894:
886:
878:
1003:
transition state is minimized by the α-carbon configuration holding the largest group away from (trans to) the congested carbonyl group and the allylmetal group approaching past the smallest group on the α-carbon centre. In the example below (Figure "An example of substrate controlled addition of achiral allyl-boron to α-chiral aldehyde"), (R)-2-methylbutanal (1) reacts with the allylboron reagent (2) with two possible diastereomers of which the (R, R)-isomer is the major product. The Cram model of this reaction is shown with the carbonyl group placed trans to the
31:
178:
400:
836:
369:
934:, however, simple qualitative factors may also be used to explain the predominant trends seen for some synthetic steps. The ease and accuracy of this qualitative approach means it is more commonly applied in synthesis and substrate design. Examples of appropriate molecular frameworks are alpha chiral aldehydes and the use of chiral auxiliaries.
1194:
1173:
1023:
Asymmetric stereoinduction can be achieved with the use of chiral auxiliaries. Chiral auxiliaries may be reversibly attached to the substrate, inducing a diastereoselective reaction prior to cleavage, overall producing an enantioselective process. Examples of chiral auxiliaries include, Evans’ chiral
929:
or species react, the precise 3D configurations of the chemical entities involved will determine how they may approach one another. Any restrictions as to how these species may approach each other will determine the configuration of the product of the reaction. In the case of asymmetric induction, we
783:
However, in the case of the syn-substrate, the Felkin–Anh and the Evans model predict different products (non-stereoreinforcing case). It has been found that the size of the incoming nucleophile determines the type of control exerted over the stereochemistry. In the case of a large nucleophile, the
511:
moiety. However, many examples exist of reactions that display stereoselectivity opposite of what is predicted by the basic tenets of the Cram and Felkin–Anh models. Although both of the models include attempts to explain these reversals, the products obtained are still referred to as "anti-Felkin"
142:
Several models exist to describe chiral induction at carbonyl carbons during nucleophilic additions. These models are based on a combination of steric and electronic considerations and are often in conflict with each other. Models have been devised by Cram (1952), Cornforth (1959), Felkin (1969) and
962:
The polar Felkin–Anh model is applied in the scenario where X is an electronegative group. The polar Felkin–Anh model postulates that the observed stereochemistry arises due to hyperconjugative stabilization arising from the anti-periplanar interaction between the C-X antibonding σ* orbital and the
1189:
has been used to prepare chiral allyltitanium compounds for asymmetric allylation with aldehydes. Jim
Leighton has developed chiral allysilicon compounds in which the release of ring strain facilitated the stereoselective allylation reaction, 95% to 98% enantiomeric excess could be achieved for a
458:
2 reactions, without offering justifications as to why this phenomenon was observed. Anh's solution was to offer the antiperiplanar effect as a consequence of asymmetric induction being controlled by both substituent and orbital effects. In this effect, the best nucleophile acceptor σ* orbital is
161:
In certain non-catalytic reactions that diastereomer will predominate, which could be formed by the approach of the entering group from the least hindered side when the rotational conformation of the C-C bond is such that the double bond is flanked by the two least bulky groups attached to the
1738:
Kim, Hyoungsu; Lee, Hyunjoo; Kim, Jayoung; Kim, Sanghee; Kim, Deukjoon (2006-12-01). "A General
Strategy for Synthesis of Both (6Z)- and (6E)-Cladiellin Diterpenes: Total Syntheses of (−)-Cladiella-6,11-dien-3-ol, (+)-Polyanthellin A, (−)-Cladiell-11-ene-3,6,7-triol, and (−)-Deacetoxyalcyonin
1002:
Cram’s rule explains the stereoselectivity by considering the transition state depicted in figure 3. In the transition state the oxygen lone pair is able to interact with the boron centre whilst the allyl group is able to add to the carbon end of the carbonyl group. The steric demand of this
483:
ideas allowed Anh to postulate a non-perpendicular attack by the nucleophile on the carbonyl center, anywhere from 95° to 105° relative to the oxygen-carbon double bond, favoring approach closer to the smaller substituent and thereby solve the problem of predictability for aldehydes.
