46:
of the root cause of the non-conformance. Non-conformance may be a market complaint or customer complaint or failure of machinery or a quality management system, or misinterpretation of written instructions to carry out work. The corrective and preventive action is designed by a team that includes quality assurance personnel and personnel involved in the actual observation point of non-conformance. It must be systematically implemented and observed for its ability to eliminate further recurrence of such non-conformation. The
203:'s code FDA 21 CFR 820.100 medical device companies need to establish a CAPA process within their QMS. This part of the system may be paper or digital, but it is something that is looked for during an FDA visit. In 2015 there were over 450 issues found with the CAPA systems for medical device companies. To have an FDA-compliant QMS system required the ability to capture, review, approve, control, and retrieve closed-loop processes.
148:
167:
Investigations to root cause may conclude that no corrective or preventive actions are required, and additionally may suggest simple corrections to a problem with no identified systemic root cause. When multiple investigations end in no corrective action, a new problem statement with expanded scope
45:
or other undesirable situations. It is usually a set of actions, laws or regulations required by an organization to take in manufacturing, documentation, procedures, or systems to rectify and eliminate recurring non-conformance. Non-conformance is identified after systematic evaluation and analysis
171:
Implementation of corrective and preventive actions is the path towards improvement and effectiveness of
Quality Management Systems. Corrective actions are nothing but actions based on problem identification. The problem or a non-conformance can be identified internally through staff suggestions,
190:
Preventive action includes the prediction of problems and attempts to avoid such occurrences (fail-safe) through self-initiated actions and analysis related to the processes or products. This can be initiated with the help of active participation by staff members and workers through improvement
67:
In certain markets and industries, CAPA may be required as part of the quality management system, such as the
Medical Devices and Pharmaceutical industries in the United States. In this case, failure to adhere to proper CAPA handling is considered a violation of US Federal regulations on good
159:
discrepancies before they occur and to ensure that they do not happen (thereby including, for example, preventive maintenance, management review or other common forms of risk avoidance). Corrective and preventive actions include stages for investigation, action, review, and further action is
172:
management reviews, document reviews or internal audits. External leads to finding the root cause of the problem can include
Customer complaints and suggestions; customer rejections; non-conformities raised in customer or third-party audits; recommendations by auditors.
68:
manufacturing practices. As a consequence, a medicine or medical device can be termed as adulterated or substandard if the company has failed to investigate, record and analyze the root cause of a non-conformance, and failed to design and implement an effective CAPA.
191:
teams, improvement meetings, opportunities for improvement during internal audits, management review, customer feedback and deciding own goals quantized in terms of business growth, reducing rejections, utilizing the equipment effectively, etc.
175:
A root cause is the identification and investigation of the source of the problem where the person(s), system, process, or external factor is identified as the cause of the nonconformity. The root cause analysis can be done via
71:
CAPA is used to bring about improvements to an organization's processes, and is often undertaken to eliminate causes of non-conformities or other undesirable situations. CAPA is a concept within
532:
80:
117:
To ensure that corrective and preventive actions are effective, the systematic investigation of the root causes of failure is pivotal. CAPA is part of the overall
206:
A corrective action can also be a field correction, an action taken to correct problems with non-conforming products. An example is the pharmaceutical company
76:
316:
106:
Corrective actions are implemented in response to customer complaints, unacceptable levels of product non-conformance, issues identified during an
505:
294:
140:
A common misconception is that the purpose of preventive action is to avert the occurrence of a similar potential problem. This process is
144:
part of corrective action because it is a process of determining such similarities that should take place in the event of a discrepancy.
560:
284:
47:
415:
210:, which in 2022 conducted a voluntary field correction after reports of 60 injuries and 23 patient deaths related to misplaced
56:: Action taken to eliminate the causes of non-conformities or other undesirable situations, so as to prevent recurrence.
200:
586:
581:
289:
111:
72:
533:"Avanos Medical Recalls Cortrak*2 Enteral Access System for Risk of Misplaced Enteral Tubes Could Cause Patient Harm"
114:(SPC). Preventive actions are implemented in response to the identification of potential sources of non-conformity.
