249:. Werner's work included two important changes to the Blomstrand theory. The first was that Werner described the two possibilities in terms of location in the coordination sphere. He claimed that if the ions were to form a chain, this would occur outside of the coordination sphere while the ions that bound directly to the metal would do so within the coordination sphere. In one of his most important discoveries however Werner disproved the majority of the chain theory. Werner discovered the spatial arrangements of the ligands that were involved in the formation of the complex hexacoordinate cobalt. His theory allows one to understand the difference between a coordinated ligand and a charge balancing ion in a compound, for example the chloride ion in the cobaltammine chlorides and to explain many of the previously inexplicable isomers.
844:
816:
798:
778:
656:
627:
714:
685:
1921:, at least some of the ligands are water molecules. It is true that the focus of mineralogy, materials science, and solid state chemistry differs from the usual focus of coordination or inorganic chemistry. The former are concerned primarily with polymeric structures, properties arising from a collective effects of many highly interconnected metals. In contrast, coordination chemistry focuses on reactivity and properties of complexes containing individual metal atoms or small ensembles of metal atoms.
204:
1146:. In a d–d transition, an electron in a d orbital on the metal is excited by a photon to another d orbital of higher energy, therefore d–d transitions occur only for partially-filled d-orbital complexes (d). For complexes having d or d configuration, charge transfer is still possible even though d–d transitions are not. A charge transfer band entails promotion of an electron from a metal-based orbital into an empty ligand-based orbital (
174:, then the number of donor atoms equals the number of ligands. For example, the cobalt(II) hexahydrate ion or the hexaaquacobalt(II) ion is a hydrated-complex ion that consists of six water molecules attached to a metal ion Co. The oxidation state and the coordination number reflect the number of bonds formed between the metal ion and the ligands in the complex ion. However, the coordination number of Pt(
5240:
5264:
31:
348:, the number of ligands attached to the metal (more specifically, the number of donor atoms). Usually one can count the ligands attached, but sometimes even the counting can become ambiguous. Coordination numbers are normally between two and nine, but large numbers of ligands are not uncommon for the lanthanides and actinides. The number of bonds depends on the size, charge, and
273:
5276:
5252:
363:). The maximum coordination number for a certain metal is thus related to the electronic configuration of the metal ion (to be more specific, the number of empty orbitals) and to the ratio of the size of the ligands and the metal ion. Large metals and small ligands lead to high coordination numbers, e.g.
2187:
Any anionic group can be electronically stabilized by any cation. An anionic complex can be stabilised by a hydrogen cation, becoming an acidic complex which can dissociate to release the cationic hydrogen. This kind of complex compound has a name with "ic" added after the central metal. For example,
1554:
complexes are similar to those of the transition metals in that some are colored. However, for the common Ln ions (Ln = lanthanide) the colors are all pale, and hardly influenced by the nature of the ligand. The colors are due to 4f electron transitions. As the 4f orbitals in lanthanides are "buried"
505:
was invented by
Addison et al. This index depends on angles by the coordination center and changes between 0 for the square pyramidal to 1 for trigonal bipyramidal structures, allowing to classify the cases in between. This system was later extended to four-coordinated complexes by Houser et al. and
1691:
Complexes that have unfilled or half-filled orbitals are often capable of reacting with substrates. Most substrates have a singlet ground-state; that is, they have lone electron pairs (e.g., water, amines, ethers), so these substrates need an empty orbital to be able to react with a metal centre.
1682:
with respect to the ligands (Zn), or lack covalency (Ln, where Ln is any lanthanide). The lability of a metal complex also depends on the high-spin vs. low-spin configurations when such is possible. Thus, high-spin Fe(II) and Co(III) form labile complexes, whereas low-spin analogues are inert.
397:
where the corners of that shape are the locations of the ligands), where orbital overlap (between ligand and metal orbitals) and ligand-ligand repulsions tend to lead to certain regular geometries. The most observed geometries are listed below, but there are many cases that deviate from a regular
190:
Any donor atom will give a pair of electrons. There are some donor atoms or groups which can offer more than one pair of electrons. Such are called bidentate (offers two pairs of electrons) or polydentate (offers more than two pairs of electrons). In some cases an atom or a group offers a pair of
1917: – as they apply to metal ions – are subsets of coordination chemistry in the sense that the metals are surrounded by ligands. In many cases these ligands are oxides or sulfides, but the metals are coordinated nonetheless, and the principles and guidelines discussed below apply. In
1677:
One important indicator of reactivity is the rate of degenerate exchange of ligands. For example, the rate of interchange of coordinate water in complexes varies over 20 orders of magnitude. Complexes where the ligands are released and rebound rapidly are classified as labile. Such labile
517:
stabilization, certain geometries (in which the coordination atoms do not follow a points-on-a-sphere pattern) are stabilized relative to the other possibilities, e.g. for some compounds the trigonal prismatic geometry is stabilized relative to octahedral structures for six-coordination.
167:. The most common coordination numbers are 2, 4, and especially 6. A hydrated ion is one kind of a complex ion (or simply a complex), a species formed between a central metal ion and one or more surrounding ligands, molecules or ions that contain at least one lone pair of electrons.
1627:
In bi- and polymetallic complexes, in which the individual centres have an odd number of electrons or that are high-spin, the situation is more complicated. If there is interaction (either direct or through ligand) between the two (or more) metal centres, the electrons may couple
225:
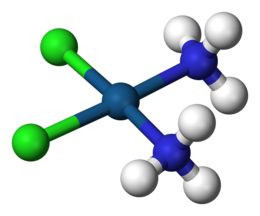
In considering metal amine complexes, he theorized that the ammonia molecules compensated for the charge of the ion by forming chains of the type , where X is the coordination number of the metal ion. He compared his theoretical ammonia chains to hydrocarbons of the form
241:
made improvements to it. In his version of the theory, Jørgensen claimed that when a molecule dissociates in a solution there were two possible outcomes: the ions would bind via the ammonia chains
Blomstrand had described or the ions would bind directly to the metal.
3089:
Addison, A. W.; Rao, N. T.; Reedijk, J.; van Rijn, J.; Verschoor, G. C. (1984). "Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aquacopper(II) perchlorate".
463:
The idealized descriptions of 5-, 7-, 8-, and 9- coordination are often indistinct geometrically from alternative structures with slightly differing L-M-L (ligand-metal-ligand) angles, e.g. the difference between square pyramidal and trigonal bipyramidal structures.
2332:
coefficients of each species. M stands for metal / metal ion , the L for Lewis bases , and finally Z for complex ions. Formation constants vary widely. Large values indicate that the metal has high affinity for the ligand, provided the system is at equilibrium.
1571:
d orbitals). f-f absorption bands are extremely sharp which contrasts with those observed for transition metals which generally have broad bands. This can lead to extremely unusual effects, such as significant color changes under different forms of lighting.
1075:
Many of the properties of transition metal complexes are dictated by their electronic structures. The electronic structure can be described by a relatively ionic model that ascribes formal charges to the metals and ligands. This approach is the essence of
1110:
Chemists tend to employ the simplest model required to predict the properties of interest; for this reason, CFT has been a favorite for the discussions when possible. MO and LF theories are more complicated, but provide a more realistic perspective.
1683:
Cr(III) can exist only in the low-spin state (quartet), which is inert because of its high formal oxidation state, absence of electrons in orbitals that are M–L antibonding, plus some "ligand field stabilization" associated with the d configuration.
884: – the isomers give different ions in solution although they have the same composition. This type of isomerism occurs when the counter ion of the complex is also a potential ligand. For example, pentaamminebromocobalt(III) sulphate
1118:
1590:. Considering only monometallic complexes, unpaired electrons arise because the complex has an odd number of electrons or because electron pairing is destabilized. Thus, monomeric Ti(III) species have one "d-electron" and must be
2351:
As a result of these complex ions forming in solutions they also can play a key role in solubility of other compounds. When a complex ion is formed it can alter the concentrations of its components in the solution. For example:
2442:
for the original reactions. The solubility is found essentially by combining the two separate equilibria into one combined equilibrium reaction and this combined reaction is the one that determines the new solubility. So
114:
Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the
1594:, regardless of the geometry or the nature of the ligands. Ti(II), with two d-electrons, forms some complexes that have two unpaired electrons and others with none. This effect is illustrated by the compounds TiX
123:(multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These complexes are called
398:
geometry, e.g. due to the use of ligands of diverse types (which results in irregular bond lengths; the coordination atoms do not follow a points-on-a-sphere pattern), due to the size of ligands, or due to
770:) as a prefix for the right-handed propeller twist. The third and fourth molecules are a similar pair of Λ and Δ isomers, in this case with two bidentate ligands and two identical monodentate ligands.
877:
occurs when the bonds are themselves different. Four types of structural isomerism are recognized: ionisation isomerism, solvate or hydrate isomerism, linkage isomerism and coordination isomerism.
3499:
1088:
based attempt at understanding complexes. But crystal field theory treats all interactions in a complex as ionic and assumes that the ligands can be approximated by negative point charges.
2336:
Sometimes the stability constant will be in a different form known as the constant of destability. This constant is expressed as the inverse of the constant of formation and is denoted as K
2323:
1034: – this occurs when both positive and negative ions of a salt are complex ions and the two isomers differ in the distribution of ligands between the cation and the anion. For example,
3113:
Yang, L.; Powell, D. R.; Houser, R. P. (2007). "Structural variation in copper(I) complexes with pyridylmethylamide ligands: structural analysis with a new four-coordinate geometry index,
3031:
Roald. Hoffmann; Barbara F. Beier; Earl L. Muetterties; Angelo R. Rossi (1977). "Seven-coordination. A molecular orbital exploration of structure, stereochemistry, and reaction dynamics".
3153:
Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. (2015). "Coordination polymers and molecular structures among complexes of mercury(II) halides with selected 1-benzoylthioureas".
2635:
1099:(MO). Ligand field theory, introduced in 1935 and built from molecular orbital theory, can handle a broader range of complexes and can explain complexes in which the interactions are
2612:
complexes. Metals can also be separated using the selective precipitation and solubility of complex ions. Cyanide is used chiefly for extraction of gold and silver from their ores.
921: – the isomers have the same composition but differ with respect to the number of molecules of solvent that serve as ligand vs simply occupying sites in the crystal. Examples:
160:. As applied to coordination chemistry, this meaning has evolved. Some metal complexes are formed virtually irreversibly and many are bound together by bonds that are quite strong.
1746:. Thus, coordination chemistry is the chemistry of the majority of the periodic table. Metals and metal ions exist, in the condensed phases at least, only surrounded by ligands.
2058:
The oxidation state of the central atom is to be specified (when it is one of several possible, or zero), and should be written as a Roman numeral (or 0) enclosed in parentheses.