930:
are considering the effects of one asymmetric centre on a molecule on the reactivity of other functional groups on that molecule. The closer together these two sites are, the larger an influence is expected to be observed. A more holistic approach to evaluating these factors is by
590:, even if the substituent is not the largest of the 3 bonded to the α-carbon. Each model offers a slightly different explanation for this phenomenon. A polar effect was postulated by the Cornforth model and the original Felkin model, which placed the EWG substituent and incoming
1067:. Even a single methyl group is often sufficient to bias the diastereomeric outcome of the reaction. These studies, among others, helped challenge the widely-held scientific belief that large rings are too floppy to provide any kind of stereochemical control.
1184:
was the first to report the chiral allylboron reagents for asymmetric allylation reactions with aldehydes. The chiral allylboron reagents were synthesized from the natural product (+)-a-pinene in two steps. The TADDOL ligands developed by
453:
to correct for two key weaknesses in Felkin's model. The first weakness addressed was the statement by Felkin of a strong polar effect in nucleophilic addition transition states, which leads to the complete inversion of stereochemistry by
1152:
of the newly generated alcohol carbon is determined by the chirality of the allymetal reagents (Figure 1). The chirality of the allymetals usually comes from the asymmetric ligands used. The metals in the allylmetal reagents include
1047:
exist in defined geometries despite having many degrees of freedom. Because of these properties, it is often easier to achieve asymmetric induction with macrocyclic substrates rather than linear ones. Early experiments performed by
946:
and addition of allylmetals. The stereoselectivity of nucleophilic attack at alpha-chiral aldehydes may be described by the Felkin–Anh or polar Felkin Anh models and addition of achiral allylmetals may be described by Cram’s rule.
665:
It has been observed that the stereoelectronic environment at the β-carbon of can also direct asymmetric induction. A number of predictive models have evolved over the years to define the stereoselectivity of such reactions.
674:
According to Reetz, the Cram-chelate model for 1,2-inductions can be extended to predict the chelated complex of a β-alkoxy aldehyde and metal. The nucleophile is seen to attack from the less sterically hindered side and
784:
interaction of the α-stereocenter with the incoming nucleophile becomes dominant; therefore, the Felkin product is the major one. Smaller nucleophiles, on the other hand, result in 1,3 control determining the asymmetry.
710:
Cram and Reetz demonstrated that 1,3-stereocontrol is possible if the reaction proceeds through an acyclic transition state. The reaction of β-alkoxy aldehyde with allyltrimethylsilane showed good selectivity for the
389:(Pitzer strain) involving partial bonds (in transition states) represents a substantial fraction of the strain between fully formed bonds, even when the degree of bonding is quite low. The conformation in the TS is
970:
or organolithium nucleophiles. Claude Spino and co-workers have demonstrated significant stereoselectivity improvements upon switching from vinylgrignard to vinylalane reagents with a number of chiral aldehydes.
769:
If the substrate has both an α- and β-stereocenter, the Felkin–Anh rule (1,2-induction) and the Evans model (1,3-induction) should considered at the same time. If these two stereocenters have an
343:
between the carbonyl substituent (the hydrogen atom in aldehydes) and the largest α-carbonyl substituent. He demonstrated that by increasing the steric bulk of the carbonyl substituent from
2043:
955:
Selectivity in nucleophilic additions to chiral aldehydes is often explained by the Felkin–Anh model (see figure). The nucleophile approaches the carbon of the carbonyl group at the
893:
2048:
646:
interactions in the stabilization of the preferred transition state. A typical reaction illustrating the potential anti-Felkin selectivity of this effect, along with its proposed
1692:
a) Anh, N. T. Top. Curr. Chem. 1980, 88, 145–162; (b) Anh, N. T.; Eisenstein, O. Nouv. J. Chim. 1977, 1, 61–70; (c) Anh, N. T.; Eisenstein, O. Tetrahedron Lett. 1976, 26, 155–158.
1116:
conformation. Substrate-controlled synthetic schemes have many advantages, since they do not require the use of complex asymmetric reagents to achieve selective transformations.
543:
will then attack from the side with the smallest free α-carbon substituent. If the chelating R group is identified as the largest, this will result in an "anti-Felkin" product.
