224:
201:
2834:
475:
230:
3685:
988:
40:
1312:
residues within the structural framework of CPOX. At least 32 of these mutations are considered to be disease-causing mutations. In terms of the molecular basis of HCP and harderoporphyria, mutations of CPOX in patients with harderoporphyria were demonstrated in the region of exon 6, where mutations
66:
1300:
has been recorded in biochemical tests. HCP is an autosomal dominant inherited disorder, whereas harderoporphyria is a rare erythropoietic variant form of HCP and is inherited in an autosomal recessive fashion. Clinically, it is characterized by neonatal haemolytic
1291:
are two phenotypically separate disorders that concern partial deficiency of CPOX. Neurovisceral symptomatology predominates in HCP. Additionally, it may be associated with abdominal pain and/or skin photosensitivity. Hyper-excretion of coproporphyrin III in
2140:
Fujita H, Kondo M, Taketani S, Nomura N, Furuyama K, Akagi R, Nagai T, Terajima M, Galbraith RA, Sassa S (October 1994). "Characterization and expression of cDNA encoding coproporphyrinogen oxidase from a patient with hereditary coproporphyria".
1854:
Kim DH, Hino R, Adachi Y, Kobori A, Taketani S (December 2013). "The enzyme engineering of mutant homodimer and heterodimer of coproporphyinogen oxidase contributes to new insight into hereditary coproporphyria and harderoporphyria".
2099:
Heyer NJ, Bittner AC, Echeverria D, Woods JS (February 2006). "A cascade analysis of the interaction of mercury and coproporphyrinogen oxidase (CPOX) polymorphism on the heme biosynthetic pathway and porphyrin production".
3176:
1415:
Lamoril J, Martasek P, Deybach JC, Da Silva V, Grandchamp B, Nordmann Y (February 1995). "A molecular defect in coproporphyrinogen oxidase gene causing harderoporphyria, a variant form of hereditary coproporphyria".
2391:
Daimon M, Gojyou E, Sugawara M, Yamatani K, Tominaga M, Sasaki H (February 1997). "A novel missense mutation in exon 4 of the human coproporphyrinogen oxidase gene in two patients with hereditary coproporphyria".
2833:
2236:
Martasek P, Nordmann Y, Grandchamp B (March 1994). "Homozygous hereditary coproporphyria caused by an arginine to tryptophane substitution in coproporphyrinogen oxidase and common intragenic polymorphisms".
1783:
Martasek P, Nordmann Y, Grandchamp B (March 1994). "Homozygous hereditary coproporphyria caused by an arginine to tryptophane substitution in coproporphyrinogen oxidase and common intragenic polymorphisms".
2645:"Characterization of mutations in the CPO gene in British patients demonstrates absence of genotype-phenotype correlation and identifies relationship between hereditary coproporphyria and harderoporphyria"
2725:"Two novel mutations and coexistence of the 991C>T and the 1339C>T mutation on a single allele in the coproporphyrinogen oxidase gene in Swedish patients with hereditary coproporphyria"
2170:
Cacheux V, Martasek P, Fougerousse F, Delfau MH, Druart L, Tachdjian G, Grandchamp B (November 1994). "Localization of the human coproporphyrinogen oxidase gene to chromosome band 3q12".
1313:
in those with HCP were also identified. As only patients with mutation in this region (K404E) would develop harderoporphyria, this mutation led to diminishment of the second step of the
2874:
2472:
Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library".
3070:
2536:
Susa S, Daimon M, Kondo H, Kondo M, Yamatani K, Sasaki H (November 1998). "Identification of a novel mutation of the CPO gene in a
Japanese hereditary coproporphyria family".
1819:
Taketani S, Kohno H, Furukawa T, Yoshinaga T, Tokunaga R (January 1994). "Molecular cloning, sequencing and expression of cDNA encoding human coproporphyrinogen oxidase".
237:
2058:
Schmitt C, Gouya L, Malonova E, Lamoril J, Camadro JM, Flamme M, Rose C, Lyoumi S, Da Silva V, Boileau C, Grandchamp B, Beaumont C, Deybach JC, Puy H (October 2005).
1079:
3109:
2207:
Delfau-Larue MH, Martasek P, Grandchamp B (August 1994). "Coproporphyrinogen oxidase: gene organization and description of a mutation leading to exon 6 skipping".
3143:
2686:
Elkon H, Don J, Melamed E, Ziv I, Shirvan A, Offen D (June 2002). "Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase".
2867:
723:
1605:
Guo R, Lim CK, Peters TJ (October 1988). "Accurate and specific HPLC assay of coproporphyrinogen III oxidase activity in human peripheral leucocytes".
704:
3286:
1512:
148:
2266:
Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides".
2501:
Lamoril J, Puy H, Gouya L, Rosipal R, Da Silva V, Grandchamp B, Foint T, Bader-Meunier B, Dommergues JP, Deybach JC, Nordmann Y (February 1998).
1893:
Hasanoglu A, Balwani M, Kasapkara CS, Ezgü FS, Okur I, Tümer L, Cakmak A, Nazarenko I, Yu C, Clavero S, Bishop DF, Desnick RJ (February 2011).
2860:
2813:
1397:
1379:
3156:
3152:
1640:
Madsen O, Sandal L, Sandal NN, Marcker KA (October 1993). "A soybean coproporphyrinogen oxidase gene is highly expressed in root nodules".
1305:. Sometimes, the presence of skin lesions with marked faecal excretion of harderoporphyrin is also described in harderoporphyric patients.
2431:"Hereditary coproporphyria: exon screening by heteroduplex analysis detects three novel mutations in the coproporphyrinogen oxidase gene"
223:
63:
3371:
3102:
2549:
2060:"Mutations in human CPO gene predict clinical expression of either hepatic hereditary coproporphyria or erythropoietic harderoporphyria"
2760:
2969:
926:
200:
3404:
3244:
3139:
2447:
2430:
2583:
2566:
2366:
2349:
1498:
1366:
3161:
3095:
3560:
1362:
1099:
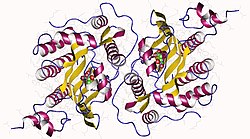
2842:: The 1.58A Crystal Structure of Human Coproporphyrinogen Oxidase Reveals the Structural Basis of Hereditary Coproporphyria
3239:
2964:
3675:
2003:"Refinement of evolutionary medicine predictions based on clinical evidence for the manifestations of Mendelian diseases"
3366:
3199:
2922:
128:
3229:
2565:
Rosipal R, Lamoril J, Puy H, Da Silva V, Gouya L, De Rooij FW, Te Velde K, Nordmann Y, Martàsek P, Deybach JC (1999).
1334:
1456:"Coproporphyrinogen oxidase. Purification, molecular cloning, and induction of mRNA during erythroid differentiation"
236:
3361:
3249:
3209:
2994:
2295:
Martasek P, Camadro JM, Delfau-Larue MH, Dumas JB, Montagne JJ, de
Verneuil H, Labbe P, Grandchamp B (April 1994).
