241:
frameworks that synergistically enhance the properties of the precursors, which, in turn, offers many advantages in terms of improved performance in different applications. As a result, the COF material is highly modular and tuned efficiently by varying the SBUs’ identity, length, and functionality depending on the desired property change on the framework scale. Ergo, there exists the ability to introduce diverse functionality directly into the framework scaffold to allow for a variety of functions which would be cumbersome, if not impossible, to achieve through a top-down method, such as lithographic approaches or chemical-based nanofabrication. Through reticular synthesis, it is possible to molecularly engineer modular, framework materials with highly porous scaffolds that exhibit unique electronic, optical, and magnetic properties while simultaneously integrating desired functionality into the COF skeleton.
224:, a material is built from atomic or molecular components synthetically as opposed to a top-down approach, which forms a material from the bulk through approaches such as exfoliation, lithography, or other varieties of post-synthetic modification. The bottom-up approach is especially advantageous with respect to materials such as COFs because the synthetic methods are designed to directly result in an extended, highly crosslinked framework that can be tuned with exceptional control at the nanoscale level. Geometrical and dimensional principles govern the framework's resulting topology as the SBUs combine to form predetermined structures. This level of synthetic control has also been termed "
367:
405:
401:(TFP) is used as one of the SBUs, two complementary tautomerizations occur (an enol to keto and an imine to enamine) which result in a β-ketoenamine moiety as depicted in the DAAQ-TFP framework. Both DAAQ-TFP and TpOMe-DAQ COFs are stable in acidic aqueous conditions and contain the redox active linker 2,6-diaminoanthroquinone which enables these materials to reversibly store and release electrons within a characteristic potential window. Consequently, both of these COFs have been investigated as electrode materials for potential use in supercapacitors.
232:
36:
structures with highly preferential structural orientation and properties which could be synergistically enhanced and amplified. With judicious selection of COF secondary building units (SBUs), or precursors, the final structure could be predetermined, and modified with exceptional control enabling fine-tuning of emergent properties. This level of control facilitates the COF material to be designed, synthesized, and utilized in various applications, many times with metrics on scale or surpassing that of the current state-of-the-art approaches.
462:
control, hereby enabling crystalline growth. This was employed by Yaghi and coworkers for 3D imine-based COFs (COF-300, COF 303, LZU-79, and LZU-111). However, the vast majority of COFs are not able to crystallize into single crystals but instead are insoluble powders. The improvement of crystallinity of these polycrystalline materials can be improved through tuning the reversibility of the linkage formation to allow for corrective particle growth and self-healing of defects that arise during COF formation.
339:
146:. The research team synthesized and designed the first 3D-COF ever; COF-103 and COF-108, helping unleash this new field. Unlike 0D and 1D systems, which are soluble, the insolubility of 2D and 3D structures precludes the use of stepwise synthesis, making their isolation in crystalline form very difficult. This first challenge, however, was overcome by judiciously choosing building blocks and using reversible condensation reactions to crystallize COFs.
326:
258:
630:
better delivery amount have been designed in the lab of
William A. Goddard III, and they have been shown to be stable and overcome the DOE target in delivery basis. COF-103-Eth-trans and COF-102-Ant, are found to exceed the DOE target of 180 v(STP)/v at 35 bar for methane storage. They reported that using thin vinyl bridging groups aids performance by minimizing the interaction methane-COF at low pressure.
471:
279:
197:
3576:
375:
629:
storage of 180 v/v at 298 K and 35 bar. The best COFs on a delivery amount basis (volume adsorbed from 5 to 100 bar) are COF-102 and COF-103 with values of 230 and 234 v(STP: 298 K, 1.01 bar)/v, respectively, making these promising materials for practical methane storage. More recently, new COFs with
605:
is important to their stability. If the metalation with alkali meals is performed in the COFs, Goddard et al. calculated that some COFs can reach 2010 DOE gravimetric target in delivery units at 298 K of 4.5 wt %: COF102-Li (5.16 wt %), COF103-Li (4.75 wt %), COF102-Na (4.75 wt %)
187:
COFs are another class of porous polymeric materials, consisting of porous, crystalline, covalent bonds that usually have rigid structures, exceptional thermal stabilities (to temperatures up to 600 °C), are stable in water and low densities. They exhibit permanent porosity with specific surface
160:
solids consist of secondary building units (SBUs) which assemble to form a periodic and porous framework. An almost infinite number of frameworks can be formed through various SBU combinations leading to unique material properties for applications in separations, storage, and heterogeneous catalysis.
30:
that form two- or three-dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials. COFs emerged as a field from the overarching domain of organic materials as researchers optimized both synthetic control
528:
enables probing of linkage formation as well and is well suited for large, insoluble materials like COFs. Gas adsorption-desorption studies quantify the porosity of the material via calculation of the
Brunauer–Emmett–Teller (BET) surface area and pore diameter from gas adsorption isotherms. Electron
479:
Integration of SBUs into a covalent framework results in the synergistic emergence of conductivities much greater than the monomeric values. The nature of the SBUs can improve conductivity. Through the use of highly conjugated linkers throughout the COF scaffold, the material can be engineered to be
274:
Since Yaghi and coworkers’ seminal work in 2005, COF synthesis has expanded to include a wide range of organic connectivity such as boron-, nitrogen-, other atom-containing linkages. The linkages in the figures shown are not comprehensive as other COF linkages exist in the literature, especially for
35:
to advance into the construction of porous, crystalline materials with rigid structures that granted exceptional material stability in a wide range of solvents and conditions. Through the development of reticular chemistry, precise synthetic control was achieved and resulted in ordered, nano-porous
461:
There are several COF single crystals synthesized to date. There are a variety of techniques employed to improve crystallinity of COFs. The use of modulators, monofunctional version of precursors, serve to slow the COF formation to allow for more favorable balance between kinetic and thermodynamic
448:
A defining advantage of COFs is the exceptional porosity that results from the substitution of analogous SBUs of varying sizes. Pore sizes range from 7-23 Å and feature a diverse range of shapes and dimensionalities that remain stable during the evacuation of solvent. The rigid scaffold of the COF
430:
during synthesis. To date, researchers have attempted to establish better control through different synthetic methods such as solvothermal synthesis, interface-assisted synthesis, solid templation as well as seeded growth. First one of the precursors is deposited onto the solid support followed by
244:
Reticular synthesis is different from retrosynthesis of organic compounds, because the structural integrity and rigidity of the building blocks in reticular synthesis remain unaltered throughout the construction process—an important aspect that could help to fully realize the benefits of design in
484:
and coworkers synthesized a COF material (NiPc-Pyr COF) from nickel phthalocyanine (NiPc) and pyrene organic linkers that had a conductivity of 2.51 x 10 S/m, which was several orders of magnitude larger than the undoped molecular NiPc, 10 S/m. A similar COF structure made by Jiang and coworkers,
757:
Due to the ability to introduce diverse functionality into COFs’ structure, catalytic sites can be fine-tuned in conjunction with other advantageous properties like conductivity and stability to afford efficient and selective catalysts. COFs have been used as heterogeneous catalysts in organic,
240:
It has been established in the literature that, when integrated into an isoreticular framework, such as a COF, properties from monomeric compounds can be synergistically enhanced and amplified. COF materials possess the unique ability for bottom-up reticular synthesis to afford robust, tunable
265:
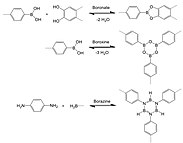
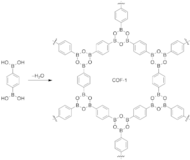
Reticular synthesis was used by Yaghi and coworkers in 2005 to construct the first two COFs reported in the literature: COF-1, using a dehydration reaction of benzenediboronic acid (BDBA), and COF-5, via a condensation reaction between hexahydroxytriphenylene (HHTP) and BDBA. These framework
744:
Due to defining molecule-framework interactions, COFs can be used as chemical sensors in a wide range of environments and applications. Properties of the COF change when their functionalities interact with various analytes enabling the materials to serve as devices in various conditions: as
3189:
Yang, Hui; Zhang, Shengliang; Han, Liheng; Zhang, Zhou; Xue, Zheng; Gao, Juan; Li, Yongjun; Huang, Changshui; Yi, Yuanping; Liu, Huibiao; Li, Yuliang (16 February 2016). "High
Conductive Two-Dimensional Covalent Organic Framework for Lithium Storage with Large Capacity".
601:. Such strategy consists of metalating the COF with alkali metals such as Li. These complexes composed of Li, Na and K with benzene ligands (such as 1,3,5-benzenetribenzoate, the ligand used in MOF-177) have been synthesized by Krieck et al. and Goddard showed that the
485:
CoPc-Pyr COF, exhibited a conductivity of 3.69 x 10 S/m. In both previously mentioned COFs, the 2D lattice allows for full π-conjugation in the x and y directions as well as π-conduction along the z axis due to the fully conjugated, aromatic scaffold and
638:
In addition to storage, COF materials are exceptional at gas separation. For instance, COFs like imine-linked COF LZU1 and azine-linked COF ACOF-1 were used as a bilayer membrane for the selective separation of the following mixtures:
524:(NMR) spectroscopy. Precursor and COF IR spectra enables comparison between vibrational peaks to ascertain that certain key bonds present in the COF linkages appear and that peaks of precursor functional groups disappear. In addition,
417:
The solvothermal approach is the most common used in the literature but typically requires long reaction times due to the insolubility of the organic SBUs in nonorganic media and the time necessary to reach thermodynamic COF products.