578:
A non-chelating electron-withdrawing substituent effect can also result in anti-Felkin selectivity. If a substituent on the α-carbon is sufficiently electron withdrawing, the
715:
1,3-diol, which was explained by the Cram polar model. The polar benzyloxy group is oriented anti to the carbonyl to minimize dipole interactions and the nucleophile attacks
1268:
Cram, Donald J.; Elhafez, Fathy Ahmed Abd (1952). "Studies in
Stereochemistry. X. The Rule of "Steric Control of Asymmetric Induction" in the Syntheses of Acyclic Systems".
557:
control was recognized and discussed in the first paper establishing the Cram model, causing Cram to assert that his model requires non-chelating conditions. An example of
38:
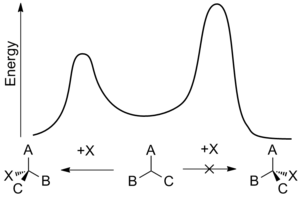
plot of an enantioselective addition reaction. The effect of asymmetric induction is to lower the transition state energy for the formation of one enantiomer over the other
177:
697:
To make such chelates, the metal center must have at least two free coordination sites and the protecting ligands should form a bidentate complex with the Lewis acid.
1909:
885:
1052:
and colleagues showed that medium- and large-ring organic molecules can provide striking levels of stereo induction as substrates in reactions such as kinetic
399:
561:
control of a reaction can be seen here, from a 1987 paper that was the first to directly observe such a "Cram-chelate" intermediate, vindicating the model:
966:
Improving Felkin–Anh selectivity for organometal additions to aldehydes can be achieved by using organo-aluminum nucleophiles instead of the corresponding
1881:
987:
on the aldehyde substrate (Figure "Substrate control: addition of achiral allylmetals to α-chiral aldehydes"). The allylmetal reagents used include
469:
The second weakness in the Felkin Model was the assumption of substituent minimization around the carbonyl R, which cannot be applied to aldehydes.
738:
More recently, Evans presented a different model for nonchelate 1,3-inductions. In the proposed transition state, the β-stereocenter is oriented
164:
The rule indicates that the presence of an asymmetric center in a molecule induces the formation of an asymmetric center adjacent to it based on
1817:
Still, W. Clark; Murata, Shizuaki; Revial, Gilbert; Yoshihara, Kazuo (1983-02-01). "Synthesis of the cytotoxic germacranolide eucannabinolide".
413:
Attack of the nucleophile occurs according to the Dunitz angle (107 degrees), eclipsing the hydrogen, rather than perpendicular to the carbonyl.
512:
products. One of the most common examples of altered asymmetric induction selectivity requires an α-carbon substituted with a component with
1782:
Still, W. Clark (1979-04-01). "(.+-.)-Periplanone-B. Total synthesis and structure of the sex excitant pheromone of the
American cockroach".
1668:
877:
1295:
Torsional strain involving partial bonds. The stereochemistry of the lithium aluminium hydride reduction of some simple open-chain ketones
789:
778:
759:
914:
properties of a molecule may determine the chirality of subsequent chemical reactions on that molecule. This principal is used to design
728:
692:
655:
610:
566:
548:
489:
474:
464:
110:
the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called
1902:
1256:
835:
2081:
1093:, in the presence of an unstrained olefin. En route to (±)-periplanone B, chemists achieved a facial selective epoxidation of an
1321:
It bears mentioning that in
Vietnamese, the surname is given first, and so this would be better called the Felkin–Nguyen Model.
959:. At this trajectory, attack from the bottom face is disfavored due to steric bulk of the adjacent, large, functional group.
642:
The improved Felkin–Anh model, as discussed above, makes a more sophisticated assessment of the polar effect by considering
368:
2066:
1895:
503:
considered and other assumptions, they both attempt to explain the same basic phenomenon: the preferential addition of a
2076:
1015:
reaction happens at the face where the hydrogen (the small group) is, producing the (R, R)-isomer as the major product.
1007:
group (the large group) and the allyl boron approaching past the hydrogen (the small group). The structure is shown in
854:
and the methyl group. The approach of the electrophile preferentially occurs from the same side of the medium group (R
307:
anion (H) is the nucleophile attacking from the least hindered side (imagine hydrogen entering from the paper plane).