1724:
Martasek P, Camadro JM, Delfau-Larue MH, Dumas JB, Montagne JJ, de
Verneuil H, Labbe P, Grandchamp B (April 1994).
1325:, implying that the active site of the enzyme involved in the second step of decarboxylation is located in exon 6.
1308:
To date, over 50 CPOX mutations causing HCP have been described. Most of these mutations result in substitution of
3545:
3122:
3726:
3661:
3648:
3635:
3622:
3609:
3596:
3583:
3317:
3303:
3259:
3186:
3171:
3135:
2959:
2806:
2567:"Systematic analysis of coproporphyrinogen oxidase gene defects in hereditary coproporphyria and mutation update"
1284:
1134:
229:
3555:
3509:
3452:
3126:
2954:
2785:
766:
172:
136:
2297:"Molecular cloning, sequencing, and functional expression of a cDNA encoding human coproporphyrinogen oxidase"
1726:"Molecular cloning, sequencing, and functional expression of a cDNA encoding human coproporphyrinogen oxidase"
1087:
3457:
3271:
3064:
2852:
2781:
2503:"Neonatal hemolytic anemia due to inherited harderoporphyria: clinical characteristics and molecular basis"
1520:
3322:
2610:"Expression of coproporphyrinogen oxidase and synthesis of hemoglobin in human erythroleukemia K562 cells"
1185:
1157:
1145:
3478:
3397:
3224:
1322:
1265:
1189:
1149:
3550:
1083:
3721:
3234:
3166:
3042:
2799:
2308:
2014:
1955:
1737:
115:
3514:
1685:"Purification and properties of coproporphyrinogen oxidase from the yeast Saccharomyces cerevisiae"
1318:
3087:
3447:
3312:
2887:
2711:
2596:
2460:
2417:
2379:
2195:
1665:
160:
904:
883:
857:
836:
2643:
Lamoril J, Puy H, Whatley SD, Martin C, Woolf JR, Da Silva V, Deybach JC, Elder GH (May 2001).
3339:
3214:
2746:
2703:
2674:
2631:
2588:
2553:
2524:
2489:
2452:
2409:
2371:
2336:
2283:
2254:
2224:
2187:
2158:
2117:
2081:
2040:
1983:
1924:
1872:
1836:
1801:
1765:
1706:
1657:
1622:
1587:
1477:
1433:
1338:
1074:
108:
56:
1895:"Harderoporphyria due to homozygosity for coproporphyrinogen oxidase missense mutation H327R"
3493:
3488:
3462:
3390:
3219:
2736:
2695:
2664:
2656:
2621:
2578:
2545:
2514:
2481:
2442:
2401:
2361:
2326:
2316:
2275:
2246:
2216:
2179:
2150:
2109:
2071:
2030:
2022:
1973:
1963:
1914:
1906:
1864:
1828:
1793:
1755:
1745:
1696:
1649:
1614:
1577:
1467:
1425:
1288:
316:
247:
191:
1066:
3540:
3524:
3437:
1314:
1181:
1153:
474:
291:
178:
156:
17:
2312:
2018:
1959:
1741:
3689:
3578:
3519:
3118:
3037:
2999:
2669:
2644:
2035:
2002:
1978:
1943:
1919:
1894:
1701:
1684:
2485:
1582:
1565:
1472:
1455:
638:
633:
628:
623:
618:
613:
608:
603:
598:
593:
577:
572:
567:
562:
557:
552:
536:
531:
526:
521:
516:
3715:
3483:
3442:
2768:
2626:
2609:
2331:
2296:
2279:
1832:
1760:
1725:
1618:
503:
2715:
2464:
2383:
2199:
1669:
1016:
3432:
2600:
2421:
1253:
1248:
Human CPOX is a mitochondrial enzyme encoded by a 14 kb CPOX gene containing seven
1062:
309:
140:
1942:
Lee DS, Flachsová E, Bodnárová M, Demeler B, Martásek P, Raman CS (October 2005).
3705:
2113:
1028:
3656:
3591:
3427:
1402:
National Center for
Biotechnology Information, U.S. National Library of Medicine
1384:
National Center for
Biotechnology Information, U.S. National Library of Medicine
1269:
1232:
1197:
1161:
3684:
2699:
2301:
Proceedings of the
National Academy of Sciences of the United States of America
2026:
1948:
Proceedings of the
National Academy of Sciences of the United States of America
1730:
Proceedings of the
National Academy of Sciences of the United States of America
1133:
in animals. The medical condition associated with this enzyme defect is called
392:
1910:
1309:
1228:
208:
144:
2519:
2502:
1227:
acceptor. The enzyme is widely distributed having been found in a variety of
3630:
3604:
2903:
2895:
2764:
2759:
2154:
1968:
1273:
1177:
1173:
668:
275:
262:
152:
2750:
2707:
2678:
2635:
2550:
10.1002/(SICI)1096-8628(19981116)80:3<204::AID-AJMG4>3.0.CO;2-G
2321:
2220:
2121:
2085:
2044:
1987:
1928:
1876:
1750:
1591:
2741:
2724:
2592:
2557:
2528:
2493:
2456:
2413:
2405:
2375:
2340:
2287:
2258:
2250:
2228:
2191:
2162:
1840:
1805:
1797:
1769:
1710:
1661:
1626:
1481:
1437:
1429:
987:
966:
961:
3701:
2076:
2059:
1454:
Kohno H, Furukawa T, Yoshinaga T, Tokunaga R, Taketani S (October 1993).
1224:
1212:
1023:
950:
811:
792:
1868:
3263:
2448:
10.1002/(SICI)1098-1004(1997)10:3<196::AID-HUMU3>3.0.CO;2-H
2183:
1653:
1566:"Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation"
1337:
with the atypical keto-isocoproporphyrin (KICP) in human subjects with
1302:
1201:
1040:
1035:
778:
735:
2584:
10.1002/(SICI)1098-1004(1999)13:1<44::AID-HUMU5>3.0.CO;2-Q
2367:
10.1002/(SICI)1098-1004(1997)9:1<78::AID-HUMU17>3.0.CO;2-M
85:
3643:
3413:
3354:
3349:
3344:
3327:
3190:
2883:
1297:
1268:
and contains an amino terminal mitochondrial targeting signal. After
1220:
1216:
1119:
1094:
934:
690:
164:
1129:. A genetic defect in the enzyme results in a reduced production of
2660:
1542:
3617:
3332:
2932:
2927:
2758:
1293:
1249:
1607:
Clinica
Chimica Acta; International Journal of Clinical Chemistry
653:
649:
3697:
3291:
3281:
3276:
3018:
3013:
2891:
1208:
1205:
1193:
1141:
1130:
1126:
1056:
1011:
132:
3386:
3091:
2856:
2795:
2348:
Lamoril J, Deybach JC, Puy H, Grandchamp B, Nordmann Y (1997).