103:
in an attempt to prevent their recurrence (for corrective action) or to prevent occurrence (for preventive action).
591:
391:
118:
64:: Action taken to prevent the occurrence of such non-conformities, generally as a result of a risk analysis.
42:
576:
265:
211:
110:, as well as adverse or unstable trends in product and process monitoring such as would be identified by
596:
133:
96:
454:
360:
17:
433:
60:
275:
In some cases, a combination of such actions may be necessary to fully correct the problem.
181:
100:
481:
419:
229:
207:
136:
that identifies the cause of a discrepancy or deviation, and suggest corrective actions
107:
41:) consists of improvements to an organization's processes taken to eliminate causes of
27:
Improvements in an organization to eliminate non-conformities or undesirable situations
570:
214:
50:
method, or 8D framework, can be used as an effective method of structuring a CAPA.
187:
Correction is the action to eliminate a detected nonconformity or nonconformance.
224:
The voluntary field correction led Avanos
Medical to recall the product. The
168:
may be generated, and a more thorough investigation to root cause performed.
241:
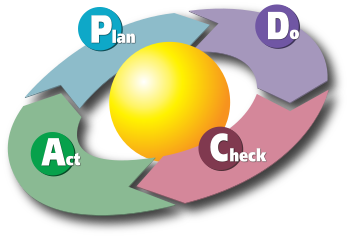
164:(plan-do-check-act) philosophy as determined by the Deming-Shewhart cycle.
258:
130:
Clearly identified sources of data that identify problems to investigate
147:
95:
business standards. It focuses on the systematic investigation of the
177:
146:
88:
84:
506:"URGENT: FIELD CORRECTION CORTRAK* 2 Enteral Access System (EAS)"
347:
ISO 9000 Quality management system - Fundamentals and vocabulary
161:
225:
92:
261:
or enhancement or modification of existing training programs
155:
Preventive action is any proactive method used to determine
561:
Quality
Systems Approach to Pharmaceutical CGMP Regulations
455:"Guidance for Industry- Q10 Pharmaceutical Quality System"
81:Hazard Analysis and Risk-based Preventive Controls
160:required. It can be seen that both fit into the
8:
392:"CFR - Code of Federal Regulations Title 21"
271:Improvements to material handling or storage
77:Hazard Analysis and Critical Control Points
307:
434:"Does Your CAPA Process Need a CAPA?"
295:Good automated manufacturing practice
99:of identified problems or identified
7:
232:, the most severe type of recall.
195:Medical devices and FDA compliance
25:
361:"Taking the First Step with PDCA"
285:Eight disciplines problem solving
199:To comply with the United States
48:Eight disciplines problem solving
255:Define and Implement Action Plan
219:CORTRAK* 2 Enteral Access System
31:Corrective and preventive action
18:Corrective and Preventive Action
315:Pruitt, W. Frazier (May 2019).
236:Examples of corrective actions
1:
482:"Field Correction definition"
201:Food and Drug Administration
290:Good documentation practice
112:statistical process control
73:good manufacturing practice
613:
180:or other methods, e.g. an
246:Visible or Audible Alarms
119:quality management system
317:"A Disciplined Approach"
418:. 2020. Archived from
396:www.accessdata.fda.gov
381:ISO 9000:2015 (3.12.3)
152:
150:
228:identified it as a
134:Root cause analysis
587:Quality management
582:Drug manufacturing
513:static.foxnews.com
217:while using their
153:
592:Change management
363:. 2 February 2009
61:Preventive action
54:Corrective action
39:corrective action
16:(Redirected from
604:
548:
547:
545:
544:
529:
523:
522:
520:
519:
510:
502:
496:
495:
493:
492:
478:
472:
471:
469:
468:
459:
451:
445:
444:
442:
441:
430:
424:
423:
412:
406:
405:
403:
402:
388:
382:
379:
373:
372:
370:
368:
357:
351:
350:
343:
337:
336:
334:
332:
321:Quality Progress
312:
264:Improvements to
252:Product Redesign
249:Process Redesign
182:Ishikawa diagram
43:non-conformities
21:
612:
611:
607:
606:
605:
603:
602:
601:
567:
566:
557:
552:
551:
542:
540:
531:
530:
526:
517:
515:
508:
504:
503:
499:
490:
488:
480:
479:
475:
466:
464:
457:
453:
452:
448:
439:
437:
432:
431:
427:
414:
413:
409:
400:
398:
390:
389:
385:
380:
376:
366:
364:
359:
358:
354:
345:
344:
340:
330:
328:
314:
313:
309:
304:
281:
238:
197:
127:
91:) and numerous
28:
23:
22:
15:
12:
11:
5:
610:
608:
600:
599:
594:
589:
584:
579:
569:
568:
565:
564:
556:
555:External links
553:
550:
549:
524:
497:
486:lawinsider.com
473:
446:
425:
422:on 2022-01-05.