30:
230:
The preference for the formation of the threo isomer can be explained by the rule stated above by having the active
1919:
1079:
587:
459:
aligned parallel to both the π and π* orbitals of the carbonyl, which provide stabilization of the incoming anion.
421:
1074:
to achieve desired reaction products. In the synthesis of (−)-cladiella-6,11-dien-3-ol, a strained trisubstituted
480:
393:
and not eclipsed with the substituent R skew with respect to two adjacent groups one of them the smallest in TS A.
2038:
1207:
1071:
292:
742:
to the incoming nucleophile, as seen in the Felkin–Anh model. The polar X group at the β-stereocenter is placed
2143:
1964:
500:
266:
820:
1656:
931:
1180:
Various chiral ligands have been developed to prepare chiral allylmetals for the reaction with aldehydes.
1025:
983:
forms a chiral alcohol, the stereochemical outcome of this reaction is determined by the chirality of the
420:
or electronic effect stabilizes a transition state with maximum separation between the nucleophile and an
390:
246:
410:
The main steric interactions involve those around R and the nucleophile but not the carbonyl oxygen atom.
2102:
2016:
1933:
1141:
1012:
328:
103:
66:
1869:
Kinnaird, J. W. A.; Ng, P. Y.; Kubota, K.; Wang, X.; Leighton, J. L. J. Am. Chem. Soc. 2002, 124, 7920.
2112:
1948:
1109:
1060:
800:
647:
602:
449:
is an extension of the Felkin model that incorporates improvements suggested by Nguyễn Trọng Anh and
131:
85:
78:
1197:
Fig. 2: Example of chiral allylmetals used: (a) allylboron, (b) allyltitanium, and (c) allyl silicon
906:
Asymmetric induction by the molecular framework of an acyclic substrate is the idea that asymmetric
2010:
1298:
943:
911:
2148:
2071:
1969:
1098:
956:
915:
598:
571:
Here, the methyl titanium chloride forms a Cram-chelate. The methyl group then dissociates from
386:
224:
1660:
1649:
1112:
proceeded as predicted by molecular modelling calculations that accounted for the lowest energy
2058:
1834:
1799:
1764:
1756:
1664:
1252:
1133:
1125:
1008:
967:
926:
643:
616:
360:
281:
274:
239:
62:
50:
35:
1039:
often exist in much more rigid conformations than their linear counterparts. Even very large
2107:
2033:
1943:
1826:
1791:
1748:
1710:
Spino, C.; Granger, M. C.; Boisvert, L.; Beaulieu, C. Tetrahedron Lett. 2002, 43, 4183–4185.
1306:
1277:
1090:
620:
450:
380:
340:
262:
197:
165:
119:
111:
2117:
2005:
1985:
1959:
1036:
807:
and enolate alkylation. The substituents around the alkene can favour the approach of the
554:
324:
58:
1887:
897:
An example of substrate controlled addition of achiral allyl-boron to α-chiral aldehyde.
1212:
1186:
1049:
428:
do not obey Cram's rule, and, in the example above, replacing the electron-withdrawing
235:
156:
1310:
1024:
oxazolidinone auxiliaries (for asymmetric aldol reactions) pseudoephedrine amides and
773:
relationship, both models predict the same diastereomer (the stereoreinforcing case).
295:, which results in the same reaction product as above but now with preference for the
2137:
2000:
1701:
Burgi, H. B.; Dunitz, J. D.; Lehn, J. M.; Wipff, G. Tetrahedron. 1974. 12, 1563–1572.
1106:
1064:
847:
127:
99:
2122:
1995:
1938:
1129:
1044:
919:
816:
808:
417:
213:
201:
89:
1097:
intermediate using tert-butyl hydroperoxide in the presence of two other alkenes.
902:
Substrate control: asymmetric induction by molecular framework in acyclic systems
1181:
1032:
Substrate control: asymmetric induction by molecular framework in cyclic systems
1004:
984:
804:
788:
777:
758:
628:
591:
579:
540:
504:
348:
320:
258:
231:
102:
and remains so during the reaction. The starting material is often derived from
17:
727:
691:
654:
609:
565:
547:
488:
473:
463:
27:
Preferential formation of one chiral isomer over another in a chemical reaction
1990:
1719:
Evans, D. A.; Bartroli, J.; Shih, T. L., Am. Chem. Soc., 1981, 103, 2127-2129.