1722:
Metal complexes, also known as coordination compounds, include virtually all metal compounds. The study of "coordination chemistry" is the study of "inorganic chemistry" of all
1563:. This contrasts to the transition metals where the ground state is split by the crystal field. Absorptions for Ln are weak as electric dipole transitions are parity forbidden (
4066:
2501:
821:
764:) is used as a prefix to describe the left-handed propeller twist formed by three bidentate ligands. The second molecule is the mirror image of the first, with the symbol Δ (
4103:
2769:
2740:
1555:
in the xenon core and shielded from the ligand by the 5s and 5p orbitals they are therefore not influenced by the ligands to any great extent leading to a much smaller
3607:
2344:. This constant represents the reverse reaction for the decomposition of a complex ion into its individual metal and ligand components. When comparing the values for K
3953:
4335:
4133:
456:
4170:
2527:, coordination complexes serve either structural or catalytic functions. An estimated 30% of proteins contain metal ions. Examples include the intensely colored
494:
488:
1138:
Transition metal complexes often have spectacular colors caused by electronic transitions by the absorption of light. For this reason they are often applied as
1559:
splitting than in the transition metals. The absorption spectra of an Ln ion approximates to that of the free ion where the electronic states are described by
899:
is red violet and in solution gives a precipitate with barium chloride, confirming the presence of sulphate ion, while pentaamminesulphatecobalt(III) bromide
288:(or a combination thereof), depending on how many electrons they provide for the bond between ligand and central atom. L ligands provide two electrons from a
4436:
2639:
478:
4441:
4189:
4260:
3984:
2656:
444:
3438:
3506:
2206:
The affinity of metal ions for ligands is described by a stability constant, also called the formation constant, and is represented by the symbol K
4419:
450:
359:
of the donor-atoms in the ligands and the d orbitals of the metal ions. The s, p, and d orbitals of the metal can accommodate 18 electrons (see
4463:
4125:
1678:
complexes can be quite stable thermodynamically. Typical labile metal complexes either have low-charge (Na), electrons in d-orbitals that are
2511:
As metals only exist in solution as coordination complexes, it follows then that this class of compounds is useful in a wide variety of ways.
3933:
3804:
3242:
2897:
2872:
2815:
1813:
534:
528:
432:
213:
Coordination complexes have been known since the beginning of modern chemistry. Early well-known coordination complexes include dyes such as
4414:
2431:, this causes the equilibrium reaction for the dissolving of the silver chloride, which has silver ion as a product, to shift to the right.
2423:
If these reactions both occurred in the same reaction vessel, the solubility of the silver chloride would be increased by the presence of NH
843:
4475:
4239:
4090:
4011:
3958:
3600:
2214:
for its assembly from the constituent metal and ligands, and can be calculated accordingly, as in the following example for a simple case:
815:
474:
4328:
3976:
3928:
468:
4251:
4206:
4162:
4146:
3948:
3915:
3905:
3792:
3473:
2427:
OH because formation of the
Diammine argentum(I) complex consumes a significant portion of the free silver ions from the solution. By
2156:
The coordination number of ligands attached to more than one metal (bridging ligands) is indicated by a subscript to the Greek symbol
1107:
can aid in the understanding of crystal or ligand field theory, by allowing simple, symmetry based solutions to the formal equations.
416:
3194:
546:
The arrangement of the ligands is fixed for a given complex, but in some cases it is mutable by a reaction that forms another stable
4292:
4201:
4058:
3857:
3406:
3372:
3340:
3319:
3295:
3271:
3220:
2990:
2725:
2690:
deals with a special class of coordination compounds where organic fragments are bonded to a metal at least through one C atom.
4154:
4117:
4112:
4078:
3920:
3869:
3834:
3817:
3809:
3745:
3685:
2201:
426:
5317:
5302:
5280:
3963:
3736:
3724:
3695:
3690:
3593:
484:
1696:, which results that metals with half-filled orbitals have a tendency to react with such substrates (it must be said that the
5159:
4321:
4039:
3943:
3897:
3780:
3700:
3654:
2631:
2627:
1795:
1659:
1655:
1198:
1126:
797:
422:
192:
1150:
or MLCT). The converse also occurs: excitation of an electron in a ligand-based orbital into an empty metal-based orbital (
777:
4602:
4356:
3712:
3062:
3033:
3004:
655:
438:
5307:
4231:
4053:
3767:
2566:. Synthetic coordination compounds are also used to bind to proteins and especially nucleic acids (e.g. anticancer drug
1807:: Ligands are organic (alkenes, alkynes, alkyls) as well as "organic-like" ligands such as phosphines, hydride, and CO.
218:
4879:
4366:
2524:
2594:
2428:
2005:), but this rule has been modified in the 2005 IUPAC recommendations and the correct forms for these ligands are now
1636:). When there is no interaction, the two (or more) individual metal centers behave as if in two separate molecules.
750:
occurs when a complex is not superimposable with its mirror image. It is so called because the two isomers are each
626:
403:
2236:
915:
is red and tests negative for sulphate ion in solution, but instead gives a precipitate of AgBr with silver nitrate.
5312:
4805:
4776:
4756:
4709:
3401:
R. G. Wilkins
Kinetics and Mechanism of Reactions of Transition Metal Complexes, 2nd Edition, VCH, Weinheim, 1991.
1567:) but can gain intensity due to the effect of a low-symmetry ligand field or mixing with higher electronic states (
713:
684:
1159:
603:
to each other. If these three ligands and the metal ion are in one plane, the isomer is said to be meridional, or
4394:
1560:
410:
5149:
5065:
4704:
4215:
3775:
3630:
2687:
1918:
1804:
1096:
977:
293:
191:
electrons to two similar or different central metal atoms or acceptors—by division of the electron pair—into a
2581:
is a major application of coordination compounds for the production of organic substances. Processes include
1900:
1749:
The areas of coordination chemistry can be classified according to the nature of the ligands, in broad terms:
576:
238:
3575:
296:. X ligands provide one electron, with the central atom providing the other electron, thus forming a regular
119:. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A
5087:
4998:
4961:
4845:
4771:
4592:
4575:
4518:
4003:
1839:
1163:
1151:
1147:
522:
3533:
De Vito, D.; Weber, J. ; Merbach, A. E. “Calculated Volume and Energy
Profiles for Water Exchange on t
514:
5005:
4993:
4884:
4749:
4523:
4389:
4098:
3910:
2937:
2671:
2601:
of ethylene and propylene to give polymers of great commercial importance as fibers, films, and plastics.
2520:
1835:
1798:
783:
393:
Most structures follow the points-on-a-sphere pattern (or, as if the central atom were in the middle of a
349:
3449:
2831:
2051:
Write the name of the central atom/ion. If the complex is an anion, the central atom's name will end in
5154:
5051:
5036:
4966:
4889:
4721:
4671:
4580:
4505:
4404:
3938:
3551:
Zumdahl, Steven S. Chemical
Principles, Fifth Edition. New York: Houghton Mifflin, 2005. 943–946, 957.
2807:
2676:
2666:
2578:
2165:
1914:
261:
5256:
2453:
1757:
Complexes"): Ligands in classical coordination chemistry bind to metals, almost exclusively, via their
1838:: Ligands are those provided by nature, especially including the side chains of amino acids, and many
203:
144:) between the ligands and the central atom. Originally, a complex implied a reversible association of
5144:
5099:
4874:
4624:
4381:
4361:
3889:
2609:
2211:
1786:
1727:
1556:
1143:
1122:
1077:
874:
591:. When three identical ligands occupy one face of an octahedron, the isomer is said to be facial, or
568:
occurs with the same bonds in distinct orientations. Stereoisomerism can be further classified into:
4279:
5167:
5121:
5046:
5019:
4917:
4899:
4852:
4790:
4686:
4666:
4535:
4530:
4431:
4297:
1092:
918:
355:
Typically the chemistry of transition metal complexes is dominated by interactions between s and p
345:
164:
131:
5263:
4302:
187:
is 4 (rather than 2) since it has two bidentate ligands, which contain four donor atoms in total.
5244:
5210:
5072:
5041:
4922:
4864:
4562:
4545:
4540:
4495:
4458:
4448:
4409:
4284:
4030:
3635:
3360:
2682:
1782:
1629:
1154:
or LMCT). These phenomena can be observed with the aid of electronic spectroscopy; also known as
989:
245:
It was not until 1893 that the most widely accepted version of the theory today was published by
1939:
Multiple occurring monodentate ligands receive a prefix according to the number of occurrences:
1936:
The ligands' names are given in alphabetical order. Numerical prefixes do not affect the order.
2061:
Name of the cation should be preceded by the name of anion. (if applicable, as in last example)
5225:
5190:
5173:
5111:
5029:
5024:
4952:
4937:
4907:
4828:
4795:
4766:
4761:
4736:
4726:
4646:
4634:
4513:
4426:
3552:
3402:
3368:
3336:
3315:
3291:
3267:
3238:
3216:
3198:
3182:
3135:
2986:
2893:
2868:
2811:
2721:
2661:
2651:
2626:
At one time, coordination compounds were used to identify the presence of metals in a sample.
2563:
2161:
2152:→ tetrachloridonickelate(II) ion (The use of chloro- was removed from IUPAC naming convention)
1910:
1651:
1085:
984:
747:
399:
356:
91:
2593:. In one example, a combination of titanium trichloride and triethylaluminium gives rise to
1142:. Most transitions that are related to colored metal complexes are either d–d transitions or
5268:
5185:
4840:
4699:
4676:
4629:
4570:
3542:
3418:
3356:
3190:
3162:
3127:
3095:
3071:
3042:
3013:
2964:
2799:
2783:
2754:
2713:
2586:
2555:
1731:
1581:
751:
510:
360:
217:. Their properties were first well understood in the late 1800s, following the 1869 work of
95:
3477:
3215:
von
Zelewsky, A. "Stereochemistry of Coordination Compounds" John Wiley: Chichester, 1995.
2913:
5126:
5082:
5077:
4971:
4947:
4781:
4744:
4597:
4587:
4470:
2605:
1778:
1672:
1663:
1523:
755:
565:
175:
124:
501:
To distinguish between the alternative coordinations for five-coordinated complexes, the
352:
of the metal ion and the ligands. Metal ions may have more than one coordination number.
333:
134:. The central atoms or ion and the donor atoms comprise the first coordination sphere.
5010:
4988:
4983:
4978:
4933:
4929:
4912:
4869:
4800:
4661:
4656:
4641:
4453:
4371:
4048:
3762:
2615:
2598:
2329:
1981:. This replaces the final 'e' when the anion ends with '-ide', '-ate' or '-ite', e.g.
1707:
1700:
molecule also has lone pairs, so it is also capable to react as a 'normal' Lewis base).