483:
39:
2791:
2350:"Three novel mutations in the coproporphyrinogen oxidase gene"
452:
330:
2763:
Heme synthesis—note that some reactions occur in the
3382:
1272:
processing, the protein is present as a mature form of a
639:
protoporphyrinogen IX biosynthetic process from glutamate
299:
119:, CPO, CPX, HCP, coproporphyrinogen oxidase, COX, HARPO
3696:
This article incorporates text from the public domain
3673:
1683:
Camadro JM, Chambon H, Jolles J, Labbe P (May 1986).
464:
3177:
Dihydroxymethyloxo-tetrahydroquinoline dehydrogenase
992:
coproporphyrinogen iii oxidase from leishmania major
3569:
3533:
3502:
3471:
3420:
3302:
3258:
3185:
3134:
3052:
3026:
3012:
2978:
2943:
2911:
2902:
1821:
Biochimica et
Biophysica Acta (BBA) - Bioenergetics
1093:
1073:
1055:
1050:
1034:
1022:
1010:
1002:
997:
980:
897:
876:
850:
829:
1358:
1356:
1354:
1156:. The activity of the CPOX enzyme, located in the
604:porphyrin-containing compound biosynthetic process
2608:Taketani S, Furukawa T, Furuyama K (March 2001).
246:
2429:Schreiber WE, Zhang X, Senz J, Jamani A (1997).
1172:CPOX is an enzyme involved in the sixth step of
1944:"Structural basis of hereditary coproporphyria"
1888:
1886:
1519:. National Institutes of Health. Archived from
1204:is a homodimer containing two internally bound
1499:"Entrez Gene: CPOX coproporphyrinogen oxidase"
1363:GRCm38: Ensembl release 89: ENSMUSG00000022742
3398:
3103:
2868:
2807:
1449:
1447:
1112:Coproporphyrinogen-III oxidase, mitochondrial
8:
1517:Genetic and Rare Diseases Information Center
3405:
3391:
3383:
3110:
3096:
3088:
3023:
2908:
2875:
2861:
2853:
2814:
2800:
2792:
1152:through two sequential steps of oxidative
1047:
986:
664:
599:protoporphyrinogen IX biosynthetic process
499:
287:
186:
74:
2784:at the U.S. National Library of Medicine
2740:
2668:
2625:
2582:
2518:
2446:
2365:
2330:
2320:
2075:
2034:
2001:Šimčíková D, Heneberg P (December 2019).
1977:
1967:
1918:
1759:
1749:
1700:
1581:
1471:
415:fetal liver hematopoietic progenitor cell
1493:
1491:
594:response to arsenic-containing substance
3680:
2829:
1350:
27:Mammalian protein found in Homo sapiens
2723:Wiman A, Floderus Y, Harper P (2002).
1899:Journal of Inherited Metabolic Disease
977:
29:
251:
212:
207:
7:
3153:Enoyl-acyl carrier protein reductase
2538:American Journal of Medical Genetics
3372:3-oxo-5beta-steroid 4-dehydrogenase
3367:Isovaleryl coenzyme A dehydrogenase
1570:The Journal of Biological Chemistry
1460:The Journal of Biological Chemistry
517:coproporphyrinogen oxidase activity
2970:Uroporphyrinogen III decarboxylase
2649:American Journal of Human Genetics
1702:10.1111/j.1432-1033.1986.tb09617.x
1317:reaction during the conversion of
894:
873:
847:
826:
802:
783:
757:
740:
714:
695:
527:structural constituent of eye lens
469:
387:
325:
304:
25:
3245:Pyrroloquinoline-quinone synthase
2688:Journal of Molecular Neuroscience
1276:with a molecular mass of 37 kDa.
1122:that in humans is encoded by the
558:mitochondrial intermembrane space
522:protein homodimerization activity
3683:
2832:
2627:10.1046/j.1432-1327.2001.02045.x
2614:European Journal of Biochemistry
1689:European Journal of Biochemistry
1564:Sano S, Granick S (April 1961).
473:
361:inferior ganglion of vagus nerve
235:
228:
222:
199:
38:
2782:Coproporphyrinogen+III+Oxidases
1144:biosynthetic pathway, converts
624:response to inorganic substance
3205:Coproporphyrinogen III oxidase
3162:7-Dehydrocholesterol reductase
2990:Coproporphyrinogen III oxidase
1264:CPOX is expressed as a 40 kDa
1140:CPOX, the sixth enzyme of the
484:More reference expression data
453:More reference expression data
1:
3240:Tryptophan alpha,beta-oxidase
2965:Uroporphyrinogen III synthase
2486:10.1016/S0378-1119(97)00411-3
1583:10.1016/S0021-9258(18)64262-0
1473:10.1016/S0021-9258(19)36931-5
1219:is active in the presence of
1051:Available protein structures:
423:epithelium of small intestine
220:
3200:Dihydroorotate dehydrogenase
2923:Aminolevulinic acid synthase
2280:10.1016/0378-1119(94)90802-8
2114:10.1016/j.toxlet.2005.09.005
1833:10.1016/0005-2728(94)90083-3
1619:10.1016/0009-8981(88)90069-1
568:mitochondrial inner membrane
3230:Tetrahydroberberine oxidase
1513:"Hereditary coproporphyria"
1200:biosynthetic pathways. The
3743:
3695:
3362:Glutaryl-CoA dehydrogenase
3250:L-galactonolactone oxidase
3210:Protoporphyrinogen oxidase
2995:Protoporphyrinogen oxidase
2027:10.1038/s41598-019-54976-4
18:Coproporphyrinogen oxidase
3561:Michaelis–Menten kinetics
3318:Butyryl-CoA dehydrogenase
3172:2,4 Dienoyl-CoA reductase
2960:Porphobilinogen deaminase
2827:
2729:Journal of Human Genetics
1911:10.1007/s10545-010-9237-9
1545:. Genetics Home Reference
1398:"Mouse PubMed Reference:"
1380:"Human PubMed Reference:"
1285:Hereditary coproporphyria
1135:hereditary coproporphyria
1046:
985:
965:
960:
956:
949:
933:
916:
901:
880:
869:
854:
833:
822:
809:
805:
790:
786:
777:
764:
760:
747:
743:
734:
721:
717:
702:
698:
689:
674:
667:
663:
647:
634:response to methylmercury
614:heme biosynthetic process
532:identical protein binding
502:
498:
481:
472:
463:
450:
399:
390:
337:
328:
298:
290:
286:
269:
256:
219:
198:
189:
185:
176:
171:
126:
123:
113:
106:
101:
82:
77:
60:
55:
50:
46:
37:
32:
3453:Diffusion-limited enzyme
2955:Porphobilinogen synthase
2786:Medical Subject Headings
2520:10.1182/blood.V91.4.1453
2239:Human Molecular Genetics
2209:Human Molecular Genetics
2143:Human Molecular Genetics
2064:Human Molecular Genetics
1786:Human Molecular Genetics
1418:Human Molecular Genetics
927:Chr 16: 58.49 – 58.54 Mb
3272:Succinate dehydrogenase
3065:glucuronosyltransferase
1969:10.1073/pnas.0506557102
1857:Journal of Biochemistry
1642:Plant Molecular Biology
1333:CPOX has been shown to
1215:of native protein. The
629:response to insecticide
537:oxidoreductase activity
3323:Acyl CoA dehydrogenase
2904:Porphyrin biosynthesis
2772:
2322:10.1073/pnas.91.8.3024
1751:10.1073/pnas.91.8.3024
1190:proto-porphyrinogen IX
1186:coproporphyrinogen III
1158:mitochondrial membrane
1146:coproporphyrinogen III
435:ventral tegmental area
3546:Eadie–Hofstee diagram
3479:Allosteric regulation
3225:Dihydrouracil oxidase
3123:CH–CH oxidoreductases
2762:
2742:10.1007/s100380200059
2406:10.1007/s004390050338
2155:10.1093/hmg/3.10.1807
1280:Clinical significance
1150:protoporphyrinogen IX
214:Chromosome 16 (mouse)
3556:Lineweaver–Burk plot
3235:Secologanin synthase
3167:Biliverdin reductase
3043:Biliverdin reductase
2914:early mitochondrial:
2700:10.1385/JMN:18:3:229
2221:10.1093/hmg/3.8.1325
619:response to lead ion
609:response to iron ion
78:List of PDB id codes
51:Available structures
3157:Enoyl ACP reductase
2981:late mitochondrial:
2313:1994PNAS...91.3024M
2251:10.1093/hmg/3.3.477
2019:2019NatSR...918577S
1960:2005PNAS..10214232L
1798:10.1093/hmg/3.3.477
1742:1994PNAS...91.3024M
1430:10.1093/hmg/4.2.275
353:right lobe of liver
3515:Enzyme superfamily
3448:Enzyme promiscuity
3313:Fumarate reductase
2773:
2184:10.1007/BF00211026
2102:Toxicology Letters
2077:10.1093/hmg/ddi342
2007:Scientific Reports
1654:10.1007/BF00021417
1323:protoporphyrinogen
1319:coproporphyrinogen
767:ENSMUSG00000022742
587:Biological process
546:Cellular component
510:Molecular function
411:tibiofemoral joint
3671:
3670:
3380:
3379:
3306:: Other acceptors
3215:Bilirubin oxidase
3085:
3084:
3081:
3080:
3008:
3007:
2850:
2849:
1869:10.1093/jb/mvt086
1160:, is measured in
1109:
1108:
1105:
1104:
1100:structure summary
976:
975:
972:
971:
945:
944:
912:
911:
891:
890:
865:
864:
844:
843:
818:
817:
799:
798:
773:
772:
754:
753:
730:
729:
711:
710:
659:
658:
494:
493:
490:
489:
459:
458:
446:
445:
384:
383:
365:bone marrow cells
282:
281:
97:
96:
93:
92:
61:Ortholog search:
16:(Redirected from
3734:
3727:Protein families
3688:
3687:
3679:
3551:Hanes–Woolf plot
3494:Enzyme activator
3489:Enzyme inhibitor
3463:Enzyme catalysis
3407:
3400:
3393:
3384:
3220:Acyl-CoA oxidase
3112:
3105:
3098:
3089:
3057:
3031:
3024:
3014:Heme degradation
2983:
2948:
2916:
2909:
2886:involved in the
2877:
2870:
2863:
2854:
2836:
2816:
2809:
2802:
2793:
2767:and some in the
2754:
2744:
2719:
2682:
2672:
2639:
2629:
2604:
2586:
2561:
2532:
2522:
2497:
2468:
2450:
2425:
2387:
2369:
2344:
2334:
2324:
2291:
2262:
2232:
2203:
2166:
2126:
2125:
2096:
2090:
2089:
2079:
2055:
2049:
2048:
2038:
1998:
1992:
1991:
1981:
1971:
1939:
1933:
1932:
1922:
1890:
1881:
1880:
1851:
1845:
1844:
1816:
1810:
1809:
1780:
1774:
1773:
1763:
1753:
1721:
1715:
1714:
1704:
1680:
1674:
1673:
1637:
1631:
1630:
1602:
1596:
1595:
1585:
1561:
1555:
1554:
1552:
1550:
1539:
1533:
1532:
1530:
1528:
1523:on 7 August 2012
1509:
1503:
1502:
1495:
1486:
1485:
1475:
1466:(28): 21359–63.
1451:
1442:
1441:
1412:
1406:
1405:
1394:
1388:
1387:
1376:
1370:
1360:
1289:harderoporphyria
1223:that acts as an
1221:molecular oxygen
1114:(abbreviated as
1048:
1006:Coprogen oxidase
990:
981:Coprogen oxidase
978:
958:
957:
929:
922:
907:
895:
886:
874:
870:RefSeq (protein)
860:
848:
839:
827:
803:
784:
758:
741:
715:
696:
665:
500:
486:
477:
470:
455:
395:
393:Top expressed in
388:
333:
331:Top expressed in
326:
305:
288:
278:
265:
254:
239:
232:
226:
215:
203:
187:
181:
167:
165:CPOX - orthologs
118:
111:
88:
75:
69:
48:
47:
42:
30:
21:
3742:
3741:
3737:
3736:
3735:
3733:
3732:
3731:
3712:
3711:
3708:
3694:
3682:
3674:
3672:
3667:
3579:Oxidoreductases
3565:
3541:Enzyme kinetics
3529:
3525:List of enzymes
3498:
3467:
3438:Catalytic triad
3416:
3411:
3381:
3376:
3298:
3254:
3181:
3130:
3119:Oxidoreductases
3116:
3086:
3077:
3053:
3048:
3045:
3027:
3016:
3004:
2979:
2974:
2944:
2939:
2912:
2898:
2881:
2851:
2846:
2843:
2837:
2823:
2820:
2778:
2757:
2722:
2685:
2642:
2607:
2564:
2535:
2500:
2480:(1–2): 149–56.
2471:
2428:
2390:
2347:
2294:
2265:
2235:
2206:
2169:
2149:(10): 1807–10.
2139:
2135:
2133:Further reading
2130:
2129:
2098:
2097:
2093:
2070:(20): 3089–98.
2057:
2056:
2052:
2000:
1999:
1995:
1954:(40): 14232–7.