606:
and COF103-Na (4.72 wt %). COFs also perform better in delivery units than MOFs because the best volumetric performance is for COF102-Na (24.9), COF102-Li (23.8), COF103-Na (22.8), and COF103-Li (21.7), all using delivery g H
474:
In a fully conjugated 2D COF material such as those synthesized from metallophthalocyanines and highly conjugated organic linkers, charge transport is increased both in-plane, as well as through the stacks, resulting in increased
235:
COF topological control through judicious selection of precursors that result in bonding directionality in the final resulting network. Adapted from Jiang and coworkers' Two- and Three-dimensional
Covalent Organic Frameworks
3143:
Zheng, Weiran; Tsang, Chui-Shan; Lee, Lawrence Yoon Suk; Wong, Kwok-Yin (June 2019). "Two-dimensional metal-organic framework and covalent-organic framework: synthesis and their energy-related applications".
2196:
Evans, Austin M.; Parent, Lucas R.; Flanders, Nathan C.; Bisbey, Ryan P.; Vitaku, Edon; Kirschner, Matthew S.; Schaller, Richard D.; Chen, Lin X.; Gianneschi, Nathan C.; Dichtel, William R. (2018-07-06).
667:
due to the inherent thermal and operational stability of the structures. It has also been shown that COFs inherently act as adsorbents, adhering to the gaseous molecules to enable storage and separation.
245:
crystalline solid-state frameworks. Similarly, reticular synthesis should be distinguished from supramolecular assembly, because in the former, building blocks are linked by strong bonds throughout the
2866:
Marco, B.; Cortizo-Lacalle, D.; Perez-Miqueo, C.; Valenti, G.; Boni, A.; Plas, J.; Strutynski, K.; De Feyter, S.; Paolucci, F.; Montes, M.; Khlobystov, K.; Melle-Franco, M.; Mateo-Alonso, A. (2017).
774:
reaction. However, such researches are still in the very early stage. Most of the efforts have been focusing on solving the key issues, such as conductivity, stability in electrochemical processes.
597:
storage materials. In 2012, the lab of
William A. Goddard III reported the uptake for COF102, COF103, and COF202 at 298 K and they also proposed new strategies to obtain higher interaction with H
397:
using an acid catalyst) can be used as a synthetic route to reach a new class of COFs. The 3D COF called COF-300 and the 2D COF named TpOMe-DAQ are good examples of this chemistry. When
378:
Reversible reactions for COF formation featuring a variety of atoms to form different linkages (a double stage connecting boronate ester and imine linkages, alkene, silicate, nitroso).
135:. COF-1 and COF-5 exhibit high thermal stability (to temperatures up to 500 to 600 °C), permanent porosity, and high surface areas (711 and 1590 square meters per gram, respectively).
52:) and Adrien P Cote published the first paper of COFs in 2005, reporting a series of 2D COFs. They reported the design and successful synthesis of COFs by condensation reactions of
2697:
Krieck, S.; Gorls, H.; Westerhausen, M., Alkali Metal-Stabilized 1,3,5-Triphenylbenzene
Monoanions: Synthesis and Characterization of the Lithium, Sodium, and Potassium Complexes.
2094:
Sharma, Rakesh Kumar; Yadav, Priya; Yadav, Manavi; Gupta, Radhika; Rana, Pooja; Srivastava, Anju; Zbořil, Radek; Varma, Rajender S.; Antonietti, Markus; Gawande, Manoj B. (2020).
692:
range of photons, and allows energy transfer and migration. Furthermore, TP-COF is electrically conductive and capable of repetitive on–off current switching at room temperature.
3225:
Diercks, Christian S.; Lin, Song; Kornienko, Nikolay; Kapustin, Eugene A.; Nichols, Eva M.; Zhu, Chenhui; Zhao, Yingbo; Chang, Christopher J.; Yaghi, Omar M. (16 January 2018).
493:
in COF structures is especially important for applications such as catalysis and energy storage where quick and efficient charge transport is required for optimal performance.
1117:
El-Kaderi, H. M.; Hunt, J. R.; Mendoza-Cortes, J. L.; Cote, A. P.; Taylor, R. E.; O'Keeffe, M.; Yaghi, O. M. (2007). "Designed
Synthesis of 3D Covalent Organic Frameworks".
501:
There exists a wide range of characterization methods for COF materials. There are several COF single crystals synthesized to date. For these highly crystalline materials,
1937:
Halder, Arjun; Ghosh, Meena; Khayum M, Abdul; Bera, Saibal; Addicoat, Matthew; Sasmal, Himadri Sekhar; Karak, Suvendu; Kurungot, Sreekumar; Banerjee, Rahul (2018-09-05).
220:
of the framework materials to introduce precise perturbations in chemical composition, resulting in the highly controlled tunability of framework properties. Through a
131:(COF-5). Their crystal structures are entirely held by strong bonds between B, C, and O atoms to form rigid porous architectures with pore sizes ranging from 7 to 27
2338:
Ma, Tianqiong; Kapustin, Eugene A.; Yin, Shawn X.; Liang, Lin; Zhou, Zhengyang; Niu, Jing; Li, Li-Hua; Wang, Yingying; Su, Jie; Li, Jian; Wang, Xiaoge (2018-07-06).
589:
and free volume, by grand canonical Monte Carlo (GCMC) simulations as a function of temperature and pressure. This is the highest value reported for associative H
798:
A prototype 2 nanometer thick COF layer on a graphene substrate was used to filter dye from industrial wastewater. Once full, the COF can be cleaned and reused.
310:
reaction which is a molecular dehydration reaction between boronic acids. In case of COF-1, three boronic acid molecules converge to form a planar six-membered B
2296:
Ben, Teng; Ren, Hao; Ma, Shengqian; Cao, Dapeng; Lan, Jianhui; Jing, Xiaofei; Wang, Wenchuan; Xu, Jun; Deng, Feng; Simmons, Jason M.; Qiu, Shilun (2009-12-07).
453:. This high surface area to volume ratio and incredible stability enables the COF structure to serve as exceptional materials for gas storage and separation.
550:
176:
minerals commonly used as commercial adsorbents. MOFs are a class of porous polymeric material, consisting of metal ions linked together by organic bridging
1994:"Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route"
810:
scaffold that showed effective drug loading and release in a simulated body fluid environment, making it useful as a nanocarrier for pharmaceutical drugs.
329:
Reversible reactions for COF formation featuring nitrogen to form a variety of linkages (imine, hydrazone, azine, squaraine, phenazine, imide, triazine).
204:
The term ‘secondary building unit’ has been used for some time to describe conceptual fragments which can be compared as bricks used to build a house of
1903:
Uribe-Romo, F. J.; Hunt, J. R.; Furukawa, H.; Klck, C.; O'Keeffe, M.; Yaghi, O. M.; A Crystalline Imine-Linked 3-D Porous
Covalent Organic Framework.
3530:
Zhao, Yu; Das, Saikat; Sekine, Taishu; Mabuchi, Haruna; Irie, Tsukase; Sakai, Jin; Wen, Dan; Zhu, Weidong; Ben, Teng; Negishi, Yuichi (2023-01-23).
610:/L units for 1–100 bar. These are the highest gravimetric molecular hydrogen uptakes for a porous material under these thermodynamic conditions.
570:
Due to the exceptional porosity of COFs, they have been used extensively in the storage and separation of gases such as hydrogen, methane, etc.
525:
366:
404:
2275:
1543:
2838:
Shun, W.; Jia, G.; Jangbae, K.; Hyotcherl, I.; Donglin, J.; A Belt-Shaped, Blue
Luminescent, and Semiconducting Covalent Organic Framework.
505:(XRD) is a powerful tool capable of determining COF crystal structure. The majority of COF materials suffer from decreased crystallinity so
266:
scaffolds were interconnected through the formation of boroxine and boronate linkages, respectively, using solvothermal synthetic methods.
3275:
Li, Xing; Wang, Hui; Chen, Zhongxin; Xu, Hai‐Sen; Yu, Wei; Liu, Cuibo; Wang, Xiaowei; Zhang, Kun; Xie, Keyu; Loh, Kian Ping (2019-10-14).
625:
per unit volume COF adsorbent is COF-1, which can store 195 v/v at 298 K and 30 bar, exceeding the U.S. Department of Energy target for CH
31:
and precursor selection. These improvements to coordination chemistry enabled non-porous and amorphous organic materials such as organic
1088:
Côté, A. P.; Benin, A. I.; Ockwig, N. W.; O'Keeffe, M.; Matzger, A. J.; Yaghi, O. M.; Porous, Crystalline, Covalent
Organic Frameworks.