1693:
1633:
766:
502:
127:; the formation of such complexes is called chelation, complexation, and coordination.
2849:
5296:
5215:
5104:
5060:
4785:
4619:
4614:
4607:
4485:
3757:
3662:
2582:
1754:
1591:
1158:. For simple compounds with high symmetry, the d–d transitions can be assigned using
580:
297:
246:
214:
207:
157:
78:
2779:
2750:
1114:
The electronic configuration of the complexes gives them some important properties:
5092:
4942:
4857:
4833:
4823:
4815:
4716:
4651:
4550:
4399:
3580:
1874:
1723:
1621:
1610:
1564:
1104:
996:
can coordinate through O or N. One pair of nitrite linkage isomers have structures
583:
complexes (but not tetrahedral). When two ligands are adjacent they are said to be
141:
1761:
of electrons residing on the main-group atoms of the ligand. Typical ligands are H
30:
4490:
3822:
2952:
2774:
2745:
2551:
2528:
2157:
1962:
Multiple occurring polydentate ligands (e.g., ethylenediamine, oxalate) receive
1877:: Ligands include all of the above as well as other metal ions or atoms as well.
1863:
1679:
17:
3166:
3060:
Jeremy K. Burdett; Roald
Hoffmann; Robert C. Fay (1978). "Eight-Coordination".
3002:
Angelo R. Rossi; Roald. Hoffmann (1975). "Transition metal pentacoordination".
1091:
More sophisticated models embrace covalency, and this approach is described by
5116:
2543:
2539:
1906:
1852:
1735:
1551:
1081:
394:
383:
272:
257:
49:, is a coordination complex of platinum(II) with two chloride and two ammonia
2968:
2778:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
2749:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
27:
Molecule or ion containing ligands datively bonded to a central metallic atom
5178:
4480:
4345:
2787:
2758:
2590:
2567:
2558:, a hydrolytic enzyme important in digestion. Another complex ion enzyme is
1843:
1758:
1743:
1739:
1711:
1706:
If the ligands around the metal are carefully chosen, the metal can aid in (
1606:
1117:
554:
289:
171:
163:
The number of donor atoms attached to the central atom or ion is called the
81:
34:
3202:
3139:
3195:
10.1002/1521-3773(20011001)40:19<3534::AID-ANIE3534>3.0.CO;2-#
2717:
5200:
3585:
3556:
3180:
Kaupp, Martin (2001). ""Non-VSEPR" Structures and
Bonding in d Systems".
3099:
2559:
1770:
1697:
1617:
1602:
1587:
1100:
387:
371:. Small metals with large ligands lead to low coordination numbers, e.g.
313:
145:
99:
4313:
3537:
Rhodium(III) and
Iridium(III) Hexaaquaions: Conclusive Evidence for an I
3075:
3046:
3017:
2630:
has largely been superseded by instrumental methods of analysis such as
1632:, resulting in a diamagnetic compound), or they may enhance each other (
5220:
2547:
1774:
1139:
993:
309:
103:
3546:
1933:
When naming a complex ion, the ligands are named before the metal ion.
390:, and early transition metals tend to have high coordination numbers.
3131:
2022:
1155:
988:
occurs with ligands with more than one possible donor atom, known as
760:
547:
305:
285:
281:
120:
86:
50:
2863:
Cotton, Frank Albert; Geoffrey Wilkinson; Carlos A. Murillo (1999).
2055:, and its Latin name will be used if available (except for mercury).
2016:
Neutral ligands are given their usual name, with some exceptions: NH
1620:
configuration). Ligands provide an important means of adjusting the
1121:
Synthesis of copper(II)-tetraphenylporphyrin, a metal complex, from
1781:. Some of the simplest members of such complexes are described in
758:
in opposite directions. In the first molecule shown, the symbol Λ (
619:, since it contains both trans and cis pairs of identical ligands.
260:, overthrowing the theory that only carbon compounds could possess
3445:
3422:
1902:, in which a cluster is embedded in a biologically active species.
1116:
271:
253:
202:
70:
29:
1869:
Many natural ligands are "classical" especially including water.
344:
In coordination chemistry, a structure is first described by its
130:
The central atom or ion, together with all ligands, comprise the
5195:
3541:
Mechanism” Inorganic Chemistry, 2004, Volume 43, pages 858–863.
3439:"Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005"
2535:
1856:
149:
62:
4317:
3589:
1654:(ET) between metal ions can occur via two distinct mechanisms,
5205:
2608:
involving complex ions. They are extracted from their ores as
2348:, the larger the value, the more unstable the complex ion is.
280:
The ions or molecules surrounding the central atom are called
153:
66:
557:
in coordination complexes, just as in many other compounds.
2914:"Coordination compound - History of coordination compounds"
2434:
This new solubility can be calculated given the values of K
513:, due to special electronic effects such as (second-order)
2679:, in which coordination complexes are the repeating units.
1894:
In some cases there are combinations of different fields:
1162:. These assignments are gaining increased support with
252:
In 1911, Werner first resolved the coordination complex
221:. Blomstrand developed what has come to be known as the
2636:
inductively coupled plasma atomic emission spectroscopy
1714:) transformations of molecules or be used as a sensor.
2456:
2239:
53:. It is one of the most successful anticancer drugs.
5137:
4898:
4814:
4735:
4685:
4561:
4504:
4380:
4272:
4182:
4023:
3996:
3882:
3850:
3678:
3647:
3623:
3233:Miessler, Gary L.; Donald Arthur Tarr (1999). "9".
1644:Complexes show a variety of possible reactivities:
599:isomer, any two identical ligands are adjacent or
572:
Cis–trans isomerism and facial–meridional isomerism
2604:Nickel, cobalt, and copper can be extracted using
2495:
2317:
1537:
1509:
1487:
1456:
1432:
1424:
1399:
1375:
1367:
1336:
1328:
1320:
1312:
1299:
1291:
1283:
1275:
1267:
1259:
1170:Colours of Various Example Coordination Complexes
1586:Metal complexes that have unpaired electrons are
3363:; Murillo, Carlos A.; Bochmann, Manfred (1999),
2953:"Zur Kenntnis des asymmetrischen Kobaltatoms. I"
611:isomer can be considered as a combination of a
61:is a chemical compound consisting of a central
3367:(6th ed.), New York: Wiley-Interscience,
2957:Berichte der Deutschen Chemischen Gesellschaft
2888:Miessler, Gary L.; Donald Arthur Tarr (1999).
2618:complexes are an important class of pigments.
2447:, the new solubility constant, is denoted by:
2318:{\displaystyle K_{f}={\frac {^{z}}{^{x}^{y}}}}
4329:
3601:
1997:. Formerly, '-ide' was changed to '-o' (e.g.
1929:The basic procedure for naming a complex is:
1464:
1448:
1440:
1407:
1391:
1383:
1350:
1246:
1238:
1230:
1222:
1214:
1206:
312:can coordinate to metal atoms. An example is
90:or complexing agents. Many metal-containing
8:
3433:
3431:
2852:. Purdue University Department of Chemistry.
2640:inductively coupled plasma mass spectrometry
1528:
1514:
1080:(CFT). Crystal field theory, introduced by
3314:(3rd ed., Harper & Row 1983), p.513–24
2192:has the name tetracyanoplatinic (II) acid.
1609:configuration), whereas when X =
1492:
140:refers to the "coordinate covalent bonds" (
4336:
4322:
4314:
3608:
3594:
3586:
3448:. section 1.6.4 (p. 10-11). Archived from
3266:(3rd ed., Harper & Row 1983), p.524–5
2129:→ dicyanidobis(ethylenediamine)cadmium(II)
1478:
3258:
3256:
3254:
2657:IUPAC nomenclature of inorganic chemistry
2487:
2474:
2461:
2455:
2306:
2297:
2288:
2279:
2268:
2259:
2253:
2244:
2238:
1866:contains a porphyrin complex of magnesium
1692:Some substrates (e.g., molecular oxygen)
3281:
3279:
2985:5th edition Oxford Science Publications
2160:placed before the ligand name. Thus the
2136:→ pentaamminechloridocobalt(III) sulfate
2067:
1168:
1061:
1057:
1053:
1045:
1041:
1037:
1023:
1019:
1003:
999:
967:
963:
959:
951:
947:
943:
939:
932:
928:
924:
910:
906:
902:
895:
891:
887:
859:
855:
832:
828:
805:
786:
732:
728:
724:
703:
699:
695:
674:
670:
666:
645:
641:
637:
378:
374:
366:
327:
323:
319:
237:Following this theory, Danish scientist
3500:"Solubility and Complex-ion Equilibria"
2867:. John Wiley & Sons. p. 1355.
2700:
773:
622:
2710:Introduction to Coordination Chemistry
1925:Nomenclature of coordination complexes
2507:Application of coordination compounds
195:. These are called bridging ligands.
7:
5251:
3237:. Prentice Hall. pp. 315, 316.
2562:, which decomposes the cell's waste
2142:→ amminepentachloridocuprate(II) ion
754:, that is, they rotate the plane of
102:that belong to the periodic table's
5275:
3565:. 1989 New York, Dover Publications
3331:Harris, D.; Bertolucci, M. (1989).
2832:"Definition of coordination sphere"
1134:Color of transition metal complexes
84:or ions, that are in turn known as
2850:"What Is A Coordination Compound?"
2775:Compendium of Chemical Terminology
2746:Compendium of Chemical Terminology
2149:→ potassium hexacyanidoferrate(II)
25:
3389:Lanthanide and Actinide Chemistry
2496:{\displaystyle K_{c}=K_{sp}K_{f}}
2328:where : x, y, and z are the
1662:. In an inner sphere reaction, a
5274:
5262:
5250:
5239:
5238:
2202:Stability constants of complexes
842:
814:
796:
776:
712:
683:
654:
625:
94:, especially those that include
3581:Transition Metal Complex Colors
3290:. McGraw-Hill. pp. 357–9.
1660:outer sphere electron transfers
1605:, the complex is paramagnetic (
1152:ligand-to-metal charge transfer
1148:metal-to-ligand charge transfer
1103:. The chemical applications of
106:), are coordination complexes.
2983:Structural Inorganic Chemistry
2892:. Prentice Hall. p. 642.
2708:Lawrance, Geoffrey A. (2010).
2632:atomic absorption spectroscopy
2628:Qualitative inorganic analysis
2303:
2294:
2285:
2276:
2265:
2256:
1546:Colors of lanthanide complexes
1127:copper(II) acetate monohydrate
193:three-center two-electron bond
1:
4603:Interface and colloid science
4357:Glossary of chemical formulae
3576:Naming Coordination Compounds
1859:, a porphyrin complex of iron
772:
621:
300:. The ligands are said to be
77:, and a surrounding array of
3561:Harris, D., Bertolucci, M.,
3391:. John Wiley & Sons Ltd.