1941:
1940:
1936:
1892:
1891:
1884:
1853:
1852:
1848:
1818:
1817:
1813:
1782:
1781:
1777:
1723:
1722:
1718:
1682:
1681:
1677:
1639:
1638:
1634:
1604:
1603:
1599:
1563:
1562:
1558:
1548:
1546:
1541:
1540:
1536:
1526:
1524:
1511:
1510:
1506:
1497:
1496:
1489:
1453:
1452:
1445:
1414:
1413:
1409:
1396:
1395:
1391:
1378:
1377:
1373:
1361:
1352:
1347:
1341:(Hg) exposure.
1331:
1315:decarboxylation
1282:
1262:
1246:
1241:
1182:decarboxylation
1170:
1154:decarboxylation
993:
967:View/Edit Mouse
962:View/Edit Human
925:
920:
917:Location (UCSC)
903:
882:
856:
835:
643:
582:
541:
482:
451:
442:
437:
433:
429:
425:
421:
417:
413:
409:
405:
391:
380:
375:
371:
367:
363:
359:
355:
351:
349:corpus callosum
347:
343:
341:trabecular bone
329:
273:
260:
253:16|16 C1.2
252:
242:
241:
240:
233:
213:
190:Gene location (
177:
127:
114:
107:
84:
62:
28:
23:
22:
15:
12:
11:
5:
3740:
3738:
3730:
3729:
3724:
3714:
3713:
3693:
3692:
3669:
3668:
3666:
3665:
3652:
3639:
3626:
3613:
3600:
3587:
3573:
3571:
3567:
3566:
3564:
3563:
3558:
3553:
3548:
3543:
3537:
3535:
3531:
3530:
3528:
3527:
3522:
3517:
3512:
3506:
3504:
3503:Classification
3500:
3499:
3497:
3496:
3491:
3486:
3481:
3475:
3473:
3469:
3468:
3466:
3465:
3460:
3455:
3450:
3445:
3440:
3435:
3430:
3424:
3422:
3418:
3417:
3412:
3410:
3409:
3402:
3395:
3387:
3378:
3377:
3375:
3374:
3369:
3364:
3359:
3358:
3357:
3352:
3347:
3337:
3336:
3335:
3330:
3320:
3315:
3309:
3307:
3300:
3299:
3297:
3296:
3295:
3294:
3289:
3284:
3279:
3268:
3266:
3256:
3255:
3253:
3252:
3247:
3242:
3237:
3232:
3227:
3222:
3217:
3212:
3207:
3202:
3196:
3194:
3183:
3182:
3180:
3179:
3174:
3169:
3164:
3159:
3149:
3147:
3132:
3131:
3117:
3115:
3114:
3107:
3100:
3092:
3083:
3082:
3079:
3078:
3076:
3075:
3074:
3073:
3060:
3058:
3050:
3049:
3047:
3046:
3040:
3038:Heme oxygenase
3034:
3032:
3021:
3010:
3009:
3006:
3005:
3003:
3002:
3000:Ferrochelatase
2997:
2992:
2986:
2984:
2976:
2975:
2973:
2972:
2967:
2962:
2957:
2951:
2949:
2941:
2940:
2938:
2937:
2936:
2935:
2930:
2919:
2917:
2906:
2900:
2899:
2882:
2880:
2879:
2872:
2865:
2857:
2848:
2847:
2845:
2844:
2838:
2831:
2828:
2825:
2824:
2821:
2819:
2818:
2811:
2804:
2796:
2790:
2789:
2777:
2776:External links
2774:
2756:
2755:
2720:
2683:
2661:10.1086/320118
2640:
2620:(6): 1705–11.
2605:
2571:Human Mutation
2562:
2533:
2498:
2469:
2441:(3): 196–200.
2435:Human Mutation
2426:
2400:(2): 199–201.
2394:Human Genetics
2388:
2354:Human Mutation
2345:
2292:
2274:(1–2): 171–4.
2263:
2233:
2215:(8): 1325–30.
2204:
2172:Human Genetics
2167:
2136:
2134:
2131:
2128:
2127:
2091:
2050:
1993:
1934:
1882:
1846:
1811:
1775:
1716:
1675:
1632:
1597:
1576:(4): 1173–80.
1556:
1534:
1504:
1487:
1443:
1407:
1389:
1371:
1349:
1348:
1346:
1343:
1330:
1327:
1281:
1278:
1261:
1258:
1245:
1242:
1240:
1237:
1180:the oxidative
1176:metabolism it
1169:
1166:
1107:
1106:
1103:
1102:
1097:
1091:
1090:
1077:
1071:
1070:
1060:
1053:
1052:
1044:
1043:
1038:
1032:
1031:
1026:
1020:
1019:
1014:
1008:
1007:
1004:
1000:
999:
995:
994:
991:
983:
982:
974:
973:
970:
969:
964:
954:
953:
947:
946:
943:
942:
940:
938:
931:
930:
923:
918:
914:
913:
910:
909:
899:
898:
892:
889:
888:
878:
877:
871:
867:
866:
863:
862:
852:
851:
845:
842:
841:
831:
830:
824:
820:
819:
816:
815:
807:
806:
800:
797:
796:
788:
787:
781:
775:
774:
771:
770:
762:
761:
755:
752:
751:
745:
744:
738:
732:
731:
728:
727:
719:
718:
712:
709:
708:
700:
699:
693:
687:
686:
681:
676:
672:
671:
661:
660:
657:
656:
645:
644:
642:
641:
636:
631:
626:
621:
616:
611:
606:
601:
596:
590:
588:
584:
583:
581:
580:
575:
570:
565:
560:
555:
549:
547:
543:
542:
540:
539:
534:
529:
524:
519:
513:
511:
507:
506:
496:
495:
492:
491:
488:
487:
479:
478:
467:
461:
460:
457:
456:
448:
447:
444:
443:
441:
440:
436:
432:
431:pontine nuclei
428:
424:
420:
416:
412:
408:
404:
400:
397:
396:
385:
382:
381:
379:
378:
374:
370:
366:
362:
358:
354:
350:
346:
345:jejunal mucosa
342:
338:
335:
334:
322:
321:
313:
302:
296:
295:
292:RNA expression
