:
411:
378:
346:
343:
334:
331:
328:
324:
320:
316:
312:
300:
273:
265:
248:
244:
211:
171:
168:mobile phase
167:
165:
114:
110:
108:
91:fluorescence
81:a series of
79:
66:
62:
50:
27:
26:
565:J Chem Educ
336:curve as a
145:(EBA). The
1137:Categories
928:Techniques
733:1079261960
675:J Org Chem
549:References
502:), where S
295:silica gel
119:silica gel
844:899240244
792:0021-9673
653:cite book
594:0021-9584
214:flow rate
127:Cellulose
115:adsorbent
83:fractions
32:chemistry
1122:Category
914:software
800:18849041
530:See also
498:)/(1 + K
293:Powdery
228:nitrogen
207:methanol
919:history
715:180–185
574:Bibcode
456:is the
428:H = L/N
420:)/(w/4)
379:where:
223:gravity
203:acetone
178:solvent
123:alumina
1045:Theory
842:
832:
798:
790:
731:
721:
682:(14).
641:
592:
416:N = (t
205:, and
191:hexane
173:eluent
137:(RP),
74:eluent
70:slurry
863:(pdf)
753:3 Jan
618:3 Jan
359:= 2(t
232:argon
230:, or
176:is a
89:, or
34:is a
840:OCLC
830:ISBN
796:PMID
788:ISSN
776:1211
755:2019
729:OCLC
719:ISBN
659:link
639:ISBN
620:2019
590:ISSN
490:= (K
482:and
367:)/(w
166:The
109:The
780:doi
688:doi
684:ACS
582:doi
514:= K
504:tot
496:tot
470:= K
371:+ w
363:– t
351:):
306:or
170:or
141:or
113:or
30:in
1139::
838:.
828:.
808:^
794:.
786:.
774:.
727:.
717:.
680:43
678:.
655:}}
651:{{
610:.
588:.
580:.
570:74
568:.
524:eq
516:eq
500:eq
492:eq
472:eq
454:eq
450:eq
392:RA
385:RB
375:),
365:RA
361:RB
201:,
197:,
193:,
133:,
125:.
51:A
895:e
888:t
881:v
846:.
802:.
782::
757:.
735:.
694:.
690::
661:)
647:.
622:.
596:.
584::
576::
522:K
494:S
474:.
448:K
418:R
406:A
404:w
399:B
397:w
390:t
383:t
373:A
369:B
357:s
355:R
349:s
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.