138:
The synthesis of 3D COFs has been hindered by longstanding practical and conceptual challenges until it was first achieved in 2007 by
3227:"Reticular Electronic Tuning of Porphyrin Active Sites in Covalent Organic Frameworks for Electrocatalytic Carbon Dioxide Reduction"
1224:
Yaghi, O. M.; O'Keeffe, M.; Ockwig, N. W.; Chae, H. K.; Eddaoudi, M.; Kim, J.; Reticular synthesis and the design of new materials.
849:
621:
and William A. Goddard III also reported COFs as exceptional methane storage materials. The best COF in terms of total volume of CH
449:
structure enables the material to be evacuated of solvent and retain its structure, resulting in high surface areas as seen by the
546:
534:
426:
Morphological control on the nanoscale is still limited as COFs lack synthetic control in higher dimensions due to the lack of
358:
in ionothermal conditions (molten zinc chloride at high temperature (400 °C)). CTF-1 is a good example of this chemistry.
2916:
829:
480:
fully conjugated, enabling high charge carrier density as well as through- and in-plane charge transport. For instance,
2615:
Han, S.; Hurukawa, H.; Yaghi, O. M.; Goddard, W. A.; Covalent Organic Frameworks as Exceptional Hydrogen Storage Materials.
2729:
Fan, Hongwei; Mundstock, Alexander; Feldhoff, Armin; Knebel, Alexander; Gu, Jiahui; Meng, Hong; Caro, Jürgen (2018-08-15).
2868:"Twisted Aromatic Frameworks: Readily Exfoliable and Solution-Processable Two-Dimensional Conjugated Microporous Polymers"
2044:
DeBlase, Catherine R.; Silberstein, Katharine E.; Truong, Thanh-Tam; Abruña, Héctor D.; Dichtel, William R. (2013-11-13).
538:
221:
217:
700:
Most studies to date have focused on the development of synthetic methodologies with the aim of maximizing pore size and
2096:"Recent development of covalent organic frameworks (COFs): synthesis and catalytic (organic-electro-photo) applications"
530:
261:
Reversible reactions for COF formation featuring boron to form a variety of linkages (boronate, boroxine, and borazine).
585:
uptakes at 77 K are 10.0 wt % at 80 bar for COF-105, and 10.0 wt % at 100 bar for COF-108, which have higher
581:
and William A. Goddard III reported COFs as exceptional hydrogen storage materials. They predicted the highest excess H
3450:
Vitaku, Edon; Gannett, Cara N.; Carpenter, Keith L.; Shen, Luxi; Abruña, Héctor D.; Dichtel, William R. (2020-01-08).
3000:
Ratiometric Electrochemical Sensors Based on Nanospheres Derived from Ferrocence-Modified Covalent Organic Frameworks"
521:
427:
398:
231:
2141:
Allendorf, Mark D.; Dong, Renhao; Feng, Xinliang; Kaskel, Stefan; Matoga, Dariusz; Stavila, Vitalie (2020-08-26).
1992:
Kandambeth, Sharath; Mallick, Arijit; Lukose, Binit; Mane, Manoj V.; Heine, Thomas; Banerjee, Rahul (2012-12-05).
732:
was reported. MOF under solvent-free conditions can also be used for catalytic activity in the cycloaddition of CO
1302:
Yaghi, Omar M.; O'Keeffe, Michael; Ockwig, Nathan W.; Chae, Hee K.; Eddaoudi, Mohamed; Kim, Jaheon (2003-06-12).
3532:"Record Ultralarge-Pores, Low Density Three-Dimensional Covalent Organic Framework for Controlled Drug Delivery"
2511:"A Stable and Conductive Metallophthalocyanine Framework for Electrocatalytic Carbon Dioxide Reduction in Water"
782:
A few COFs possess the stability and conductivity necessary to perform well in energy storage applications like
3614:
2784:
Fenton, Julie L.; Burke, David W.; Qian, Dingwen; Olvera de la Cruz, Monica; Dichtel, William R. (2021-01-27).
819:
545:(AFM) have also been used to characterize COF microstructural information as well. Additionally, methods like
3340:"A Microporous Covalent-Organic Framework with Abundant Accessible Carbonyl Groups for Lithium-Ion Batteries"
2731:"Covalent Organic Framework–Covalent Organic Framework Bilayer Membranes for Highly Selective Gas Separation"
346:
Another class of high performance polymer frameworks with regular porosity and high surface area is based on
2645:"High H 2 Uptake in Li-, Na-, K-Metalated Covalent Organic Frameworks and Metal Organic Frameworks at 298 K"
2509:
Huang, Ning; Lee, Ka Hung; Yue, Yan; Xu, Xiaoyi; Irle, Stefan; Jiang, Qiuhong; Jiang, Donglin (2020-09-14).
839:
542:
490:
169:
143:
68:
2298:"Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area"
53:
181:
124:
3599:
208:; in the context of this page it refers to the geometry of the units defined by the points of extension.
2963:
517:
294:
225:
128:
431:
the introduction of the second precursor in vapor form. This results in the deposition of the COF as a
3338:
Luo, Zhiqiang; Liu, Luojia; Ning, Jiaxin; Lei, Kaixiang; Lu, Yong; Li, Fujun; Chen, Jun (2018-07-20).
3226:
3398:
3288:
2990:
Liang, Huihui; Xu, Mengli; Zhu, Yongmei; Wang, Linyu; Xie, Yi; Song, Yonghai; Wang, Li (2020-01-24).
2656:
2351:
2210:
1684:
1626:
1577:
1464:
Feng, Liang; Wang, Kun-Yu; Lv, Xiu-Liang; Yan, Tian-Hao; Li, Jian-Rong; Zhou, Hong-Cai (2020-02-12).
1126:
934:
486:
3506:
3088:"Covalent Organic Frameworks for Heterogeneous Catalysis: Principle, Current Status, and Challenges"
2255:
1523:
593:
storage of any material. Thus 3D COFs are most promising new candidates in the quest for practical H
3452:"Phenazine-Based Covalent Organic Framework Cathode Materials with High Energy and Power Densities"
2936:
Hussain, MD. Waseem; Bhardwaj, Vipin; GIRI, ARKAPRABHA; Chande, Ajit; Patra, Abhijit (2019-11-27).
824:
807:
783:
554:
537:(TEM) can resolve surface structure and morphology, and microstructural information, respectively.
3391:
Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences
2397:"Solving the COF trilemma: towards crystalline, stable and functional covalent organic frameworks"
3487:
3367:
3320:
3276:
3257:
3171:
3068:
3048:
3029:
2821:
2766:
2546:
2486:
2178:
2123:
1974:
1755:
1735:
1716:
1501:
1443:
1349:
1150:
1012:
958:
516:
In order to verify and analyze COF linkage formation, various techniques can be employed such as
506:
282:
84:
3585:
338:
985:"Molecular Engineering of Multifunctional Metallophthalocyanine-Containing Framework Materials"
3553:
3479:
3471:
3432:
3414:
3359:
3312:
3304:
3249:
3207:
3125:
3107:
3021:
2897:
2813:
2805:
2758:
2750:
2680:
2672:
2598:
2538:
2530:
2478:
2470:
2426:
2418:
2377:
2369:
2317:
2271:
2236:
2228:
2170:
2162:
2115:
2073:
2065:
2021:
2013:
1966:
1958:
1888:
1855:
1847:
1806:
1708:
1700:
1650:
1642:
1595:
1539:
1493:
1485:
1435:
1396:
1388:
1341:
1333:
1284:
1276:
1142:
1062:
1054:
1004:
950:
900:
892:
510:
502:
325:
2510:
2046:"β-Ketoenamine-Linked Covalent Organic Frameworks Capable of Pseudocapacitive Energy Storage"
3543:
3463:
3422:
3406:
3387:"Metal- and covalent-organic frameworks as solid-state electrolytes for metal-ion batteries"
3351:
3296:
3241:
3199:
3161:
3153:
3115:
3099:
3060:
3011:
2945:
2887:
2879:
2850:
2797:
2742:
2710:
2664:
2627:
2588:
2522:
2462:
2451:"Two-Dimensional Chemiresistive Covalent Organic Framework with High Intrinsic Conductivity"
2408:
2359:
2309:
2263:
2218:
2154:
2107:
2057:
2005:
1950:
1939:"Interlayer Hydrogen-Bonded Covalent Organic Frameworks as High-Performance Supercapacitors"
1916:
1837:
1798:
1747:
1692:
1634:
1585:
1531:
1477:
1427:
1380:
1323:
1315:
1266:
1237:
1206:
1179:
1134:
1101:
1046:
996:
942:
884:
708:. That means the functions of COFs have not yet been well explored, but COFs can be used as
509:(PXRD) is used. In conjunction with simulated powder packing models, PXRD can determine COF
481:
1614:
2976:
771:
767:
729:
664:
257:
173:
984:
923:"Covalent organic frameworks: a materials platform for structural and functional designs"
3581:
3402:
3292:
2660:
2577:"Eyes of covalent organic frameworks: cooperation between analytical chemistry and COFs"
2355:
2214:
1688:
1630:
1581:
1130:
938:
3427:
3386:
3120:
3087:
2892:
2867:
2267:
2142:
1535:
725:
713:
27:
3064:
1672:
983:
Aykanat, Aylin; Meng, Zheng; Benedetto, Georganna; Mirica, Katherine A. (2020-07-14).