284:
283:
280:
279:
271:
267:
266:
258:
255:
250:
244:
243:
234:
227:
221:
217:
216:
211:
205:
204:
196:
195:
183:
182:
175:
169:
168:
125:
121:
120:
112:
104:
103:
99:
98:
95:
94:
91:
90:
80:
79:
71:
70:
59:
53:
52:
44:
43:
35:
34:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
3739:
3728:
3725:
3723:
3720:
3719:
3717:
3710:
3707:
3703:
3699:
3691:
3686:
3681:
3677:
3663:
3659:
3658:
3653:
3650:
3646:
3645:
3640:
3637:
3633:
3632:
3627:
3624:
3620:
3619:
3614:
3611:
3607:
3606:
3601:
3598:
3594:
3593:
3588:
3585:
3581:
3580:
3575:
3574:
3572:
3568:
3562:
3559:
3557:
3554:
3552:
3549:
3547:
3544:
3542:
3539:
3538:
3536:
3532:
3526:
3523:
3521:
3520:Enzyme family
3518:
3516:
3513:
3511:
3508:
3507:
3505:
3501:
3495:
3492:
3490:
3487:
3485:
3484:Cooperativity
3482:
3480:
3477:
3476:
3474:
3470:
3464:
3461:
3459:
3456:
3454:
3451:
3449:
3446:
3444:
3443:Oxyanion hole
3441:
3439:
3436:
3434:
3431:
3429:
3426:
3425:
3423:
3419:
3415:
3408:
3403:
3401:
3396:
3394:
3389:
3388:
3385:
3373:
3370:
3368:
3365:
3363:
3360:
3356:
3353:
3351:
3348:
3346:
3343:
3342:
3341:
3338:
3334:
3331:
3329:
3326:
3325:
3324:
3321:
3319:
3316:
3314:
3311:
3310:
3308:
3305:
3301:
3293:
3290:
3288:
3285:
3283:
3280:
3278:
3275:
3274:
3273:
3270:
3269:
3267:
3265:
3261:
3257:
3251:
3248:
3246:
3243:
3241:
3238:
3236:
3233:
3231:
3228:
3226:
3223:
3221:
3218:
3216:
3213:
3211:
3208:
3206:
3203:
3201:
3198:
3197:
3195:
3192:
3188:
3184:
3178:
3175:
3173:
3170:
3168:
3165:
3163:
3160:
3158:
3154:
3151:
3150:
3148:
3145:
3141:
3137:
3133:
3128:
3124:
3120:
3113:
3108:
3106:
3101:
3099:
3094:
3093:
3090:
3072:
3069:
3068:
3067:
3066:
3062:
3061:
3059:
3056:
3051:
3044:
3041:
3039:
3036:
3035:
3033:
3030:
3025:
3022:
3020:
3015:
3011:
3001:
2998:
2996:
2993:
2991:
2988:
2987:
2985:
2982:
2977:
2971:
2968:
2966:
2963:
2961:
2958:
2956:
2953:
2952:
2950:
2947:
2942:
2934:
2931:
2929:
2926:
2925:
2924:
2921:
2920:
2918:
2915:
2910:
2907:
2905:
2901:
2897:
2893:
2889:
2885:
2878:
2873:
2871:
2866:
2864:
2859:
2858:
2855:
2841:
2835:
2830:
2826:
2817:
2812:
2810:
2805:
2803:
2798:
2797:
2794:
2787:
2783:
2780:
2779:
2775:
2770:
2769:mitochondrion
2766:
2761:
2752:
2748:
2743:
2738:
2735:(8): 407–12.
2734:
2730:
2726:
2721:
2717:
2713:
2709:
2705:
2701:
2697:
2694:(3): 229–38.
2693:
2689:
2684:
2680:
2676:
2671:
2666:
2662:
2658:
2655:(5): 1130–8.
2654:
2650:
2646:
2641:
2637:
2633:
2628:
2623:
2619:
2615:
2611:
2606:
2602:
2598:
2594:
2590:
2585:
2580:
2576:
2572:
2568:
2563:
2559:
2555:
2551:
2547:
2543:
2539:
2534:
2530:
2526:
2521:
2516:
2513:(4): 1453–7.
2512:
2508:
2504:
2499:
2495:
2491:
2487:
2483:
2479:
2475:
2470:
2466:
2462:
2458:
2454:
2449:
2444:
2440:
2436:
2432:
2427:
2423:
2419:
2415:
2411:
2407:
2403:
2399:
2395:
2389:
2385:
2381:
2377:
2373:
2368:
2363:
2359:
2355:
2351:
2346:
2342:
2338:
2333:
2328:
2323:
2318:
2314:
2310:
2307:(8): 3024–8.
2306:
2302:
2298:
2293:
2289:
2285:
2281:
2277:
2273:
2269:
2264:
2260:
2256:
2252:
2248:
2245:(3): 477–80.
2244:
2240:
2234:
2230:
2226:
2222:
2218:
2214:
2210:
2205:
2201:
2197:
2193:
2189:
2185:
2181:
2177:
2173:
2168:
2164:
2160:
2156:
2152:
2148:
2144:
2138:
2137:
2132:
2123:
2119:
2115:
2111:
2108:(2): 159–66.
2107:
2103:
2095:
2092:
2087:
2083:
2078:
2073:
2069:
2065:
2061:
2054:
2051:
2046:
2042:
2037:
2032:
2028:
2024:
2020:
2016:
2012:
2008:
2004:
1997:
1994:
1989:
1985:
1980:
1975:
1970:
1965:
1961:
1957:
1953:
1949:
1945:
1938:
1935:
1930:
1926:
1921:
1916:
1912:
1908:
1905:(1): 225–31.
1904:
1900:
1896:
1889:
1887:
1883:
1878:
1874:
1870:
1866:
1862:
1858:
1850:
1847:
1842:
1838:
1834:
1830:
1826:
1822:
1815:
1812:
1807:
1803:
1799:
1795:
1792:(3): 477–80.
1791:
1787:
1779:
1776:
1771:
1767:
1762:
1757:
1752:
1747:
1743:
1739:
1736:(8): 3024–8.
1735:
1731:
1727:
1720:
1717:
1712:
1708:
1703:
1698:
1695:(3): 579–87.
1694:
1690:
1686:
1679:
1676:
1671:
1667:
1663:
1659:
1655:
1651:
1647:
1643:
1636:
1633:
1628:
1624:
1620:
1616:
1613:(3): 245–52.