3365:Advanced Inorganic Chemistry
3092:J. Chem. Soc., Dalton Trans.
2865:Advanced Inorganic Chemistry
2606:hydrometallurgical processes
919:Solvate or hydrate isomerism
587:, when opposite each other,
457:Tricapped trigonal prismatic
284:. Ligands are classified as
219:Christian Wilhelm Blomstrand
110:Nomenclature and terminology
4880:Bioorganometallic chemistry
4367:List of inorganic compounds
3183:Angew. Chem. Int. Ed. Engl.
2525:bioorganometallic chemistry
1694:have a triplet ground state
1666:serves as a conduit for ET.
495:Capped square antiprismatic
489:bicapped trigonal prismatic
382:. Due to their large size,
5334:
4806:Dynamic covalent chemistry
4777:Enantioselective synthesis
4757:Physical organic chemistry
4710:Organolanthanide chemistry
3288:Modern Inorganic Chemistry
3286:Jolly, William L. (1984).
3167:10.1016/j.poly.2015.01.035
2199:
1630:antiferromagnetic coupling
1579:
553:There exist many kinds of
5234:
4395:Electroanalytical methods
4352:
3563:Symmetry and Spectroscopy
3417:Exception: metal vapors,
3333:Symmetry and Spectroscopy
2804:Chemistry of the Elements
2802:; Earnshaw, Alan (1997).
579:occurs in octahedral and
479:capped trigonal prismatic
223:complex ion chain theory.
5150:Nobel Prize in Chemistry
5066:Supramolecular chemistry
4705:Organometallic chemistry
3474:"Complex Ion Equilibria"
2969:10.1002/cber.19110440297
2836:chemistry-dictionary.com
2688:Organometallic chemistry
2429:Le Chatelier's principle
2139:→ hexaaquacopper(II) ion
1805:Organometallic chemistry
1097:Molecular orbital theory
978:water of crystallization
294:coordinate covalent bond
5088:Combinatorial chemistry
4999:Food physical chemistry
4962:Environmental chemistry
4846:Bioorthogonal chemistry
4772:Retrosynthetic analysis
4593:Chemical thermodynamics
4576:Spectroelectrochemistry
4519:Computational chemistry
2951:Werner, A. (May 1911).
2938:"Coordination Compound"
2918:Encyclopedia Britannica
2788:10.1351/goldbook.C01330
2759:10.1351/goldbook.C01203
2595:Ziegler–Natta catalysts
1164:computational chemistry
506:also Okuniewski et al.
170:If all the ligands are
5318:Coordination chemistry
5303:Coordination complexes
5160:of element discoveries
5006:Agricultural chemistry
4994:Carbohydrate chemistry
4885:Bioinorganic chemistry
4750:Alkane stereochemistry
4695:Coordination chemistry
4524:Mathematical chemistry
4390:Instrumental chemistry
3617:Coordination complexes
3387:Cotton, Simon (2006).
3335:. Dover Publications.
2672:Coordination isomerism
2521:bioinorganic chemistry
2515:Bioinorganic chemistry
2497:
2319:
1836:Bioinorganic chemistry
1787:metal ammine complexes
1688:Associative processes
1634:ferromagnetic coupling
1160:Tanabe–Sugano diagrams
1130:
1032:Coordination isomerism
531:for three-coordination
491:for eight-coordination
481:for seven-coordination
453:for eight-coordination
447:for seven-coordination
445:Pentagonal bipyramidal
419:for three-coordination
404:Jahn–Teller distortion
350:electron configuration
277:
210:
54:
5155:Timeline of chemistry
5052:Post-mortem chemistry
5037:Clandestine chemistry
4967:Atmospheric chemistry
4890:Biophysical chemistry
4722:Solid-state chemistry
4672:Equilibrium chemistry
4581:Photoelectrochemistry
2808:Butterworth-Heinemann
2800:Greenwood, Norman N.
2718:10.1002/9780470687123
2677:Coordination polymers
2667:Coordination geometry
2579:Homogeneous catalysis
2498:
2320:
2166:aluminium trichloride
1915:solid state chemistry
1728:alkaline earth metals
1616:, it is diamagnetic (
1601:: when X =
1144:charge transfer bands
1120:
1071:Electronic properties
497:for nine-coordination
471:for five-coordination
459:for nine-coordination
435:for five-coordination
429:for four-coordination
275:
239:Sophus Mads Jørgensen
206:
33:
5145:History of chemistry
5100:Chemical engineering
4875:Bioorganic chemistry
4625:Structural chemistry
4362:List of biomolecules
3100:10.1039/dt9840001349
2454:
2237:
2212:equilibrium constant
1783:metal aquo complexes
1123:tetraphenylporphyrin
1086:quantum mechanically
1078:crystal field theory
882:Ionisation isomerism
875:Structural isomerism
870:Structural isomerism
537:for six-coordination
525:for two-coordination
509:In systems with low
451:Square antiprismatic
441:for six-coordination
433:Trigonal bipyramidal
413:for two-coordination
59:coordination complex
5308:Inorganic chemistry
5168:The central science
5122:Ceramic engineering
5047:Forensic toxicology
5020:Chemistry education
4918:Radiation chemistry
4900:Interdisciplinarity
4853:Medicinal chemistry
4791:Fullerene chemistry
4667:Microwave chemistry
4536:Molecular mechanics
4531:Molecular modelling
3361:Wilkinson, Geoffrey
3312:Inorganic Chemistry
3264:Inorganic Chemistry
3235:Inorganic Chemistry
3076:10.1021/ic50187a041
3063:Inorganic Chemistry
3047:10.1021/ic50169a002
3034:Inorganic Chemistry
3018:10.1021/ic50144a032
3005:Inorganic Chemistry
2890:Inorganic Chemistry
2780:coordination entity
2683:Inclusion compounds
1648:Electron transfers
1561:spin-orbit coupling
1510:Precipitate+bubbles
1306:(OH), concentrated
1171:
1093:ligand field theory
1016:(nitro isomer) and
990:ambidentate ligands
976:is dark green. See
956:is blue-green, and
936:is violet colored,
577:Cis–trans isomerism
346:coordination number
165:coordination number
132:coordination sphere
75:coordination centre
69:, which is usually
5211:Chemical substance
5073:Chemical synthesis
5042:Forensic chemistry
4923:Actinide chemistry
4865:Clinical chemistry
4546:Molecular geometry
4541:Molecular dynamics
4496:Elemental analysis
4449:Separation process
3310:Huheey, James E.,
3262:Huheey, James E.,
2981:Wells A.F. (1984)
2493:
2315:
2196:Stability constant
2168:is described by Al
1169:
1131:
535:Trigonal prismatic
529:Trigonal pyramidal
400:electronic effects
357:molecular orbitals
290:lone electron pair
278:
276:Structure of hexol
211:
156:through such weak
73:and is called the
55:
5313:Transition metals
5290:
5289:
5226:Quantum mechanics
5191:Chemical compound
5174:Chemical reaction
5112:Materials science
5030:General chemistry
5025:Amateur chemistry
4953:Photogeochemistry
4938:Stellar chemistry
4908:Nuclear chemistry
4829:Molecular biology
4796:Polymer chemistry
4767:Organic synthesis
4762:Organic reactions
4727:Ceramic chemistry
4717:Cluster chemistry
4647:Chemical kinetics
4635:Molecular physics
4514:Quantum chemistry
4427:Mass spectrometry
4311:
4310:
3547:10.1021/ic035096n
3357:Cotton, F. Albert
3244:978-0-13-841891-5
2899:978-0-13-841891-5
2874:978-0-471-19957-1
2817:978-0-08-037941-8
2662:Coordination cage
2652:Activated complex
2564:hydrogen peroxide
2313:
2300:
2282:
2262:
2126:
2125:
1911:materials science
1875:Cluster chemistry
1732:transition metals
1652:Electron transfer
1565:Laporte forbidden
1543:
1542:
1084:in 1929, gives a
1028:(nitrito isomer).
985:Linkage isomerism
748:Optical isomerism
743:Optical isomerism
475:Capped octahedral
304:to the atom. For
292:, resulting in a
125:chelate complexes
96:transition metals
16:(Redirected from
5325:
5278:
5277:
5266:
5254:
5253:
5242:
5241:
5186:Chemical element
4841:Chemical biology
4700:Magnetochemistry
4677:Mechanochemistry
4630:Chemical physics
4571:Electrochemistry
4476:Characterization
4338:
4331:
4324:
4315:
3610:
3603:
3596:
3587:
3521:
3520:
3518:
3517:
3511:
3505:. Archived from
3504:
3495:
3489:
3488:
3486:
3485:
3476:. Archived from
3470:
3464:
3463:
3461:
3460:
3454:
3443:
3435:
3426:
3415:
3409:
3399:
3393:
3392:
3384:
3378:
3377:
3353:
3347:
3346:
3328:
3322:
3308:
3302:
3301:
3283:
3274:
3260:
3249:
3248:
3230:
3224:
3213:
3207:
3206:
3189:(1): 3534–3565.
3177:
3171:
3170:
3150:
3144:
3143:
3132:10.1039/b617136b
3121:
3110:
3104:
3103:
3094:(7): 1349–1356.
3086:
3080:
3079:
3070:(9): 2553–2568.
3057:
3051:
3050:
3028:
3022:
3021:
2999:
2993:
2979:
2973:
2972:
2963:(2): 1887–1898.
2948:
2942:
2941:
2934:
2928:
2927:
2925:
2924:
2910:
2904:
2903:
2885:
2879:
2878:
2860:
2854:
2853:
2846:
2840:
2839:
2828:
2822:
2821:
2806:(2nd ed.).