416:"CAPA Process"
407:
383:
374:
352:
338:
306:
305:
303:
300:
299:
298:
292:
287:
280:
277:
273:
272:
269:
262:
256:
253:
250:
247:
244:
242:Error Proofing
237:
234:
230:Class I recall
208:Avanos Medical
196:
193:
151:The PDCA cycle
138:
137:
131:
126:
123:
108:internal audit
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
609:
598:
595:
593:
590:
588:
585:
583:
580:
578:
577:ISO standards
575:
574:
572:
562:
559:
558:
554:
539:. 16 May 2022
538:
534:
528:
525:
514:
507:
501:
498:
487:
483:
477:
474:
463:
456:
450:
447:
435:
429:
426:
421:
417:
411:
408:
397:
393:
387:
384:
378:
375:
362:
356:
353:
348:
342:
339:
326:
322:
318:
311:
308:
301:
296:
293:
291:
288:
286:
283:
282:
278:
276:
270:
267:
263:
260:
257:
254:
251:
248:
245:
243:
240:
239:
235:
233:
231:
227:
222:
220:
216:
215:feeding tubes
213:
209:
204:
202:
194:
192:
188:
185:
183:
179:
173:
169:
165:
163:
158:
149:
145:
143:
135:
132:
129:
128:
124:
122:
120:
115:
113:
109:
104:
102:
98:
94:
90:
86:
82:
78:
74:
69:
65:
63:
62:
57:
55:
51:
49:
44:
40:
36:
32:
19:
541:. Retrieved
536:
527:
516:. Retrieved
512:
500:
489:. Retrieved
485:
476:
465:. Retrieved
461:
449:
438:. Retrieved
428:
420:the original
410:
399:. Retrieved
395:
386:
377:
365:. Retrieved
355:
349:. ISO. 2005.
346:
341:
329:. Retrieved
324:
320:
310:
274:
223:
218:
205:
198:
189:
186:
174:
170:
166:
156:
154:
141:
139:
116:
105:
70:
66:
59:
58:
53:
52:
38:
34:
30:
29:
462:www.fda.gov
266:maintenance
212:nasogastric
97:root causes
597:Prevention
571:Categories
543:2023-03-09
518:2023-03-09
491:2023-03-09
467:2016-08-29
440:2016-08-29
401:2016-05-20
331:31 October
302:References
37:or simply
268:schedules
157:potential
436:. SOLABS
367:17 March
279:See also
259:Training
125:Concepts
537:fda.gov
327:(5): 64
121:(QMS).
75:(GMP),
297:(GAMP)
178:5 Whys
563:(FDA)
509:(PDF)
458:(PDF)
101:risks
89:HARPC
85:HACCP
369:2011
333:2019
162:PDCA
35:CAPA
226:FDA
221:.
142:all
93:ISO
573::
535:.
511:.
484:.
460:.
394:.
325:52
323:.
319:.
184:.
546:.
521:.
494:.
470:.
443:.
404:.
371:.
335:.
87:/
83:(
79:/
33:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.