1612:
1608:
1601:
1598:
1593:
1589:
1584:
1579:
1575:
1571:
1567:
1560:
1557:
1544:
1538:
1535:
1522:
1518:
1514:
1508:
1505:
1500:
1494:
1492:
1488:
1483:
1479:
1474:
1469:
1465:
1461:
1457:
1450:
1448:
1444:
1439:
1435:
1431:
1427:
1423:
1419:
1411:
1408:
1403:
1399:
1393:
1390:
1385:
1381:
1375:
1372:
1368:
1364:
1359:
1357:
1355:
1351:
1344:
1342:
1340:
1336:
1328:
1326:
1324:
1320:
1316:
1311:
1306:
1304:
1299:
1295:
1290:
1286:
1279:
1277:
1275:
1271:
1267:
1259:
1257:
1255:
1251:
1243:
1238:
1236:
1234:
1230:
1226:
1222:
1218:
1214:
1210:
1207:
1203:
1199:
1195:
1191:
1187:
1183:
1179:
1175:
1167:
1165:
1163:
1159:
1155:
1151:
1147:
1143:
1138:
1136:
1132:
1128:
1125:
1121:
1117:
1113:
1101:
1098:
1096:
1092:
1089:
1085:
1081:
1078:
1076:
1072:
1068:
1064:
1061:
1058:
1054:
1049:
1045:
1042:
1039:
1037:
1033:
1030:
1027:
1025:
1021:
1018:
1015:
1013:
1009:
1005:
1001:
996:
989:
984:
979:
968:
963:
959:
955:
952:
948:
941:
939:
936:
932:
928:
924:
919:
915:
908:
906:
900:
896:
893:
887:
885:
879:
875:
872:
868:
861:
859:
853:
849:
846:
840:
838:
832:
828:
825:
823:RefSeq (mRNA)
821:
814:
813:
808:
804:
801:
795:
794:
789:
785:
782:
780:
776:
769:
768:
763:
759:
756:
750:
746:
742:
739:
737:
733:
726:
725:
720:
716:
713:
707:
706:
701:
697:
694:
692:
688:
685:
682:
680:
677:
673:
670:
666:
662:
655:
651:
646:
640:
637:
635:
632:
630:
627:
625:
622:
620:
617:
615:
612:
610:
607:
605:
602:
600:
597:
595:
592:
591:
589:
586:
585:
579:
576:
574:
571:
569:
566:
564:
563:mitochondrion
561:
559:
556:
554:
551:
550:
548:
545:
544:
538:
535:
533:
530:
528:
525:
523:
520:
518:
515:
514:
512:
509:
508:
505:
504:Gene ontology
501:
497:
485:
480:
476:
471:
468:
466:
462:
454:
449:
438:
434:
430:
426:
422:
418:
414:
410:
406:
402:
401:
398:
394:
389:
386:
376:
372:
368:
364:
360:
356:
352:
348:
344:
340:
339:
336:
332:
327:
324:
323:
320:
318:
314:
312:
311:
307:
306:
303:
301:
297:
293:
289:
285:
277:
272:
268:
264:
259:
249:
245:
238:
231:
225:
218:
210:
206:
202:
197:
193:
188:
184:
180:
174:
170:
166:
162:
158:
154:
150:
146:
142:
138:
134:
130:
122:
117:
110:
105:
100:
89:
87:
81:
76:
73:
72:
68:
65:
58:
54:
49:
45:
41:
36:
31:
19:
3709:
3657:Translocases
3654:
3641:
3628:
3615:
3602:
3592:Transferases
3589:
3576:
3433:Binding site
3340:5α-reductase
3204:
3063:
3054:
3028:
2989:
2980:
2945:
2913:
2839:
2732:
2728:
2691:
2687:
2652:
2648:
2617:
2613:
2577:(1): 44–53.
2574:
2570:
2544:(3): 204–6.
2541:
2537:
2510:
2506:
2477:
2473:
2438:
2434:
2397:
2393:
2360:(1): 78–80.
2357:
2353:
2304:
2300:
2271:
2267:
2242:
2238:
2212:
2208:
2178:(5): 557–9.
2175:
2171:
2146:
2142:
2105:
2101:
2094:
2067:
2063:
2053:
2013:(1): 18577.
2010:
2006:
1996:
1951:
1947:
1937:
1902:
1898:
1863:(6): 551–9.
1860:
1856:
1849:
1827:(3): 547–9.
1824:
1820:
1814:
1789:
1785:
1778:
1733:
1729:
1719:
1692:
1688:
1678:
1648:(1): 35–43.
1645:
1641:
1635:
1610:
1606:
1600:
1573:
1569:
1559:
1547:. Retrieved
1537:
1525:. Retrieved
1521:the original
1516:
1507:
1463:
1459:
1424:(2): 275–8.
1421:
1417:
1410:
1401:
1392:
1383:
1374:
1332:
1329:Interactions
1307:
1283:
1263:
1254:chromosome 3
1247:
1171:
1139:
1123:
1115:
1111:
1110:
902:
881:
855:
834:
810:
791:
765:
748:
722:
703:
683:
678:
427:right kidney
315:
308:
124:External IDs
83:
3722:Human genes
3428:Active site
2822:PDB gallery
1270:proteolytic
1252:located on
1233:prokaryotic
1198:chlorophyll
1162:lymphocytes
998:Identifiers
403:human fetus
357:bone marrow
274:58,537,999
261:58,490,655
102:Identifiers
3716:Categories
3631:Isomerases
3605:Hydrolases
3472:Regulation
2946:cytosolic:
2888:metabolism
1369:, May 2017
1345:References
1310:amino acid
1287:(HCP) and
1256:at q11.2.
1229:eukaryotic
1063:structures
319:(ortholog)
145:HomoloGene
3706:IPR001260
3510:EC number
2896:porphyrin
2765:cytoplasm
1274:homodimer
1266:precursor
1239:Structure
1235:sources.