2796:
2790:
2767:
2761:
2738:
2732:
2731:
2705:
2587:hydroformylation
2556:carboxypeptidase
2502:
2500:
2499:
2494:
2492:
2491:
2482:
2481:
2466:
2465:
2419:
2418:
2410:
2409:
2385:
2384:
2368:
2364:
2363:
2324:
2322:
2321:
2316:
2314:
2312:
2311:
2310:
2301:
2298:
2293:
2292:
2283:
2280:
2274:
2273:
2272:
2263:
2260:
2254:
2249:
2248:
2068:
1582:magnetochemistry
1539:
1533:
1525:
1519:
1511:
1505:
1489:
1483:
1466:
1458:
1450:
1442:
1434:
1426:
1409:
1401:
1393:
1385:
1377:
1369:
1352:
1338:
1330:
1322:
1314:
1301:
1293:
1285:
1277:
1269:
1261:
1248:
1240:
1232:
1224:
1216:
1208:
1172:
1065:
1049:
1027:
1015:
1014:
1013:
1010:
975:
955:
935:
914:
898:
863:
846:
836:
818:
809:
800:
790:
780:
752:optically active
736:
716:
707:
687:
678:
658:
649:
629:
511:d electron count
503:τ geometry index
469:Square pyramidal
381:
370:
361:18-Electron rule
331:
186:
185:
21:
18:Complex compound
5333:
5332:
5328:
5327:
5326:
5324:
5323:
5322:
5293:
5292:
5291:
5286:
5230:
5133:
5127:Polymer science
5083:Click chemistry
5078:Green chemistry
4972:Ocean chemistry
4948:Biogeochemistry
4894:
4810:
4782:Total synthesis
4745:Stereochemistry
4731:
4681:
4598:Surface science
4588:Thermochemistry
4557:
4500:
4471:Crystallography
4376:
4348:
4342:
4312:
4307:
4288:
4268:
4255:
4247:
4243:
4235:
4227:
4223:
4219:
4210:
4197:
4193:
4178:
4174:
4166:
4158:
4150:
4141:
4137:
4129:
4121:
4107:
4094:
4086:
4082:
4074:
4070:
4062:
4043:
4034:
4019:
4015:
4007:
3992:
3980:
3971:
3967:
3924:
3901:
3893:
3878:
3873:
3865:
3861:
3846:
3842:
3838:
3830:
3826:
3813:
3800:
3796:
3788:
3784:
3771:
3753:
3749:
3740:
3732:
3728:
3720:
3716:
3708:
3704:
3674:
3670:
3666:
3658:
3643:
3639:
3619:
3614:
3572:
3540:
3536:
3530:
3528:Further reading
3525:
3524:
3515:
3513:
3509:
3502:
3498:Stretton, Tom.
3497:
3496:
3492:
3483:
3481:
3472:
3471:
3467:
3458:
3456:
3452:
3441:
3437:
3436:
3429:
3416:
3412:
3400:
3396:
3386:
3385:
3381:
3375:
3355:
3354:
3350:
3343:
3330:
3329:
3325:
3309:
3305:
3298:
3285:
3284:
3277:
3261:
3252:
3245:
3232:
3231:
3227:
3214:
3210:
3179:
3178:
3174:
3152:
3151:
3147:
3120:
3114:
3112:
3111:
3107:
3088:
3087:
3083:
3059:
3058:
3054:
3030:
3029:
3025:
3001:
3000:
2996:
2980:
2976:
2950:
2949:
2945:
2936:
2935:
2931:
2922:
2920:
2912:
2911:
2907:
2900:
2887:
2886:
2882:
2875:
2862:
2861:
2857:
2848:
2847:
2843:
2830:
2829:
2825:
2818:
2798:
2797:
2793:
2768:
2764:
2739:
2735:
2728:
2707:
2706:
2702:
2697:
2648:
2624:
2597:, used for the
2576:
2532:
2517:
2509:
2483:
2470:
2457:
2452:
2451:
2446:
2441:
2437:
2426:
2417:
2414:
2413:
2412:
2408:
2405:
2404:
2403:
2401:
2397:
2393:
2383:
2380:
2379:
2378:
2376:
2372:
2366:
2362:
2359:
2358:
2357:
2347:
2343:
2339:
2302:
2284:
2275:
2264:
2255:
2240:
2235:
2234:
2229:
2225:
2221:
2209:
2204:
2198:
2191:
2183:
2179:
2175:
2171:
2148:
2135:
2029:
2019:
1927:
1889:
1885:
1829:
1825:
1821:
1817:
1768:
1764:
1753:Classical (or "
1720:
1673:ligand exchange
1664:bridging ligand
1642:
1614:
1600:
1597:
1584:
1578:
1548:
1536:
1534:
1532:
1522:
1520:
1518:
1508:
1506:
1504:
1500:
1496:
1486:
1484:
1482:
1474:
1463:
1461:
1455:
1453:
1447:
1445:
1439:
1437:
1431:
1429:
1423:
1421:
1418:, concentrated
1417:
1406:
1404:
1398:
1396:
1390:
1388:
1382:
1380:
1374:
1372:
1366:
1364:
1360:
1349:
1347:
1343:
1341:
1335:
1333:
1327:
1325:
1319:
1317:
1311:
1309:
1298:
1296:
1290:
1288:
1282:
1280:
1274:
1272:
1266:
1264:
1258:
1256:
1245:
1243:
1237:
1235:
1229:
1227:
1221:
1219:
1213:
1211:
1205:
1203:
1136:
1073:
1063:
1059:
1055:
1051:
1047:
1043:
1039:
1035:
1025:
1021:
1017:
1011:
1008:
1007:
1005:
1001:
997:
992:. For example,
973:
969:
965:
961:
957:
953:
949:
945:
941:
937:
934:
930:
926:
922:
912:
908:
904:
900:
897:
893:
889:
885:
872:
867:
864:
861:
857:
849:
847:
838:
834:
830:
822:
819:
810:
807:
803:
801:
792:
788:
784:
781:
756:polarized light
745:
740:
737:
734:
730:
726:
719:
717:
708:
705:
701:
697:
690:
688:
679:
676:
672:
668:
661:
659:
650:
647:
643:
639:
632:
630:
574:
566:Stereoisomerism
563:
561:Stereoisomerism
544:
417:Trigonal planar
380:
376:
372:
368:
364:
342:
329:
325:
321:
317:
316:in the complex
270:
258:optical isomers
233:
229:
201:
184:
181:
180:
179:
112:
98:(elements like
48:
44:
40:
28:
23:
22:
15:
12:
11:
5:
5331:
5329:
5321:
5320:
5315:
5310:
5305:
5295:
5294:
5288:
5287:
5285:
5284:
5272:
5260:
5248:
5235:
5232:
5231:
5229:
5228:
5223:
5218:
5213:
5208:
5203:
5198:
5193:
5188:
5183:
5182:
5181:
5171:
5164:
5163:
5162:
5152:
5147:
5141:
5139:
5135:
5134:
5132:
5131:
5130:
5129:
5124:
5119:
5109:
5108:
5107:
5097:
5096:
5095:
5090:
5085:
5080:
5070:
5069:
5068:
5057:
5056:
5055:
5054:
5049:
5039:
5034:
5033:
5032:
5027:
5016:
5015:
5014:
5013:
5011:Soil chemistry
5003:
5002:
5001:
4996:
4989:Food chemistry
4986:
4984:Carbochemistry
4981:
4979:Clay chemistry
4976:
4975:
4974:
4969:
4958:
4957:
4956:
4955:
4950:
4940:
4934:Astrochemistry
4930:Cosmochemistry
4927:
4926:
4925:
4920:
4915:
4913:Radiochemistry
4904:
4902:
4896:
4895:
4893:
4892:
4887:
4882:
4877:
4872:
4870:Neurochemistry
4867:
4862:
4861:
4860:
4850:
4849:
4848:
4838:
4837:
4836:
4831:
4820:
4818:
4812:
4811:
4809:
4808:
4803:
4801:Petrochemistry
4798:
4793:
4788:
4779:
4774:
4769:
4764:
4759:
4754:
4753:
4752:
4741:
4739:
4733:
4732:
4730:
4729:
4724:
4719:
4714:
4713:
4712:
4702:
4697:
4691:
4689:
4683:
4682:
4680:
4679:
4674:
4669:
4664:
4662:Spin chemistry
4659:
4657:Photochemistry
4654:
4649:
4644:
4642:Femtochemistry
4639:
4638:
4637:
4627:
4622:
4617:
4612:
4611:
4610:
4600:
4595:
4590:
4585:
4584:
4583:
4578:
4567:
4565:
4559:
4558:
4556:
4555:
4554:
4553:
4543:
4538:
4533:
4528:
4527:
4526:
4516:
4510:
4508:
4502:
4501:
4499:
4498:
4493:
4488:
4483:
4478:
4473:
4468:
4467:
4466:
4461:
4454:Chromatography
4451:
4446:
4445:
4444:
4439:
4434:
4424:
4423:
4422:
4417:
4412:
4407:
4397:
4392:
4386:
4384:
4378:
4377:
4375:
4374:
4372:Periodic table
4369:
4364:
4359:
4353:
4350:
4349:
4343:
4341:
4340:
4333:
4326:
4318:
4309:
4308:
4306:
4305:
4300:
4295:
4290:
4286:
4282:
4276:
4274:
4273:Halide donors:
4270:
4269:
4267:
4266:
4258:
4253:
4249:
4245:
4241:
4237:
4233:
4229:
4225:
4221:
4217:
4213:
4208:
4204:
4199:
4195:
4191:
4186:
4184:
4180:
4179:
4177:
4176:
4172:
4168:
4164:
4160:
4156:
4152:
4148:
4144:
4139:
4135:
4131:
4127:
4123:
4119:
4115:
4110:
4105:
4101:
4096:
4092:
4088:
4084:
4080:
4076:
4072:
4068:
4064:
4060:
4056:
4051:
4046:
4041:
4037:
4032:
4027:
4025:
4021:
4020:
4018:
4017:
4013:
4009:
4005:
4000:
3998:
3994:
3993:
3991:
3990:
3982:
3978:
3974:
3969:
3965:
3961:
3956:
3951:
3946:
3941:
3936:
3931:
3926:
3922:
3918:
3913:
3908:
3903:
3899:
3895:
3891:
3886:
3884:
3880:
3879:
3877:
3876:
3871:
3867:
3863:
3859:
3854:
3852:
3848:
3847:
3845:
3844:
3840:
3836:
3832:
3828:
3824:
3820:
3815:
3811:
3807:
3802:
3798:
3794:
3790:
3786:
3782:
3778:
3773:
3769:
3765:
3760:
3755:
3751:
3747:
3743:
3738:
3734:
3730:
3726:
3722:
3718:
3714:
3710:
3706:
3702:
3698:
3693:
3688:
3682:
3680:
3676:
3675:
3673:
3672:
3668:
3664:
3660:
3656:
3651:
3649:
3645:
3644:
3642:
3641:
3637:
3633:
3627:
3625:
3621:
3620:
3615:
3613:
3612:
3605:
3598:
3590:
3584:
3583:
3578:
3571:
3570:External links
3568:
3567:
3566:
3559:
3549:
3538:
3534:
3529:
3526:
3523:
3522:
3490:
3465:
3427:
3410:
3394:
3379:
3373:
3348:
3341:
3323:
3303:
3296:
3275:
3250:
3243:
3225:
3208:
3172:
3145:
3118:
3105:
3081:
3052:
3041:(3): 511–522.
3023:
3012:(2): 365–374.