3608:
3491:
3371:
3324:
3261:
3175:
3072:
3033:
2825:
2550:
2490:
2182:
2127:
1978:
1773:
Jackson, Karl T.; Rabbani, Mohammad G.; Reich, Thomas E.; El-Kaderi, Hani M. (2011).
1759:
1505:
1415:
1255:"Reticular Chemistry—Construction, Properties, and Precision Reactions of Frameworks"
1016:
962:
834:
746:
618:
578:
351:
139:
45:
2770:
1720:
1465:
1447:
1154:
2786:"Polycrystalline Covalent Organic Framework Films Act as Adsorbents, Not Membranes"
1353:
736:
and epoxides into cyclic organic carbonates with enhanced catalyst recyclability.
701:
681:
586:
470:
394:
386:
307:
1000:
922:
278:
3157:
1751:
1638:
684:
functionalities alternately linked in a mesoporous hexagonal skeleton, is highly
123:(COF-5) revealed 2-dimensional expanded porous graphitic layers that have either
3451:
2937:
2785:
2158:
1938:
1876:; Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis.
1873:
705:
685:
157:
3103:
2949:
1775:"Synthesis of highly porous borazine-linked polymers and their application to H
1734:
Yusran, Yusran; Li, Hui; Guan, Xinyu; Fang, Qianrong; Qiu, Shilun (June 2020).
946:
196:
3339:
2297:
1590:
1565:
1431:
1167:
Kitagawa, S.; Kitaura, R.; Noro, S.; Functional Porous Coordination Polymers.
689:
450:
83:). Powder X-ray diffraction studies of the highly crystalline products having
49:
3475:
3418:
3308:
3111:
3025:
2809:
2754:
2676:
2644:
2602:
2534:
2474:
2422:
2373:
2232:
2199:"Seeded growth of single-crystal two-dimensional covalent organic frameworks"
2166:
2119:
2069:
2045:
2017:
1962:
1851:
1810:
1704:
1646:
1599:
1489:
1439:
1392:
1337:
1303:
1280:
1058:
1008:
954:
896:
770:
for energy-related catalysis, including carbon dioxide electro-reduction and
2593:
2576:
2364:
2340:"Single-crystal x-ray diffraction structures of covalent organic frameworks"
2339:
2223:
2198:
1696:
1416:"From Top-Down to Bottom-Up to Hybrid Nanotechnologies: Road to Nanodevices"
1138:
1105:
432:
3557:
3548:
3531:
3483:
3436:
3410:
3363:
3355:
3316:
3300:
3253:
3211:
3203:
3129:
3016:
2991:
2917:"New Technology to Capture, Convert Carbon Dioxide | MIT Technology Review"
2901:
2883:
2854:
2817:
2762:
2684:
2542:
2526:
2482:
2430:
2381:
2321:
2313:
2240:
2174:
2077:
2025:
1970:
1892:
1859:
1712:
1654:
1497:
1400:
1345:
1288:
1183:
1146:
1066:
904:
3507:"Nano-sponges on graphene make efficient filters of industrial wastewater"
2643:
Mendoza-Cortés, José L.; Han, Sang Soo; Goddard, William A. (2012-02-16).
2256:"Synthesis of 2D Covalent Organic Frameworks at the Solid–Vapor Interface"
374:
172:(MOFs), and covalent organic frameworks (COFs). Zeolites are microporous,
3467:
3245:
2801:
2746:
2466:
1954:
1481:
1328:
1271:
1254:
724:
In 2015 the use of highly porous, catalyst-decorated COFs for converting
709:
355:
347:
319:
290:
132:
32:
3166:
2575:
Guo, Hao; Zhang, Longwen; Xue, Rui; Ma, Baolong; Yang, Wu (2019-03-26).
1319:
1241:
2938:"Functional Ionic Porous Frameworks Based on Triaminoguanidinium for CO
2730:
2450:
2413:
2396:
2111:
2095:
1842:
1825:
1802:
1774:
1384:
1368:
1367:
Yu, Hai-Dong; Regulacio, Michelle D.; Ye, Enyi; Han, Ming-Yong (2013).
1050:
1034:
888:
872:
844:
787:
390:
246:
205:
177:
165:
2714:
2668:
2631:
2061:
2009:
1920:
1210:
677:
286:
154:
1993:
1566:"Reticular chemistry at the atomic, molecular, and framework scales"
188:
areas surpassing those of well-known zeolites and porous silicates.
2449:
Meng, Zheng; Stolz, Robert M.; Mirica, Katherine A. (2019-07-31).
469:
383:
373:
230:
228:", abiding by the concept termed by Arthur R. von Hippel in 1956.
195:
873:"Covalent organic frameworks (COFs): from design to applications"
749:
sensors, as well as electrochemical sensors for small molecules.
1826:"On the road towards electroactive covalent organic frameworks"
1524:"Two- and Three-dimensional Covalent Organic Frameworks (COFs)"
602:
180:
and are a new development on the interface between molecular
3047:
Hu, Hui; Yan, Qianqian; Ge, Rile; Gao, Yanan (July 2018).
1564:
Zhang, Yue-Biao; Li, Qiaowei; Deng, Hexiang (2021-11-28).
557:
can be used to identify elemental composition and ratios.
806:
A 3D COF was created, characterised by an interconnected
389:
reaction which eliminates water (exemplified by reacting
3049:"Covalent organic frameworks as heterogeneous catalysts"
921:
Huang, Ning; Wang, Ping; Jiang, Donglin (2016-09-20).
322:) ring with the elimination of three water molecules.
1304:"Reticular synthesis and the design of new materials"
758:
electrochemical, as well as photochemical reactions.
676:
A highly ordered π-conjugation TP-COF, consisting of
3277:"Covalent‐Organic‐Framework‐Based Li–CO 2 Batteries"
2143:"Electronic Devices Using Open Framework Materials"
1084:
1082:
1080:
1078:
1076:
342:
Formation of CTF-1 COF featuring triazine linkages.
1673:"Porous, Crystalline, Covalent Organic Frameworks"
1033:Feng, Xiao; Ding, Xuesong; Jiang, Donglin (2012).
354:reaction of simple, cheap, and abundant aromatic
1466:"Modular Total Synthesis in Reticular Chemistry"
786:, and various different metal-ion batteries and
370:A structural representation of the TpOMe-DAQ COF
306:The most popular COF synthesis route is a boron
408:A structural representation of the DAAQ-TFP COF
3385:Miner, Elise M.; Dincă, Mircea (2019-07-15).
1369:"Chemical routes to top-down nanofabrication"
8:
2395:Haase, Frederik; Lotsch, Bettina V. (2020).
551:inductively coupled plasma mass spectrometry
1736:"Covalent Organic Frameworks for Catalysis"
350:materials which can be achieved by dynamic
164:Types of porous crystalline solids include
1522:Chen, Q.; Dalapati, S.; Jiang, D. (2017),
3547:
3426:
3165:
3119:
3015:
2891:
2592:
2412:
2363:
2222:
1841:
1589:
1528:Comprehensive Supramolecular Chemistry II
1327:
1270:
3456:Journal of the American Chemical Society
3234:Journal of the American Chemical Society
2790:Journal of the American Chemical Society
2735:Journal of the American Chemical Society
2455:Journal of the American Chemical Society
2050:Journal of the American Chemical Society
1998:Journal of the American Chemical Society
1943:Journal of the American Chemical Society
1470:Journal of the American Chemical Society
1259:Journal of the American Chemical Society
1220:
1218:
1194:James, S. L.; Metal-organic frameworks.
403:
365:
337:
324:
277:
256:
200:Schematic Figure of Reticular Chemistry.
3600:Welcome to the Yaghi Laboratory Website
3344:Angewandte Chemie International Edition
3086:Guo, Jia; Jiang, Donglin (2020-06-24).
2724:
2722:
2570:
2568:
2566:
2564:
2562:
2560:
2515:Angewandte Chemie International Edition
2504:
2502:
2500:
2444:
2442:
2440:
2333:
2331:
2302:Angewandte Chemie International Edition
2089:
2087:
1414:Teo, Boon K.; Sun, X. H. (2006-12-05).
860:
766:COFs have been studied as non-metallic
3192:ACS Applied Materials & Interfaces
2972:
2961:
2915:Martin, Richard (September 24, 2015).
1459:
1457:
2260:Encyclopedia of Interfacial Chemistry
2039:
2037:
2035:
1932:
1930:
1928:
1666:
1664:
1517:
1515:
1028:
1026:
978:
976:
974:
972:
916:
914:
866:
864:
7:
1824:Dogru, Mirjam; Bein, Thomas (2014).
2649:The Journal of Physical Chemistry A
216:Reticular synthesis enables facile
142:and colleagues, which received the
2942:Conversion and Combating Microbes"
2268:10.1016/b978-0-12-409547-2.13071-9
1536:10.1016/b978-0-12-409547-2.12608-3
871:Ding, San-Yuan; Wang, Wei (2013).