1178:catalyses
1174:porphyrin
1041:PDOC00783
1029:IPR001260
905:NP_031783
884:NP_000088
858:NM_007757
837:NM_000097
669:Orthologs
578:cytoplasm
173:EC number
153:GeneCards
3702:InterPro
3534:Kinetics
3458:Cofactor
3421:Activity
3193:acceptor
3146:acceptor
2771:(yellow)
2751:12181641
2716:42265181
2708:12059041
2679:11309681
2636:11248690
2465:32065580
2384:45889945
2200:11997203
2122:16214298
2086:16159891
2045:31819097
1988:16176984
1929:21103937
1877:24078084
1670:23011457
1592:13746277
1549:8 August
1527:8 August
1365:–
1335:interact
1225:electron
1213:molecule
1168:Function
1118:) is an
1080:RCSB PDB
1024:InterPro
951:Wikidata
648:Sources:
553:membrane
439:habenula
419:yolk sac
377:duodenum
373:monocyte
3690:Biology
3644:Ligases
3414:Enzymes
3264:Quinone
3029:spleen:
2884:Enzymes
2670:1226094
2601:2705450
2593:9888388
2558:9843038
2529:9454777
2494:9373149
2457:9298818
2422:1813242
2414:9048920
2376:8990017
2341:8159699
2309:Bibcode
2288:8125298
2259:8012360
2229:7987309
2192:7959694
2163:7849704
2036:6901466
2015:Bibcode
1979:1224704
1956:Bibcode
1920:3091031
1841:8286403
1806:8012360
1770:8159699
1738:Bibcode
1711:3516695
1662:8219054
1627:3233772
1482:8407975
1438:7757079
1367:Ensembl
1339:mercury
1303:anaemia
1260:Protein
1202:protein
1192:in the
1036:PROSITE
1017:PF01218
779:UniProt
736:Ensembl
675:Species
654:QuickGO
573:cytosol
294:pattern
179:1.3.3.3
109:Aliases
3676:Portal
3618:Lyases
3355:SRD5A3
3350:SRD5A2
3345:SRD5A1
3328:ACADSB
3304:1.3.99
3191:Oxygen
3071:UGT1A1
3055:liver:
2788:(MeSH)
2749:
2714:
2706:
2677:
2667:
2634:
2599:
2591:
2556:
2527:
2492:
2463:
2455:
2420:
2412:
2382:
2374:
2339:
2329:
2286:
2257:
2227:
2198:
2190:
2161:
2120:
2084:
2043:
2033:
1986:
1976:
1927:
1917:
1875:
1839:
1804:
1768:
1758:
1709:
1668:
1660:
1625:
1590:
1543:"CPOX"
1480:
1436:
1298:faeces
1217:enzyme
1120:enzyme
1095:PDBsum
1069:
1059:
1003:Symbol
937:search
935:PubMed
812:P36552
793:P36551
691:Entrez
465:BioGPS
407:atrium
369:rectum
141:104841
133:612732
3570:Types
3333:ACADS
3260:1.3.5
3187:1.3.3
3136:1.3.1
2933:ALAS2
2928:ALAS1
2712:S2CID
2597:S2CID
2507:Blood
2461:S2CID
2418:S2CID
2380:S2CID
2332:43507
2196:S2CID
1761:43507
1666:S2CID
1294:urine
1250:exons
1209:atoms
724:12892
684:Mouse
679:Human
650:Amigo
317:Mouse
310:Human
257:Start
192:Mouse
3700:and
3698:Pfam
3662:list
3655:EC7
3649:list
3642:EC6
3636:list
3629:EC5
3623:list
3616:EC4
3610:list
3603:EC3
3597:list
3590:EC2
3584:list
3577:EC1
3292:SDHD
3287:SDHC
3282:SDHB
3277:SDHA
3144:NADP
3129:1.3)
3019:bile
2894:and
2892:heme
2840:2aex
2747:PMID
2704:PMID
2675:PMID
2632:PMID
2589:PMID
2554:PMID
2525:PMID
2490:PMID
2474:Gene
2453:PMID
2410:PMID
2372:PMID
2337:PMID
2284:PMID
2268:Gene
2255:PMID
2225:PMID
2188:PMID
2159:PMID
2118:PMID
2082:PMID
2041:PMID
1984:PMID
1925:PMID
1873:PMID
1837:PMID
1825:1183
1802:PMID
1766:PMID
1707:PMID
1658:PMID
1623:PMID
1588:PMID
1551:2011
1529:2011
1478:PMID
1434:PMID
1296:and
1244:Gene
1231:and
1211:per
1206:iron
1196:and
1194:haem
1142:haem
1131:heme
1127:gene
1124:CPOX
1116:CPOX
1088:PDBj
1084:PDBe
1067:ECOD
1057:Pfam
1012:Pfam
705:1371
300:Bgee
248:Band
209:Chr.
157:CPOX
129:OMIM
116:CPOX
86:2AEX
67:RCSB
64:PDBe
33:CPOX
3140:NAD
3017:to
2890:of
2737:doi
2696:doi
2665:PMC
2657:doi
2622:doi
2618:268
2579:doi
2546:doi
2515:doi
2482:doi
2478:200
2443:doi
2402:doi
2362:doi
2327:PMC
2317:doi
2276:doi
2272:138
2247:doi
2217:doi
2180:doi
2151:doi
2110:doi
2106:161
2072:doi
2031:PMC
2023:doi
1974:PMC
1964:doi
1952:102
1915:PMC
1907:doi
1865:doi
1861:154
1829:doi
1794:doi
1756:PMC
1746:doi
1697:doi
1693:156
1650:doi
1615:doi
1611:177
1578:doi
1574:236
1468:doi
1464:268
1426:doi
1321:to
1188:to
1184:of
1148:to
1075:PDB
921:n/a
749:n/a
270:End
161:OMA
137:MGI
57:PDB
3718::
3704::
3262::
3189::
3138::
3127:EC
3121::
2745:.
2733:47
2731:.
2727:.
2710:.
2702:.
2692:18
2690:.
2673:.
2663:.
2653:68
2651:.
2647:.
2630:.
2616:.
2612:.
2595:.
2587:.
2575:13
2573:.
2569:.
2552:.
2542:80
2540:.
2523:.
2511:91
2509:.
2505:.
2488:.
2476:.
2459:.
2451:.
2439:10
2437:.
2433:.
2416:.
2408:.
2398:99
2396:.
2378:.
2370:.
2356:.
2352:.
2335:.
2325:.
2315:.
2305:91
2303:.
2299:.
2282:.
2270:.
2253:.
2241:.
2223:.
2211:.
2194:.
2186:.
2176:94
2174:.
2157:.
2145:.
2116:.
2104:.
2080:.
2068:14
2066:.
2062:.
2039:.
2029:.
2021:.
2009:.
2005:.
1982:.
1972:.
1962:.
1950:.
1946:.
1923:.
1913:.
1903:34
1901:.
1897:.
1885:^
1871:.
1859:.
1835:.
1823:.
1800:.
1788:.
1764:.
1754:.
1744:.
1734:91
1732:.
1728:.
1705:.
1691:.
1687:.
1664:.
1656:.
1646:23
1644:.
1621:.
1609:.
1586:.
1572:.
1568:.
1515:.
1490:^
1476:.
1462:.
1458:.
1446:^
1432:.
1420:.
1400:.
1382:.
1353:^
1164:.
1137:.
1086:;
1082:;
1065:/
652:/
276:bp
263:bp
159:;
155::
151:;
149:76
147::
143:;
139::
135:;
131::
3678::
3664:)
3660:(
3651:)
3647:(
3638:)
3634:(
3625:)
3621:(
3612:)
3608:(
3599:)
3595:(
3586:)
3582:(
3406:e
3399:t
3392:v
3155:/
3142:/
3125:(
3111:e
3104:t
3097:v
2876:e
2869:t
2862:v
2815:e
2808:t
2801:v
2753:.
2739::
2718:.
2698::
2681:.
2659::
2638:.
2624::
2603:.
2581::
2560:.
2548::
2531:.
2517::
2496:.
2484::
2467:.
2445::
2424:.
2404::
2386:.
2364::
2358:9
2343:.
2319::
2311::
2290:.
2278::
2261:.
2249::
2243:3
2231:.
2219::
2213:3
2202:.
2182::
2165:.
2153::
2147:3
2124:.
2112::
2088:.
2074::
2047:.
2025::
2017::
2011:9
1990:.
1966::
1958::
1931:.
1909::
1879:.
1867::
1843:.
1831::
1808:.
1796::
1790:3
1772:.
1748::
1740::
1713:.
1699::
1672:.
1652::
1629:.
1617::
1594:.
1580::
1553:.
1531:.
1501:.
1484:.
1470::
1440:.
1428::
1422:4
1404:.
1386:.
194:)
163::
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.