2994:
2974:
2943:
2929:
2905:
2898:
2880:
2873:
2855:
2841:
2823:
2816:
2791:
2762:
2733:
2726:
2699:
2698:
2696:
2693:
2692:
2691:
2685:
2680:
2674:
2669:
2664:
2659:
2654:
2647:
2644:
2638:(ICP-AES) and
2623:
2620:
2616:Phthalocyanine
2599:polymerization
2575:
2572:
2530:
2516:
2513:
2508:
2505:
2504:
2503:
2490:
2486:
2480:
2477:
2473:
2469:
2464:
2460:
2444:
2439:
2435:
2424:
2421:
2420:
2415:
2406:
2399:
2395:
2391:
2387:
2386:
2381:
2374:
2370:
2360:
2345:
2341:
2337:
2330:stoichiometric
2326:
2325:
2309:
2305:
2296:
2291:
2287:
2278:
2271:
2267:
2258:
2252:
2247:
2243:
2231:
2230:
2227:
2223:
2219:
2207:
2200:Main article:
2197:
2194:
2189:
2181:
2177:
2173:
2169:
2154:
2153:
2150:
2146:
2143:
2140:
2137:
2133:
2130:
2124:
2123:
2120:
2116:
2115:
2112:
2108:
2107:
2104:
2100:
2099:
2096:
2092:
2091:
2088:
2084:
2083:
2080:
2076:
2075:
2072:
2063:
2062:
2059:
2056:
2049:
2048:
2047:
2027:
2017:
2014:
1977:Anions end in
1975:
1960:
1934:
1926:
1923:
1904:
1903:
1896:
1895:
1891:
1890:
1887:
1883:
1879:
1878:
1871:
1870:
1867:
1860:
1848:
1847:
1832:
1831:
1827:
1823:
1819:
1815:
1809:
1808:
1801:
1800:
1791:
1790:
1766:
1762:
1719:
1718:Classification
1716:
1708:stoichiometric
1704:
1703:
1702:
1701:
1686:
1685:
1684:
1669:
1668:
1667:
1641:
1638:
1612:
1598:
1595:
1592:(para)magnetic
1580:Main article:
1577:
1574:
1550:Superficially
1547:
1544:
1541:
1540:
1530:
1526:
1516:
1512:
1502:
1498:
1494:
1490:
1480:
1476:
1472:
1468:
1467:
1459:
1451:
1443:
1438:Straw coloured
1435:
1427:
1419:
1415:
1411:
1410:
1402:
1394:
1386:
1381:Straw coloured
1378:
1370:
1362:
1358:
1354:
1353:
1345:
1339:
1331:
1323:
1315:
1307:
1303:
1302:
1294:
1286:
1278:
1270:
1262:
1254:
1250:
1249:
1241:
1233:
1225:
1217:
1209:
1201:
1195:
1194:
1191:
1188:
1185:
1182:
1179:
1176:
1135:
1132:
1072:
1069:
1068:
1067:
1029:
981:
971:
916:
871:
868:
866:
865:
848:
841:
839:
820:
813:
811:
802:
795:
793:
782:
775:
744:
741:
739:
738:
718:
711:
709:
689:
682:
680:
660:
653:
651:
631:
624:
573:
570:
562:
559:
543:
540:
539:
538:
532:
526:
499:
498:
492:
482:
472:
461:
460:
454:
448:
442:
436:
430:
420:
414:
341:
338:
269:
266:
231:
227:
200:
197:
182:
158:chemical bonds
111:
108:
46:
42:
38:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
5330:
5319:
5316:
5314:
5311:
5309:
5306:
5304:
5301:
5300:
5298:
5283:
5282:
5273:
5271:
5270:
5265:
5261:
5259:
5258:
5249:
5247:
5246:
5237:
5236:
5233:
5227:
5224:
5222:
5219:
5217:
5216:Chemical bond
5214:
5212:
5209:
5207:
5204:
5202:
5199:
5197:
5194:
5192:
5189:
5187:
5184:
5180:
5177:
5176:
5175:
5172:
5169:
5165:
5161:
5158:
5157:
5156:
5153:
5151:
5148:
5146:
5143:
5142:
5140:
5136:
5128:
5125:
5123:
5120:
5118:
5115:
5114:
5113:
5110:
5106:
5105:Stoichiometry
5103:
5102:
5101:
5098:
5094:
5091:
5089:
5086:
5084:
5081:
5079:
5076:
5075:
5074:
5071:
5067:
5064:
5063:
5062:
5061:Nanochemistry
5059:
5058:
5053:
5050:
5048:
5045:
5044:
5043:
5040:
5038:
5035:
5031:
5028:
5026:
5023:
5022:
5021:
5018:
5017:
5012:
5009:
5008:
5007:
5004:
5000:
4997:
4995:
4992:
4991:
4990:
4987:
4985:
4982:
4980:
4977:
4973:
4970:
4968:
4965:
4964:
4963:
4960:
4959:
4954:
4951:
4949:
4946:
4945:
4944:
4941:
4939:
4935:
4931:
4928:
4924:
4921:
4919:
4916:
4914:
4911:
4910:
4909:
4906:
4905:
4903:
4901:
4897:
4891:
4888:
4886:
4883:
4881:
4878:
4876:
4873:
4871:
4868:
4866:
4863:
4859:
4856:
4855:
4854:
4851:
4847:
4844:
4843:
4842:
4839:
4835:
4832:
4830:
4827:
4826:
4825:
4822:
4821:
4819:
4817:
4813:
4807:
4804:
4802:
4799:
4797:
4794:
4792:
4789:
4787:
4786:Semisynthesis
4783:
4780:
4778:
4775:
4773:
4770:
4768:
4765:
4763:
4760:
4758:
4755:
4751:
4748:
4747:
4746:
4743:
4742:
4740:
4738:
4734:
4728:
4725:
4723:
4720:
4718:
4715:
4711:
4708:
4707:
4706:
4703:
4701:
4698:
4696:
4693:
4692:
4690:
4688:
4684:
4678:
4675:
4673:
4670:
4668:
4665:
4663:
4660:
4658:
4655:
4653:
4650:
4648:
4645:
4643:
4640:
4636:
4633:
4632:
4631:
4628:
4626:
4623:
4621:
4620:Sonochemistry
4618:
4616:
4615:Cryochemistry
4613:
4609:
4608:Micromeritics
4606:
4605:
4604:
4601:
4599:
4596:
4594:
4591:
4589:
4586:
4582:
4579:
4577:
4574:
4573:
4572:
4569:
4568:
4566:
4564:
4560:
4552:
4549:
4548:
4547:
4544:
4542:
4539:
4537:
4534:
4532:
4529:
4525:
4522:
4521:
4520:
4517:
4515:
4512:
4511:
4509:
4507:
4503:
4497:
4494:
4492:
4489:
4487:
4486:Wet chemistry
4484:
4482:
4479:
4477:
4474:
4472:
4469:
4465:
4462:
4460:
4457:
4456:
4455:
4452:
4450:
4447:
4443:
4440:
4438:
4435:
4433:
4430:
4429:
4428:
4425:
4421:
4418:
4416:
4413:
4411:
4408:
4406:
4403:
4402:
4401:
4398:
4396:
4393:
4391:
4388:
4387:
4385:
4383:
4379:
4373:
4370:
4368:
4365:
4363:
4360:
4358:
4355:
4354:
4351:
4347:
4339:
4334:
4332:
4327:
4325:
4320:
4319:
4316:
4304:
4301:
4299:
4296:
4294:
4291:
4289:
4283:
4281:
4278:
4277:
4275:
4271:
4265:
4264:
4259:
4257:
4250:
4248:
4238:
4236:
4230:
4228:
4214:
4212:
4205:
4203:
4200:
4198:
4188:
4187:
4185:
4181:
4175:
4169:
4167:
4161:
4159:
4153:
4151:
4145:
4143:
4132:
4130:
4124:
4122:
4116:
4114:
4111:
4109:
4102:
4100:
4097:
4095:
4089:
4087:
4077:
4075:
4065:
4063:
4057:
4055:
4052:
4050:
4047:
4045:
4038:
4036:
4029:
4028:
4026:
4022:
4016:
4010:
4008:
4002:
4001:
3999:
3995:
3989:
3987:
3983:
3981:
3975:
3973:
3962:
3960:
3957:
3955:
3952:
3950:
3947:
3945:
3942:
3940:
3937:
3935:
3932:
3930:
3927:
3925:
3919:
3917:
3914:
3912:
3909:
3907:
3904:
3902:
3896:
3894:
3888:
3887:
3885:
3881:
3875:
3868:
3866:
3856:
3855:
3853:
3849:
3843:
3833:
3831:
3821:
3819:
3816:
3814:
3808:
3806:
3803:
3801:
3791:
3789:
3779:
3777:
3774:
3772:
3766:
3764:
3761:
3759:
3756:
3754:
3744:
3742:
3735:
3733:
3723:
3721:
3711:
3709:
3699:
3697:
3694:
3692:
3689:
3687:
3684:
3683:
3681:
3677:
3671:
3661:
3659:
3653:
3652:
3650:
3646:
3640:
3634:
3632:
3629:
3628:
3626:
3622:
3618:
3611:
3606:
3604:
3599:
3597:
3592:
3591:
3588:
3582:
3579:
3577:
3574:
3573:
3569:
3564:
3560:
3558:
3554:
3550:
3548:
3544:
3532:
3531:
3527:
3512:on 2018-09-20
3508:
3501:
3494:
3491:
3480:on 2015-03-08
3479:
3475:
3469:
3466:
3455:on 2014-12-22
3451:
3447:
3440:
3434:
3432:
3428:
3424:
3420:
3414:
3411:
3408:
3407:1-56081-125-0
3404:
3398:
3395:
3390:
3383:
3380:
3376:
3374:0-471-19957-5
3370:
3366:
3362:
3358:
3352:
3349:
3344:
3342:9780486661445
3338:
3334:
3327:
3324:
3321:
3320:0-06-042987-9
3317:
3313:
3307:
3304:
3299:
3297:0-07-032760-2
3293:
3289:
3282:
3280:
3276:
3273:
3272:0-06-042987-9
3269:
3265:
3259:
3257:
3255:
3251:
3246:
3240:
3236:
3229:
3226:
3222:
3221:0-471-95599-X
3218:
3212:
3209:
3204:
3200:
3196:
3192:
3188:
3185:
3184:
3176:
3173:
3168:
3164:
3160:
3156:
3149:
3146:
3141:
3137:
3133:
3129:
3126:(9): 955–64.
3125:
3124:Dalton Trans.
3117:
3109:
3106:
3101:
3097:
3093:
3085:
3082:
3077:
3073:
3069:
3065:
3064:
3056:
3053:
3048:
3044:
3040:
3036:
3035:
3027:
3024:
3019:
3015:
3011:
3007:
3006:
2998:
2995:
2992:
2991:0-19-855370-6
2988:
2984:
2978:
2975:
2970:
2966:
2962:
2959:(in German).