16:Class of solid chemical substances
14:
850:Hydrogen-bonded organic framework
44:While at University of Michigan,
3574:
547:X-ray photoelectron spectroscopy
535:transmission electron microscopy
2581:Reviews in Analytical Chemistry
451:Brunauer–Emmett–Teller analysis
3505:Irving, Michael (2022-08-05).
2262:, Elsevier, pp. 446–452,
1530:, Elsevier, pp. 271–290,
830:Conjugated microporous polymer
1:
3065:10.1016/S1872-2067(18)63057-8
1613:von Hippel, A. (1956-02-24).
1253:Yaghi, Omar M. (2016-12-07).
1035:"Covalent organic frameworks"
1001:10.1021/acs.chemmater.9b05289
696:Porosity/surface-area effects
539:Scanning tunneling microscope
399:1,3,5-triformylphloroglucinol
3158:10.1016/j.mtchem.2018.12.002
3053:Chinese Journal of Catalysis
1752:10.1016/j.enchem.2020.100035
1639:10.1126/science.123.3191.315
802:Pharmaceutical drug delivery
531:scanning electron microscope
334:Triazine based trimerization
3587:covalent organic framework
2254:Chen, T.; Wang, D. (2018),
2159:10.1021/acs.chemrev.0c00033
529:imagine techniques such as
20:Covalent organic frameworks
3631:
3104:10.1021/acscentsci.0c00463
3004:ACS Applied Nano Materials
2950:10.26434/chemrxiv.10332431
1872:Kuhn, P.; Antonietti, M.;
1671:Cote, A. P. (2005-11-18).
1420:Journal of Cluster Science
947:10.1038/natrevmats.2016.68
566:Gas storage and separation
522:nuclear magnetic resonance
275:the formation of 3D COFs.
3146:Materials Today Chemistry
1591:10.1007/s12274-020-3226-6
1432:10.1007/s10876-006-0086-5
489:, respectively. Emergent
2401:Chemical Society Reviews
1373:Chemical Society Reviews
1039:Chemical Society Reviews
927:Nature Reviews Materials
663:. The COFs outperformed
507:powder X-ray diffraction
297:of phenyldiboronic acid.
293:rings, synthesized by a
192:Secondary building units
170:metal-organic frameworks
2594:10.1515/revac-2017-0023
2365:10.1126/science.aat7679
2224:10.1126/science.aar7883
1697:10.1126/science.1120411
1615:"Molecular Engineering"
1139:10.1126/science.1139915
1106:10.1126/science.1120411
840:Metal-organic framework
543:atomic force microscopy
520:(IR) spectroscopy, and
491:electrical conductivity
285:of COF-1 consisting of
184:and materials science.
144:Newcomb Cleveland Prize
69:hexahydroxytriphenylene
3549:10.1002/anie.202300172
3411:10.1098/rsta.2018.0225
3356:10.1002/anie.201805540
3301:10.1002/adma.201905879
3204:10.1021/acsami.5b12370
3017:10.1021/acsanm.9b02117
2971:Cite journal requires
2884:10.1002/anie.201700271
2855:10.1002/anie.200890235
2527:10.1002/anie.202005274
2314:10.1002/anie.200904637
1184:10.1002/anie.200300610
989:Chemistry of Materials
820:Jose L. Mendoza-Cortes
476:
435:on the solid support.
413:Solvothermal synthesis
409:
379:
371:
343:
330:
298:
262:
237:
201:
182:coordination chemistry
125:staggered conformation
2840:Angew. Chem. Int. Ed.
1878:Angew. Chem. Int. Ed.
1169:Angew. Chem. Int. Ed.
784:lithium-ion batteries
473:
407:
377:
369:
341:
328:
295:condensation reaction
281:
260:
234:
226:molecular engineering
199:
129:eclipsed conformation
54:phenyl diboronic acid
3468:10.1021/jacs.9b08147
3246:10.1021/jacs.7b11940
2872:Angew. Chem. Int. Ed
2802:10.1021/jacs.0c11159
2747:10.1021/jacs.8b05136
2467:10.1021/jacs.9b03441
1955:10.1021/jacs.8b06460
1482:10.1021/jacs.9b12408
1272:10.1021/jacs.6b11821
3403:2019RSPTA.37780225M
3293:2019AdM....3105879L
3092:ACS Central Science
2741:(32): 10094–10098.
2661:2012JPCA..116.1621M
2521:(38): 16587–16593.
2461:(30): 11929–11937.
2356:2018Sci...361...48M
2215:2018Sci...361...52E
2056:(45): 16821–16824.
2004:(48): 19524–19527.
1949:(35): 10941–10945.
1689:2005Sci...310.1166C
1683:(5751): 1166–1170.
1631:1956Sci...123..315V
1582:2021NaRes..14..335Z
1320:10.1038/nature01650
1265:(48): 15507–15509.
1242:10.1038/nature01650
1131:2007Sci...316..268E
939:2016NatRM...116068H
825:Reticular chemistry
555:combustion analysis
422:Templated synthesis
253:Synthetic chemistry
218:bottom-up synthesis
212:Reticular synthesis
3584:has a profile for
3542:(13): e202300172.
3397:(2149): 20180225.
3281:Advanced Materials
2626:, pp 11580–11581.
2414:10.1039/D0CS01027H
2112:10.1039/C9MH00856J
2100:Materials Horizons
1843:10.1039/C3CC46767H
1803:10.1039/c1py00374g
1385:10.1039/c3cs60113g
1051:10.1039/c2cs35157a
889:10.1039/C2CS35072F
688:, harvests a wide
672:Optical properties
477:
410:
380:
372:
362:Imine condensation
344:
331:
302:Boron condensation
299:
283:Skeletal structure
263:
238:
222:bottom-up approach
202:
85:empirical formulas
3590:
3536:Angewandte Chemie
3350:(30): 9443–9446.
2878:(24): 6946–6951.
2715:10.1021/om1009632
2669:10.1021/jp206981d
2632:10.1021/ja803247y
2617:J. Am. Chem. Soc.
2407:(23): 8469–8500.
2308:(50): 9457–9460.
2277:978-0-12-809894-3
2153:(16): 8581–8640.
2062:10.1021/ja409421d
2010:10.1021/ja308278w
1921:10.1021/ja8096256
1836:(42): 5531–5546.
1791:Polymer Chemistry
1625:(3191): 315–317.
1545:978-0-12-803199-5
1314:(6941): 705–714.
1125:(5822): 268–272.
995:(13): 5372–5409.
511:crystal structure
503:X-ray diffraction
428:dynamic chemistry
26:) are a class of
3622:
3588:
3578:
3577:
3562:
3561:
3551:
3527:
3521:
3520:
3518:
3517:
3502:
3496:
3495:
3447:
3441:
3440:
3430:
3382:
3376:
3375:
3335:
3329:
3328:
3272:
3266:
3265:
3240:(3): 1116–1122.
3231:
3222:
3216:
3215:
3198:(8): 5366–5375.
3186:
3180:
3179:
3169:
3140:
3134:
3133:
3123:
3083:
3077:
3076:
3059:(7): 1167–1179.
3044:
3038:
3037:
3019:
2987:
2981:
2980:
2974:
2969:
2967:
2959:
2957:
2956:
2933:
2927:
2926:
2924:
2923:
2912:
2906:
2905:
2895:
2863:
2857:
2849:, pp 8826-8830.
2836:
2830:
2829:
2796:(3): 1466–1473.
2781:
2775:
2774:
2726:
2717:
2709:, pp 6790–6800.
2695:
2689:
2688:
2655:(6): 1621–1631.
2640:
2634:
2613:
2607:
2606:
2596:
2572:
2555:
2554:
2506:
2495:
2494:
2446:
2435:
2434:
2416:
2392:
2386:
2385:
2367:
2335:
2326:
2325:
2293:
2287:
2286:
2285:
2284:
2251:
2245:
2244:
2226:
2193:
2187:
2186:
2147:Chemical Reviews
2138:
2132:
2131:
2091:
2082:
2081:
2041:
2030:
2029:
1989:
1983:
1982:
1934:
1923:
1915:, pp 4570-4571.
1905:J. Am. Chem. Soc
1901:
1895:
1887:, pp 3450-3453.
1870:
1864:
1863:
1845:
1821:
1815:
1814:
1770:
1764:
1763:
1731:
1725:
1724:
1668:
1659:
1658:
1610:
1604:
1603:
1593:
1561:
1555:
1554:
1553:
1552:
1519:
1510:
1509:
1476:(6): 3069–3076.
1461:
1452:
1451:
1411:
1405:
1404:
1364:
1358:
1357:
1331:
1299:
1293:
1292:
1274:
1250:
1244:
1222:
1213:
1211:10.1039/B200393G
1192:
1186:
1178:, pp 2334-2375.
1165:
1159:
1158:
1114:
1108:
1100:, pp 1166-1170.
1086:
1071:
1070:
1030:
1021:
1020:
980:
967:
966:
918:
909:
908:
868:
794:Water filtration
768:electrocatalysts
762:Electrocatalysis
665:molecular sieves
574:Hydrogen storage
497:Characterization
289:rings joined by
3630:
3629:
3625:
3624:
3623:
3621:
3620:
3619:
3615:Porous polymers
3605:
3604:
3596:
3595:
3594:
3579:
3575:
3570:
3565:
3529:
3528:
3524:
3515:
3513:
3504:
3503:
3499:
3449:
3448:
3444:
3384:
3383:
3379:
3337:
3336:
3332:
3287:(48): 1905879.
3274:
3273:
3269:
3229:
3224:
3223:
3219:
3188:
3187:
3183:
3142:
3141:
3137:
3085:
3084:
3080:
3046:
3045:
3041:
2999:
2995:
2989:
2988:
2984:
2970:
2960:
2954:
2952:
2941:
2935:
2934:
2930:
2921:
2919:
2914:
2913:
2909:
2865:
2864:
2860:
2837:
2833:
2783:
2782:
2778:
2728:
2727:
2720:
2699:Organometallics
2696:
2692:
2642:
2641:
2637:
2614:
2610:
2574:
2573:
2558:
2508:
2507:
2498:
2448:
2447:
2438:
2394:
2393:
2389:
2350:(6397): 48–52.
2337:
2336:
2329:
2295:
2294:
2290:
2282:
2280:
2278:
2253:
2252:
2248:
2209:(6397): 52–57.
2195:
2194:
2190:
2140:
2139:
2135:
2093:
2092:
2085:
2043:
2042:
2033:
1991:
1990:
1986:
1936:
1935:
1926:
1902:
1898:
1871:
1867:
1823:
1822:
1818:
1786:
1782:
1778:
1772:
1771:
1767:
1733:
1732:
1728:
1670:
1669:
1662:
1612:
1611:
1607:
1563:
1562:
1558:
1550:
1548:
1546:
1521:
1520:
1513:
1463:
1462:
1455:
1413:
1412:
1408:
1379:(14): 6006–18.
1366:
1365:
1361:
1301:
1300:
1296:
1252:
1251:
1247:
1223:
1216:
1196:Chem. Soc. Rev.
1193:
1189:
1166:
1162:
1116:
1115:
1111:
1087:
1074:
1045:(18): 6010–22.
1032:
1031:
1024:
982:
981:
970:
920:
919:
912:
870:
869:
862:
858:
816:
804:
796:
780:
772:water splitting
764:
755:
742:
735:
730:carbon monoxide
722:
698:
674:
662:
658:
654:
650:
646:
642:
636:
628:
624:
616:
614:Methane storage
609:
600:
596:
592:
584:
576:
568:
563:
526:solid-state NMR
499:
468:
459:
446:
441:
424:
415:
364:
336:
317:
313:
304:
272:
255:
214:
194:
174:aluminosilicate
152:
122:
118:
114:
110:
106:
102:
98:
94:
90:
82:
78:
74:
66:
63:
59:
42:
28:porous polymers
17:
12:
11:
5:
3628:
3626:
3618:
3617:
3607:
3606:
3603:
3602:
3580:
3573:
3572:
3571:
3569:
3568:External links
3566:
3564:
3563:
3522:
3497:
3442:
3377:
3330:
3267:
3217:
3181:
3135:
3098:(6): 869–879.
3078:
3039:
3010:(1): 555–562.
2997:
2993:
2982:
2973:|journal=
2939:
2928:
2907:
2858:
2831:
2776:
2718:
2690:
2635:
2608:
2556:
2496:
2436:
2387:
2327:
2288:
2276:
2246:
2188:
2133:
2106:(2): 411–454.
2083:
2031:
1984:
1924:
1896:
1865:
1816:
1784:
1780:
1776:
1765:
1726:
1660:
1605:
1576:(2): 335–337.
1556:
1544:
1511:
1453:
1426:(4): 529–540.
1406:
1359:
1294:
1245:
1236:, pp 705-714.
1214:
1205:, pp 276-288.
1187:
1160:
1109:
1072:
1022:
968:
910:
883:(2): 548–568.
877:Chem. Soc. Rev
859:
857:
854:
853:
852:
847:
842:
837:
832:
827:
822:
815:
812:
803:
800:
795:
792:
779:
778:Energy storage
776:
763:
760:
754:
751:
747:chemiresistive
741:
738:
733:
726:carbon dioxide
721:
720:Carbon capture
718:
714:gas separation
697:
694:
673:
670:
660:
656:
652:
648:
644:
640:
635:
634:Gas separation
632:
626:
622:
615:
612:
607:
598:
594:
590:
582:
575:
572:
567:
564:
562:
559:
553:(ICP-MS), and
498:
495:
467:
464:
458:
455:
445:
442:
440:
437:
423:
420:
414:
411:
363:
360:
335:
332:
315:
311:
303:
300:
271:
268:
254:
251:
213:
210:
193:
190:
151:
148:
120:
116:
112:
108:
104:
100:
96:
92:
88:
80:
76:
72:
64:
61:
57:
48:(currently at
41:
38:
15:
13:
10:
9:
6:
4:
3:
2:
3627:
3616:
3613:
3612:
3610:
3601:
3598:
3597:
3592:
3591:
3583:
3567:
3559:
3555:
3550:
3545:
3541:
3537:
3533:
3526:
3523:
3512:
3508:
3501:
3498:
3493:
3489:
3485:
3481:
3477:
3473:
3469:
3465:
3461:
3457:
3453:
3446:
3443:
3438:
3434:
3429:
3424:
3420:
3416:
3412:
3408:
3404:
3400:
3396:
3392:
3388:
3381:
3378:
3373:
3369:
3365:
3361:
3357:
3353:
3349:
3345:
3341:
3334:
3331:
3326:
3322:
3318:
3314:
3310:
3306:
3302:
3298:
3294:
3290:
3286:
3282:
3278:
3271:
3268:
3263:
3259:
3255:
3251:
3247:
3243:
3239:
3235:
3228:
3221:
3218:
3213:
3209:
3205:
3201:
3197:
3193:
3185:
3182:
3177:
3173:
3168:
3163:
3159:
3155:
3151:
3147:
3139:
3136:
3131:
3127:
3122:
3117:
3113:
3109:
3105:
3101:
3097:
3093:
3089:
3082:
3079:
3074:
3070:
3066:
3062:
3058:
3054:
3050:
3043:
3040:
3035:
3031:
3027:
3023:
3018:
3013:
3009:
3005:
3001:
2986:
2983:
2978:
2965:
2951:
2947:
2943:
2932:
2929:
2918:
2911:
2908:
2903:
2899:
2894:
2889:
2885:
2881:
2877:
2873:
2869:
2862:
2859:
2856:
2852:
2848:
2844:
2841:
2835:
2832:
2827:
2823:
2819:
2815:
2811:
2807:
2803:
2799:
2795:
2791:
2787:
2780:
2777:
2772:
2768:
2764:
2760:
2756:
2752:
2748:
2744:
2740:
2736:
2732:
2725:
2723:
2719:
2716:
2712:
2708:
2704:
2700:
2694:
2691:
2686:
2682:
2678:
2674:
2670:
2666:
2662:
2658:
2654:
2650:
2646:
2639:
2636:
2633:
2629:
2625:
2621:
2618:
2612:
2609:
2604:
2600:
2595:
2590:
2586:
2582:
2578:
2571:
2569:
2567:
2565:
2563:
2561:
2557:
2552:
2548:
2544:
2540:
2536:
2532:
2528:
2524:
2520:
2516:
2512:
2505:
2503:
2501:
2497:
2492:
2488:
2484:
2480:
2476:
2472:
2468:
2464:
2460:
2456:
2452:
2445:
2443:
2441:
2437:
2432:
2428:
2424:
2420:
2415:
2410:
2406:
2402:
2398:
2391:
2388:
2383:
2379:
2375:
2371:
2366:
2361:
2357:
2353:
2349:
2345:
2341:
2334:
2332:
2328:
2323:
2319:
2315:
2311:
2307:
2303:
2299:
2292:
2289:
2279:
2273:
2269:
2265:
2261:
2257:
2250:
2247:
2242:
2238:
2234:
2230:
2225:
2220:
2216:
2212:
2208:
2204:
2200:
2192:
2189:
2184:
2180:
2176:
2172:
2168:
2164:
2160:
2156:
2152:
2148:
2144:
2137:
2134:
2129:
2125:
2121:
2117:
2113:
2109:
2105:
2101:
2097:
2090:
2088:
2084:
2079:
2075:
2071:
2067:
2063:
2059:
2055:
2051:
2047:
2040:
2038:
2036:
2032:
2027:
2023:
2019:
2015:
2011:
2007:
2003:
1999:
1995:
1988:
1985:
1980:
1976:
1972:
1968:
1964:
1960:
1956:
1952:
1948:
1944:
1940:
1933:
1931:
1929:
1925:
1922:
1918:
1914:
1910:
1906:
1900:
1897:
1894:
1890:
1886:
1882:
1879:
1875:
1869:
1866:
1861:
1857:
1853:
1849:
1844:
1839:
1835:
1831:
1827:
1820:
1817:
1812:
1808:
1804:
1800:
1796:
1792:
1788:
1769:
1766:
1761:
1757:
1753:
1749:
1746:(3): 100035.