2958:
2954:
2947:
2944:
2939:
2933:
2930:
2919:
2915:
2909:
2906:
2901:
2895:
2891:
2884:
2881:
2876:
2870:
2866:
2859:
2856:
2851:
2845:
2842:
2837:
2833:
2827:
2824:
2819:
2813:
2809:
2805:
2801:
2795:
2792:
2789:
2785:
2781:
2777:
2776:
2771:
2766:
2763:
2760:
2756:
2752:
2748:
2747:
2742:
2737:
2734:
2729:
2727:9780470687123
2723:
2719:
2715:
2711:
2704:
2701:
2694:
2689:
2686:
2684:
2681:
2678:
2675:
2673:
2670:
2668:
2665:
2663:
2660:
2658:
2655:
2653:
2650:
2649:
2645:
2643:
2641:
2637:
2633:
2629:
2621:
2619:
2617:
2613:
2611:
2607:
2602:
2600:
2596:
2592:
2588:
2584:
2583:hydrogenation
2580:
2573:
2571:
2569:
2565:
2561:
2557:
2553:
2549:
2548:chlorin group
2545:
2541:
2537:
2533:
2526:
2522:
2514:
2512:
2506:
2488:
2484:
2478:
2475:
2471:
2467:
2462:
2458:
2450:
2449:
2448:
2432:
2430:
2389:
2388:
2355:
2354:
2353:
2349:
2334:
2331:
2307:
2289:
2269:
2250:
2245:
2241:
2233:
2232:
2217:
2216:
2215:
2213:
2203:
2195:
2193:
2185:
2167:
2163:
2159:
2151:
2144:
2141:
2138:
2131:
2128:
2127:
2121:
2118:
2117:
2113:
2110:
2109:
2105:
2102:
2101:
2097:
2094:
2093:
2089:
2086:
2085:
2081:
2078:
2077:
2073:
2070:
2069:
2066:
2060:
2057:
2054:
2050:
2045:
2042:; NO becomes
2041:
2038:; CO becomes
2037:
2033:
2025:
2024:
2015:
2012:
2008:
2004:
2000:
1996:
1992:
1988:
1984:
1980:
1976:
1973:
1969:
1965:
1961:
1958:
1954:
1950:
1946:
1942:
1938:
1937:
1935:
1932:
1931:
1930:
1924:
1922:
1920:
1916:
1912:
1908:
1901:
1898:
1897:
1893:
1892:
1881:
1880:
1876:
1873:
1872:
1868:
1865:
1861:
1858:
1854:
1850:
1849:
1845:
1841:
1837:
1834:
1833:
1830:
1811:
1810:
1806:
1803:
1802:
1799:
1796:
1793:
1792:
1788:
1784:
1780:
1776:
1772:
1760:
1756:
1752:
1751:
1750:
1747:
1745:
1741:
1737:
1733:
1729:
1725:
1717:
1715:
1713:
1709:
1699:
1695:
1690:
1689:
1687:
1681:
1676:
1675:
1674:
1671:(Degenerate)
1670:
1665:
1661:
1657:
1653:
1650:
1649:
1647:
1646:
1645:
1639:
1637:
1635:
1631:
1625:
1623:
1619:
1615:
1608:
1604:
1593:
1589:
1583:
1575:
1573:
1570:
1566:
1562:
1558:
1557:crystal field
1553:
1545:
1527:
1513:
1491:
1477:
1470:
1469:
1460:
1452:
1444:
1436:
1428:
1420:
1413:
1412:
1403:
1395:
1387:
1379:
1371:
1363:
1356:
1355:
1346:
1340:
1332:
1324:
1316:
1308:
1305:
1304:
1295:
1287:
1279:
1271:
1263:
1255:
1253:(OH), dilute
1252:
1251:
1242:
1234:
1226:
1218:
1210:
1202:
1200:
1197:
1196:
1192:
1189:
1186:
1183:
1180:
1177:
1174:
1173:
1167:
1165:
1161:
1157:
1153:
1149:
1145:
1141:
1133:
1128:
1124:
1119:
1115:
1112:
1108:
1106:
1102:
1098:
1094:
1089:
1087:
1083:
1079:
1070:
1033:
1030:
995:
991:
987:
986:
982:
979:
920:
917:
883:
880:
879:
878:
876:
869:
853:
845:
840:
837:
826:
817:
812:
804:Δ-[Fe(ox)
799:
794:
791:
785:Λ-[Fe(ox)
779:
774:
771:
769:
768:
763:
762:
757:
753:
749:
742:
722:
715:
710:
693:
686:
681:
664:
657:
652:
635:
628:
623:
620:
618:
614:
610:
606:
602:
598:
594:
590:
586:
582:
581:square planar
578:
571:
569:
567:
560:
558:
556:
551:
549:
541:
536:
533:
530:
527:
524:
521:
520:
519:
516:
512:
507:
504:
496:
493:
490:
486:
483:
480:
476:
473:
470:
467:
466:
465:
458:
455:
452:
449:
446:
443:
440:
437:
434:
431:
428:
427:square planar
424:
421:
418:
415:
412:
409:
408:
407:
405:
401:
396:
391:
389:
385:
362:
358:
353:
351:
347:
339:
337:
335:
315:
311:
307:
303:
299:
298:covalent bond
295:
291:
287:
283:
274:
267:
265:
263:
259:
255:
250:
248:
247:Alfred Werner
243:
240:
235:
224:
220:
216:
215:Prussian blue
209:
208:Alfred Werner
205:
198:
196:
194:
188:
177:
173:
168:
166:
161:
159:
155:
151:
147:
143:
142:dipolar bonds
139:
135:
133:
128:
126:
122:
118:
109:
107:
105:
101:
97:
93:
89:
88:
83:
80:
76:
72:
68:
64:
60:
52:
36:
32:
19:
5279:
5267:
5255:
5243:
5093:Biosynthesis
4943:Geochemistry
4858:Pharmacology
4834:Cell biology
4824:Biochemistry
4694:
4652:Spectroscopy
4551:VSEPR theory
4400:Spectroscopy
4344:Branches of
4262:
3985:
3616:
3562:
3514:. Retrieved
3507:the original
3493:
3482:. Retrieved
3478:the original
3468:
3457:. Retrieved
3450:the original
3413:
3397:
3388:
3382:
3364:
3351:
3332:
3326:
3311:
3306:
3287:
3263:
3234:
3228:
3211:
3186:
3181:
3175:
3158:
3154:
3148:
3123:
3115:
3108:
3091:
3084:
3067:
3061:
3055:
3038:
3032:
3026:
3009:
3003:
2997:
2982:
2977:
2960:
2956:
2946:
2932:
2921:. Retrieved
2917:
2908:
2889:
2883:
2864:
2858:
2844:
2835:
2826:
2803:
2794:
2773:
2765:
2744:
2736:
2709:
2703:
2625:
2614:
2603:
2577:
2518:
2510:
2433:
2422:
2350:
2335:
2327:
2210:. It is the
2205:
2186:
2155:
2064:
2052:
2043:
2039:
2035:
2031:
2021:
2010:
2006:
2002:
1998:
1994:
1990:
1986:
1982:
1978:
1971:
1967:
1963:
1956:
1952:
1948:
1944:
1940:
1928:
1905:
1794:Examples: ,
1748:
1721:
1705:
1643:
1626:
1624:properties.
1622:ground state
1585:
1568:
1549:
1212:Yellow/brown
1199:Hydrated Ion
1137:
1113:
1109:
1105:group theory
1090:
1074:
1060:][Co(CN)
1044:][Cr(CN)
1031:
983:
881:
873:
851:
824:
765:
759:
746:
720:
691:
662:
633:
616:
612:
608:
604:
600:
596:
592:
588:
584:
575:
564:
552:
545:
508:
500:
485:Dodecahedral
462:
402:(see, e.g.,
392:
373:Pt[P(CMe
354:
343:
334:Zeise's salt
301:
279:
251:
244:
236:
222:
212:
189:
169:
162:
138:Coordination
137:
136:
129:
116:
113:
85:
74:
58:
56:
5281:WikiProject
4506:Theoretical
4491:Calorimetry
2552:chlorophyll
2544:cytochromes
2074:changed to
1864:chlorophyll
1736:lanthanides
1680:antibonding
1538:Precipitate
1524:Precipitate
1488:Precipitate
1457:Precipitate
1433:Precipitate
1425:Precipitate
1400:Precipitate
1376:Precipitate
1368:Precipitate
1337:Precipitate
1329:Precipitate
1321:Precipitate
1313:Precipitate
1300:Precipitate
1292:Precipitate
1284:Precipitate
1276:Precipitate
1268:Precipitate
1260:Precipitate
970:]Cl·2H
938:[CrCl(H
515:Jahn–Teller
423:Tetrahedral
384:lanthanides
365:[Mo(CN)
302:coordinated
172:monodentate
121:polydentate
5297:Categories
5117:Metallurgy
4816:Biological
4382:Analytical
3934:amino acid
3851:Si donors:
3516:2015-04-22
3484:2015-04-22
3459:2016-03-06
3155:Polyhedron
2923:2021-07-07
2695:References
2642:(ICP-MS).
2540:hemoglobin
2536:heme group
2090:aluminate
2087:aluminium
2082:cobaltate
2065:Examples:
2030:O becomes
1907:Mineralogy
1882:Example Ru
1853:hemoglobin
1844:porphyrins
1759:lone pairs
1744:metalloids
1640:Reactivity
1552:lanthanide
1535:Blue/green
1485:Dark green
1422:Dark green
1365:Dark green
1342:Colourless
1326:Blue/green
1310:Dark green
1273:Blue/green
1257:Dark green
1236:Colourless
1204:Pale green
1095:(LFT) and
1082:Hans Bethe
1052:[Cr(NH
1036:[Co(NH
901:[Co(NH
886:[Co(NH
854:-[CoCl
827:-[CoCl
723:-[CoCl
694:-[CoCl
665:-[CoCl
636:-[CoCl
439:Octahedral
395:polyhedron
268:Structures
117:donor atom
5179:Catalysis
4687:Inorganic
4481:Titration
4346:chemistry
4183:S donors:
4024:O donors:
3997:P donors:
3959:porphyrin
3906:imidazole
3883:N donors:
3679:C donors:
3648:B donors:
3624:H donors:
3161:: 47–57.
2712:. Wiley.