1745:
1741:
1737:
1730:
1727:
1722:
1718:
1714:
1710:
1706:
1702:
1698:
1694:
1690:
1686:
1682:
1678:
1674:
1667:
1665:
1661:
1656:
1652:
1648:
1644:
1640:
1636:
1632:
1628:
1624:
1620:
1616:
1609:
1606:
1601:
1597:
1592:
1587:
1583:
1579:
1575:
1571:
1570:Nano Research
1567:
1560:
1557:
1547:
1541:
1537:
1533:
1529:
1525:
1518:
1516:
1512:
1507:
1503:
1499:
1495:
1491:
1487:
1483:
1479:
1475:
1471:
1467:
1460:
1458:
1454:
1449:
1445:
1441:
1437:
1433:
1429:
1425:
1421:
1417:
1410:
1407:
1402:
1398:
1394:
1390:
1386:
1382:
1378:
1374:
1370:
1363:
1360:
1355:
1351:
1347:
1343:
1339:
1335:
1330:
1329:2027.42/62718
1325:
1321:
1317:
1313:
1309:
1305:
1298:
1295:
1290:
1286:
1282:
1278:
1273:
1268:
1264:
1260:
1256:
1249:
1246:
1243:
1239:
1235:
1231:
1227:
1221:
1219:
1215:
1212:
1208:
1204:
1200:
1197:
1191:
1188:
1185:
1181:
1177:
1173:
1170:
1164:
1161:
1156:
1152:
1148:
1144:
1140:
1136:
1132:
1128:
1124:
1120:
1113:
1110:
1107:
1103:
1099:
1095:
1091:
1085:
1083:
1081:
1079:
1077:
1073:
1068:
1064:
1060:
1056:
1052:
1048:
1044:
1040:
1036:
1029:
1027:
1023:
1018:
1014:
1010:
1006:
1002:
998:
994:
990:
986:
979:
977:
975:
973:
969:
964:
960:
956:
952:
948:
944:
940:
936:
933:(10): 16068.
932:
928:
924:
917:
915:
911:
906:
902:
898:
894:
890:
886:
882:
878:
874:
867:
865:
861:
855:
851:
848:
846:
843:
841:
838:
836:
835:Omar M. Yaghi
833:
831:
828:
826:
823:
821:
818:
817:
813:
811:
809:
801:
799:
793:
791:
789:
785:
777:
775:
773:
769:
761:
759:
752:
750:
748:
739:
737:
731:
727:
719:
717:
715:
711:
707:
703:
695:
693:
691:
687:
683:
679:
671:
669:
666:
633:
631:
620:
619:Omar M. Yaghi
613:
611:
604:
588:
580:
579:Omar M. Yaghi
573:
571:
565:
560:
558:
556:
552:
548:
544:
540:
536:
532:
527:
523:
519:
514:
512:
508:
504:
496:
494:
492:
488:
483:
475:conductivity.
472:
465:
463:
457:Crystallinity
456:
454:
452:
443:
438:
436:
434:
429:
421:
419:
412:
406:
402:
400:
396:
392:
388:
385:
376:
368:
361:
359:
357:
353:
352:trimerization
349:
340:
333:
327:
323:
321:
309:
301:
296:
292:
288:
284:
280:
276:
269:
267:
259:
252:
250:
248:
242:
233:
229:
227:
223:
219:
211:
209:
207:
198:
191:
189:
185:
183:
179:
175:
171:
167:
162:
159:
156:
149:
147:
145:
141:
140:Omar M. Yaghi
136:
134:
130:
126:
111:(COF-1) and C
86:
70:
55:
51:
47:
46:Omar M. Yaghi
39:
37:
34:
29:
25:
21:
3586:
3539:
3535:
3525:
3514:. Retrieved
3510:
3500:
3462:(1): 16–20.
3459:
3455:
3445:
3394:
3390:
3380:
3347:
3343:
3333:
3284:
3280:
3270:
3237:
3233:
3220:
3195:
3191:
3184:
3167:10397/101525
3149:
3145:
3138:
3095:
3091:
3081:
3056:
3052:
3042:
3007:
3003:
2985:
2964:cite journal
2953:. Retrieved
2931:
2920:. Retrieved
2910:
2875:
2871:
2861:
2846:
2842:
2839:
2834:
2793:
2789:
2779:
2738:
2734:
2706:
2702:
2698:
2693:
2652:
2648:
2638:
2623:
2619:
2616:
2611:
2584:
2580:
2518:
2514:
2458:
2454:
2404:
2400:
2390:
2347:
2343:
2305:
2301:
2291:
2281:, retrieved
2259:
2249:
2206:
2202:
2191:
2150:
2146:
2136:
2103:
2099:
2053:
2049:
2001:
1997:
1987:
1946:
1942:
1912:
1908:
1904:
1899:
1884:
1880:
1877:
1868:
1833:
1830:Chem. Commun
1829:
1819:
1797:(12): 2775.
1794:
1790:
1768:
1743:
1739:
1729:
1680:
1676:
1622:
1618:
1608:
1573:
1569:
1559:
1549:, retrieved
1527:
1473:
1469:
1423:
1419:
1409:
1376:
1372:
1362:
1311:
1307:
1297:
1262:
1258:
1248:
1233:
1229:
1225:
1202:
1198:
1195:
1190:
1175:
1171:
1168:
1163:
1122:
1118:
1112:
1097:
1093:
1089:
1042:
1038:
992:
988:
930:
926:
880:
876:
805:
797:
781:
765:
756:
743:
723:
702:surface area
699:
682:triphenylene
675:
637:
617:
587:surface area
577:
569:
561:Applications
515:
500:
487:π-π stacking
478:
466:Conductivity
460:
447:
425:
416:
395:benzaldehyde
387:condensation
381:
345:
308:condensation
305:
273:
270:COF linkages
264:
243:
239:
215:
203:
186:
163:
153:
137:
43:
23:
19:
18:
706:gas storage
686:luminescent
533:(SEM), and
158:crystalline
127:(COF-1) or
3589:(Q5178887)
3516:2022-08-06
2955:2022-06-22
2922:2015-09-27
2283:2021-03-01
1874:Thomas, A.
1740:EnergyChem
1551:2021-03-01
856:References
808:mesoporous
690:wavelength
541:(STM) and
439:Properties
50:UCBerkeley
3511:New Atlas
3492:209317683
3476:0002-7863
3419:1364-503X
3372:205407552
3325:204545588
3309:0935-9648
3262:207188096
3176:139305086
3152:: 34–60.
3112:2374-7943
3073:102933312
3034:214062588
3026:2574-0970
2826:231596406
2810:0002-7863
2755:0002-7863
2677:1089-5639
2603:2191-0189
2551:218765357
2535:1433-7851
2491:195694903
2475:0002-7863
2423:0306-0012
2374:0036-8075
2233:0036-8075
2183:220670221
2167:0009-2665
2128:204292382
2120:2051-6347
2070:0002-7863
2018:0002-7863
1979:207193051
1963:0002-7863
1852:1359-7345
1811:1759-9954
1760:219459194
1705:0036-8075
1647:0036-8075
1600:1998-0124
1506:210882977
1490:0002-7863
1440:1040-7278
1393:0306-0012
1338:0028-0836
1281:0002-7863
1059:0306-0012
1017:225664378
1009:0897-4756
963:138892338
955:2058-8437
897:0306-0012
753:Catalysis
712:, or for
710:catalysts
433:thin film
150:Structure
133:Angstroms
3609:Category
3558:36688253
3484:31820958
3437:31130094
3364:29863784
3317:31609043
3254:29284263
3212:26840757
3130:32607434
2902:28318084
2818:33438399
2771:51696424
2763:30021065
2685:22188543
2543:32436331
2483:31241936
2431:33155009
2382:29976818
2322:19921728
2241:29930093
2175:32692163
2078:24147596
2026:23153356
1971:30132332
1893:18330878
1860:24667827
1787:storage"
1783:, and CH
1721:35798005
1713:16293756
1655:17774519
1498:31971790
1448:98710293
1401:23653019
1346:12802325
1289:27934016
1155:19555677
1147:17431178
1067:22821129
905:23060270
814:See also
788:cathodes
518:infrared
444:Porosity
356:nitriles
348:triazine
320:boroxine
291:boroxine
206:zeolites
166:zeolites
33:polymers
3582:Scholia
3428:6562342
3399:Bibcode
3289:Bibcode
3121:7318070
2893:5485174
2657:Bibcode
2352:Bibcode
2344:Science
2211:Bibcode
2203:Science
1685:Bibcode
1677:Science
1627:Bibcode
1619:Science
1578:Bibcode
1354:4300639
1127:Bibcode
1119:Science
1090:Science
935:Bibcode
845:Zeolite
740:Sensing
716:, etc.
655:, and H
549:(XPS),
391:aniline
247:crystal
236:(COFs).