2591:oxidation
2568:cisplatin
2529:vitamin B
2106:vanadate
2103:vanadium
2098:chromate
2095:chromium
1972:tetrakis-
1899:Example:
1862:Example:
1855:contains
1851:Example:
1840:cofactors
1812:Example:
1740:actinides
1712:catalytic
1607:high-spin
1576:Magnetism
1446:Deep blue
1389:Deep blue
1361:, dilute
1344:Solution
958:[CrCl
923:[Cr(H
555:isomerism
542:Isomerism
388:actinides
318:[PtCl
262:chirality
146:molecules
92:compounds
82:molecules
35:Cisplatin
5245:Category
5201:Molecule
5138:See also
4563:Physical
3557:77760970
3203:11592184
3140:17308676
2646:See also
2622:Analysis
2574:Industry
2560:catalase
2122:ferrate
2114:cuprate
2044:nitrosyl
2040:carbonyl
2020:becomes
2007:chlorido
1993:becomes
1987:chlorido
1985:becomes
1983:chloride
1919:hydrates
1842:such as
1698:dioxygen
1618:low-spin
1588:magnetic
1465:Solution
1449:Solution
1441:Solution
1408:Solution
1392:Solution
1384:Solution
1351:Solution
1247:Solution
1239:Solution
1231:Solution
1223:Solution
1215:Solution
1207:Solution
1140:pigments
1101:covalent
340:Geometry
314:ethylene
310:pi bonds
100:titanium
71:metallic
5257:Commons
5221:Alchemy
4737:Organic
3797:& C
3419:plasmas
2751:complex
2634:(AAS),
2373:⇌ Ag(NH
2111:copper
2079:cobalt
2011:cyanido
1995:sulfato
1991:sulfate
1822:)Fe(CO)
1175:
994:nitrite
595:. In a
306:alkenes
282:ligands
199:History
104:d-block
87:ligands
51:ligands
5269:Portal
4415:UV-Vis
3705:=CH-CH
3555:
3423:alloys
3421:, and
3405:
3371:
3339:
3318:
3294:
3270:
3241:
3219:
3201:
3138:
2989:
2896:
2871:
2814:
2724:
2610:ammine
2554:, and
2546:, the
2542:, the
2534:, the
2367:
2071:metal
2023:ammine
1999:chloro
1974:, etc.
1953:penta-
1949:tetra-
1913:, and
1755:Werner
1742:, and
1724:alkali
1462:Purple
1405:Purple
1156:UV-Vis
761:lambda
615:and a
548:isomer
411:Linear
308:, the
286:L or X
37:, PtCl
4442:MALDI
4410:Raman
3696:HC(O)
3691:RC(O)
3510:(PDF)
3503:(PDF)
3453:(PDF)
3446:IUPAC
3442:(PDF)
2770:IUPAC
2741:IUPAC
2438:and K
2340:= 1/K
2226:⇌ zZ
2224:(aq)
2222:+ yL
2162:dimer
2119:iron
2003:cyano
1968:tris-
1957:hexa-
1955:, or
1765:O, NH
1656:inner
1507:Brown
1454:White
1430:Brown
1397:White
1373:Brown
1348:Green
1318:Brown
1297:Green
1289:White
1265:Brown
1244:Green
1026:CoONO
894:Br]SO
767:delta
663:trans
613:trans
589:trans
256:into
254:hexol
152:, or
150:atoms
79:bound
5196:Atom
4464:HPLC
4099:acac
4071:/HCO
3954:bipy
3713:C(CH
3553:OCLC
3403:ISBN
3369:ISBN
3337:ISBN
3316:ISBN
3292:ISBN
3268:ISBN
3239:ISBN
3217:ISBN
3199:PMID
3136:PMID
2987:ISBN
2894:ISBN
2869:ISBN
2812:ISBN
2722:ISBN
2523:and
2416:(aq)
2411:+ Cl
2407:(aq)
2402:⇌ Ag
2390:AgCl
2361:(aq)
2228:(aq)
2220:(aq)
2180:-Cl)
2053:-ate
2036:aquo
2032:aqua
2009:and
2001:and
1989:and
1964:bis-
1945:tri-
1886:(CO)
1857:heme
1726:and
1658:and
1569:e.g.
1529:CuCO
1521:Pink
1515:CoCO
1479:FeCO
1334:Blue
1281:Blue
1228:Blue
1220:Pink
1125:and
1050:and
1006:CoNO
858:(en)
831:(en)
607:. A
523:Bent
154:ions
63:atom
5206:Ion
4437:ICP
4420:NMR
4194:NCS
4171:OPR
4147:OSR
4126:ClO
4113:ONO
4091:RCO
3968:Si)
3964:(Me
3949:RCN
3916:RNO
3864:4−n
3862:SiR
3818:≡CR
3810:=CR
3805:RNC
3729:=CH
3543:doi
3191:doi
3163:doi
3128:doi
3122:".
3096:doi
3072:doi
3043:doi
3014:doi
2965:doi
2784:doi
2782:".
2755:doi
2753:".
2714:doi
2570:).
2550:in
2538:in
2519:In
2400:(l)
2394:+ H
2392:(s)
2365:+ 2
2218:xM
2164:of
2034:or
2026:; H
1941:di-
1710:or
1497:(CO
1471:(CO
1193:Cr
1190:Al
1187:Cu
1184:Co
1181:Fe
1178:Fe
1018:(NH
998:(NH
946:]Cl
931:]Cl
913:]Br
852:cis
825:cis
727:(NH
721:mer
698:(NH
692:fac
669:(NH
640:(NH
634:cis
617:cis
609:mer
605:mer
601:cis
597:fac
593:fac
585:cis
487:or
477:or
425:or
406:):
336:).
226:(CH
67:ion
65:or
41:(NH
5299::
4936:/
4932:/
4784:/
4459:GC
4432:EI
4405:IR
4298:Br
4293:Cl
4261:NC
4252:SR
4232:SO
4202:RS
4163:PO
4155:SO
4142:NO
4118:NO
4108:CO
4067:CO
4049:RO
4012:PR
4004:PR
3988:CS
3944:RN
3929:py
3921:NO
3911:NO
3890:NH
3874:Si
3799:70
3795:60
3768:CO
3763:CO
3758:CN
3737:RC
3725:CH
3701:CH
3655:BR
3535:2g
3444:.
3430:^
3359:;
3278:^
3253:^
3197:.
3187:40
3159:90
3157:.
3134:.
3068:17
3066:.
3039:16
3037:.
3010:14
3008:.
2961:44
2955:.
2916:.
2834:.
2810:.
2772:,
2743:,
2720:.
2589:,
2585:,
2531:12
2440:sp
2369:NH
2356:Ag
2184:.
2176:(μ
2172:Cl
2132:SO
1970:,
1966:,
1951:,
1947:,
1943:,
1909:,
1888:12
1826:CH
1814:(C
1797:,
1785:,
1779:en
1777:,
1775:CN
1773:,
1771:Cl
1769:,
1738:,
1734:,
1730:,
1611:CH
1603:Cl
1493:Fe
1475:)
1414:NH
1357:NH
1166:.
1009:2+
966:O)
962:(H
950:·H
942:O)
927:O)
909:SO
850:Δ-
823:Λ-
550:.
386:,
377:)]
330:)]
322:(C
264:.
234:.
176:en
148:,
57:A
5170:"
5166:"
4337:e
4330:t
4323:v
4303:I
4287:2
4285:F
4280:F
4263:S
4256:O
4254:2
4246:3
4244:O
4242:2
4240:S
4234:2
4226:2
4224:S
4222:2
4220:C
4218:2
4216:R
4211:S
4209:2
4207:R
4196:2
4192:2
4190:R
4173:3
4165:4
4157:4
4149:2
4140:5
4138:H
4136:5
4134:C
4128:4
4120:3
4106:2
4104:R
4093:2
4085:4
4083:O
4081:2
4079:C
4073:3
4069:3
4061:2
4059:O
4054:O
4044:O
4042:2
4040:R
4035:O
4033:2
4031:H
4014:2
4006:3
3986:N
3979:2
3977:N
3972:N
3970:2
3966:3
3939:N
3923:2
3900:3
3898:N
3892:3
3872:3
3870:R
3860:n
3858:H
3841:7
3839:H
3837:9
3835:C
3829:5
3827:H
3825:5
3823:C
3812:2
3793:C
3787:6
3785:R
3783:6
3781:C
3776:C
3770:2
3752:4
3750:H
3748:6
3746:C
3741:R
3739:2
3731:2
3727:2
3719:3
3717:)
3715:2
3707:2
3703:2
3686:R
3669:n
3667:H
3665:m
3663:B
3657:2
3638:2
3636:H
3631:H
3609:e
3602:t
3595:v
3545::
3539:a
3519:.
3487:.
3462:.
3425:.
3345:.
3300:.
3247:.
3223:.
3205:.
3193::
3169:.
3165::
3142:.
3130::
3119:4
3116:τ
3102:.
3098::
3078:.
3074::
3049:.
3045::
3020:.
3016::
2971:.
2967::
2940:.
2926:.
2902:.
2877:.
2838:.
2820:.
2786::
2757::
2730:.
2716::
2489:f
2485:K
2479:p
2476:s
2472:K
2468:=
2463:c
2459:K
2445:c
2443:K
2436:f
2425:4
2398:O
2396:2
2382:2
2377:)
2375:3
2371:3
2346:d
2342:f
2338:d
2308:y
2304:]
2299:L
2295:[
2290:x
2286:]
2281:M
2277:[
2270:z
2266:]
2261:Z
2257:[
2251:=
2246:f
2242:K
2208:f
2190:2
2188:H
2182:2
2178:2
2174:4
2170:2
2158:μ
2147:4
2145:K
2134:4
2046:.
2028:2
2018:3
2013:.
1979:o
1959:.
1884:3
1846:.
1828:3
1824:2
1820:5
1818:H
1816:5
1789:,
1767:3
1763:2
1628:(
1613:3
1599:2
1596:2
1531:3
1517:3
1503:3
1501:)
1499:3
1495:2
1481:3
1473:3
1416:3
1359:3
1129:.
1066:.
1064:]
1062:6
1058:6
1056:)
1054:3
1048:]
1046:6
1042:6
1040:)
1038:3
1024:5
1022:)
1020:3
1012:2
1004:5
1002:)
1000:3
980:.
974:O
972:2
968:4
964:2
960:2
954:O
952:2
948:2
944:5
940:2
933:3
929:6
925:2
911:4
907:5
905:)
903:3
896:4
892:5
890:)
888:3
862:]
860:2
856:2
835:]
833:2
829:2
808:]
806:3
789:]
787:3
735:]
733:3
731:)
729:3
725:3
706:]
704:3
702:)
700:3
696:3
677:]
675:4
673:)
671:3
667:2
648:]
646:4
644:)
642:3
638:2
379:2
375:3
369:]
367:8
332:(
328:4
326:H
324:2
320:3
232:X
230:)
228:2
183:2
178:)
47:2
45:)
43:3
39:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.