178:ligands
40:History
3556:
3490:
3482:
3474:
3435:
3425:
3417:
3370:
3362:
3323:
3315:
3307:
3260:
3252:
3210:
3174:
3128:
3118:
3110:
3071:
3032:
3024:
2900:
2890:
2824:
2816:
2808:
2769:
2761:
2753:
2683:
2675:
2601:
2549:
2541:
2533:
2489:
2481:
2473:
2429:
2421:
2380:
2372:
2320:
2274:
2239:
2231:
2181:
2173:
2165:
2126:
2118:
2076:
2068:
2024:
2016:
1977:
1969:
1961:
1891:
1858:
1850:
1809:
1758:
1719:
1711:
1703:
1653:
1645:
1598:
1542:
1504:
1496:
1488:
1446:
1438:
1399:
1391:
1352:
1344:
1336:
1308:Nature
1287:
1279:
1226:Nature
1153:
1145:
1065:
1057:
1015:
1007:
961:
953:
903:
895:
678:pyrene
482:Mirica
287:phenyl
155:Porous
67:) and
3488:S2CID
3368:S2CID
3321:S2CID
3258:S2CID
3230:(PDF)
3172:S2CID
3069:S2CID
3030:S2CID
2822:S2CID
2767:S2CID
2587:(1).
2547:S2CID
2487:S2CID
2179:S2CID
2124:S2CID
1975:S2CID
1756:S2CID
1717:S2CID
1502:S2CID
1444:S2CID
1350:S2CID
1151:S2CID
1013:S2CID
959:S2CID
728:into
393:with
384:imine
3554:PMID
3480:PMID
3472:ISSN
3433:PMID
3415:ISSN
3360:PMID
3313:PMID
3305:ISSN
3250:PMID
3208:PMID
3126:PMID
3108:ISSN
3022:ISSN
2977:help
2898:PMID
2843:2008
2814:PMID
2806:ISSN
2759:PMID
2751:ISSN
2703:2010
2681:PMID
2673:ISSN
2620:2008
2599:ISSN
2539:PMID
2531:ISSN
2479:PMID
2471:ISSN
2427:PMID
2419:ISSN
2378:PMID
2370:ISSN
2318:PMID
2272:ISBN
2237:PMID
2229:ISSN
2171:PMID
2163:ISSN
2116:ISSN
2074:PMID
2066:ISSN
2022:PMID
2014:ISSN
1967:PMID
1959:ISSN
1909:2009
1889:PMID
1881:2008
1856:PMID
1848:ISSN
1807:ISSN
1779:, CO
1709:PMID
1701:ISSN
1651:PMID
1643:ISSN
1596:ISSN
1540:ISBN
1494:PMID
1486:ISSN
1436:ISSN
1397:PMID
1389:ISSN
1342:PMID
1334:ISSN
1285:PMID
1277:ISSN
1230:2003
1199:2003
1172:2004
1143:PMID
1094:2005
1063:PMID
1055:ISSN
1005:ISSN
951:ISSN
901:PMID
893:ISSN
704:for
680:and
382:The
79:(OH)
24:COFs
3544:doi
3464:doi
3460:142
3423:PMC
3407:doi
3395:377
3352:doi
3297:doi
3242:doi
3238:140
3200:doi
3162:hdl
3154:doi
3116:PMC
3100:doi
3061:doi
3012:doi
2946:doi
2888:PMC
2880:doi
2851:doi
2798:doi
2794:143
2743:doi
2739:140
2711:doi
2665:doi
2653:116
2628:doi
2624:130
2589:doi
2523:doi
2463:doi
2459:141
2409:doi
2360:doi
2348:361
2310:doi
2264:doi
2219:doi
2207:361
2155:doi
2151:120
2108:doi
2058:doi
2054:135
2006:doi
2002:134
1951:doi
1947:140
1917:doi
1913:131
1838:doi
1799:doi
1748:doi
1693:doi
1681:310
1635:doi
1623:123
1586:doi
1532:doi
1478:doi
1474:142
1428:doi
1381:doi
1324:hdl
1316:doi
1312:423
1267:doi
1263:138
1238:doi
1234:423
1207:doi
1180:doi
1135:doi
1123:316
1102:doi
1098:310
1047:doi
997:doi
943:doi
885:doi
659:/CH
647:, H
643:/CO
603:THF
99:·(C
95:BO)
3611::
3552:.
3540:62
3538:.
3534:.
3509:.
3486:.
3478:.
3470:.
3458:.
3454:.
3431:.
3421:.
3413:.
3405:.
3393:.
3389:.
3366:.
3358:.
3348:57
3346:.
3342:.
3319:.
3311:.
3303:.
3295:.
3285:31
3283:.
3279:.
3256:.
3248:.
3236:.
3232:.
3206:.
3194:.
3170:.
3160:.
3150:12
3148:.
3124:.
3114:.
3106:.
3094:.
3090:.
3067:.
3057:39
3055:.
3051:.
3028:.
3020:.
3006:.
3002:.
2992:"H
2968::
2966:}}
2962:{{
2944:.
2896:.
2886:.
2876:56
2874:.
2870:.
2847:47
2845:,
2820:.
2812:.
2804:.
2792:.
2788:.
2765:.
2757:.
2749:.
2737:.
2733:.
2721:^
2707:29
2705:,
2701:.
2679:.
2671:.
2663:.
2651:.
2647:.
2622:,
2597:.
2585:38
2583:.
2579:.
2559:^
2545:.
2537:.
2529:.
2519:59
2517:.
2513:.
2499:^
2485:.
2477:.
2469:.
2457:.
2453:.
2439:^
2425:.
2417:.
2405:49
2403:.
2399:.
2376:.
2368:.
2358:.
2346:.
2342:.
2330:^
2316:.
2306:48
2304:.
2300:.
2270:,
2258:,
2235:.
2227:.
2217:.
2205:.
2201:.
2177:.
2169:.
2161:.
2149:.
2145:.
2122:.
2114:.
2102:.
2098:.
2086:^
2072:.
2064:.
2052:.
2048:.
2034:^
2020:.
2012:.
2000:.
1996:.
1973:.
1965:.
1957:.
1945:.
1941:.
1927:^
1911:,
1907:.
1885:47
1883:.
1854:.
1846:.
1834:50
1832:.
1828:.
1805:.
1793:.
1789:.
1754:.
1742:.
1738:.
1715:.
1707:.
1699:.
1691:.
1679:.
1675:.
1663:^
1649:.
1641:.
1633:.
1621:.
1617:.
1594:.
1584:.
1574:14
1572:.
1568:.
1538:,
1526:,
1514:^
1500:.
1492:.
1484:.
1472:.
1468:.
1456:^
1442:.
1434:.
1424:17
1422:.
1418:.
1395:.
1387:.
1377:42
1375:.
1371:.
1348:.
1340:.
1332:.
1322:.
1310:.
1306:.
1283:.
1275:.
1261:.
1257:.
1232:,
1228:.
1217:^
1203:32
1201:,
1176:43
1174:,
1149:.
1141:.
1133:.
1121:.
1096:,
1092:.
1075:^
1061:.
1053:.
1043:41
1041:.
1037:.
1025:^
1011:.
1003:.
993:32
991:.
987:.
971:^
957:.
949:.
941:.
929:.
925:.
913:^
899:.
891:.
881:42
879:.
875:.
863:^
790:.
651:/N
513:.
249:.
168:,
119:BO
105:12
87:(C
73:18
71:(C
56:(C
3593:.
3560:.
3546::
3519:.
3494:.
3466::
3439:.
3409::
3401::
3374:.
3354::
3327:.
3299::
3291::
3264:.
3244::
3214:.
3202::
3196:8
3178:.
3164::
3156::
3132:.
3102::
3096:6
3075:.
3063::
3036:.
3014::
3008:3
2998:2
2996:O
2994:2
2979:)
2975:(
2958:.
2948::
2940:2
2925:.
2904:.
2882::
2853::
2828:.
2800::
2773:.
2745::
2713::
2687:.
2667::
2659::
2630::
2605:.
2591::
2553:.
2525::
2493:.
2465::
2433:.
2411::
2384:.
2362::
2354::
2324:.
2312::
2266::
2243:.
2221::
2213::
2185:.
2157::
2130:.
2110::
2104:7
2080:.
2060::
2028:.
2008::
1981:.
1953::
1919::
1862:.
1840::
1813:.
1801::
1795:2
1785:4
1781:2
1777:2
1762:.
1750::
1744:2
1723:.
1695::
1687::
1657:.
1637::
1629::
1602:.
1588::
1580::
1534::
1508:.
1480::
1450:.
1430::
1403:.
1383::
1356:.
1326::
1318::
1291:.
1269::
1240::
1209::
1182::
1157:.
1137::
1129::
1104::
1069:.
1049::
1019:.
999::
965:.
945::
937::
931:1
907:.
887::
734:2
661:4
657:2
653:2
649:2
645:2
641:2
639:H
627:4
623:4
608:2
599:2
595:2
591:2
583:2
318:(
316:3
314:O
312:3
121:2
117:4
115:H
113:9
109:1
107:)
103:H
101:9
97:6
93:2
91:H
89:3
81:6
77:6
75:H
65:2
62:4
60:H
58:6
22:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.