1113:
1056:
1040:
750:
to the aldehyde group to minimize the steric hindrance. Consequently, the 1,3-
536:
517:
513:
433:
425:
356:
54:
1838:
1803:
1760:
1233:
1176:
Fig. 1: Reagent control: addition of chiral allylmetals to achiral aldehydes
1149:
1145:
1137:
980:
558:
528:
525:
352:
1768:
1162:
996:
889:
Substrate control. addition of achiral allylmetals to α-chiral aldehydes.
572:
532:
508:
336:
332:
254:
123:
74:
1830:
1795:
1281:
1237:
335:
groups. Felkin argued that the Cram model suffered a major drawback: an
1166:
1053:
304:
296:
193:
70:
1752:
1193:
1172:
1075:
907:
811:
from one or the other face of the molecule. This is the basis of the
429:
344:
270:
250:
220:
1120:
Reagent control: addition of chiral allylmetals to achiral aldehydes
98:
makes use of a chiral center bound to the reactive center through a
575:
and attacks the carbonyl, leading to the anti-Felkin diastereomer.
516:
character (i.e. O, N, S, P substituents). In this situation, if a
1230:
1192:
1171:
1154:
1102:
1094:
988:
892:
884:
876:
217:
746:
to the carbonyl to reduce dipole interactions, and Rβ is placed
1891:
1851:
Brown, H. C.; Jadhav, P. K. J. Am. Chem. Soc. 1983, 105, 2092.
1158:
992:
881:
Felkin–Ahn model for nucleophilic addition to chiral aldehydes
1128:, reagent control is an approach to selectively forming one
1728:
Still, W. C.; Galynker, I. Tetrahedron 1981, 37, 3981-3996.
862:), mainly producing the shown diastereoisomer. Since for a
1884:
Evans Group
Afternoon Seminar Sarah Siska February 9, 2001
1140:
of the reagent used. When chiral allylmetals are used for
184:
The experiments involved two reactions. In experiment one
77:
or environment. Asymmetric induction is a key element in
597:- to each other in order to most effectively cancel the
922:
is in place and additional stereocentres are required.
363:
also increased, which is not predicted by Cram's rule:
819:, which predicts that the selectivity is stronger for
1860:
Duthaler, R. O.; Hafner, A. Chem. Rev. 1992, 92, 807.
1392:
Bürgi, H. B.; Dunitz, J. D.; Lehn, J. M.; Wipff, G.
1353:
Anh, N. T.; Eisenstein, O.; Lefour, J-M.; Dau, M-E.
499:
Though the Cram and Felkin–Anh models differ in the
2090:
2057:
2026:
1978:
1926:
1683:Houk, K. N. et al., Science, 1986, 231, 1108-1117.
1648:
846:alkene assumes the shown conformation to minimize
2049:Ultraviolet–visible spectroscopy of stereoisomers
1626:Evans, D.A.; Dart, M.J.; Duffy, J.L.; Yang, M.G.
539:substituent in an eclipsed conformation, and the
405:For comparison TS B is the Cram transition state.
61:over the other as a result of the influence of a
754:-diol would be predicted as the major product.
196:but (R)-enantiomer shown) was reacted with the
1078:was dihydroxylated diasetereoselectively with
938:Asymmetric induction at alpha-chiral aldehydes
1903:
1882:The Evolution of Models for Carbonyl Addition
1297:Marc Chérest, Hugh Felkin and Nicole Prudent
1070:A number of total syntheses have made use of
631:, regardless of its steric bulk relative to R
436:group reduces stereoselectivity considerably.
49:) describes the preferential formation in a
8:
1329:
1327:
870:and the H group is not as large as for the
1910:
1896:
1888:
1647:Clayden; Greeves; Warren; Wothers (2001).
1373:Bürgi, H. B.; Dunitz, J. D.; Shefter, E.
942:Possible reactivity at aldehydes include
765:Carbonyl 1,2 and 1,3 asymmetric induction
507:to the most sterically favored face of a
1819:Journal of the American Chemical Society
1784:Journal of the American Chemical Society
1741:Journal of the American Chemical Society
1509:Cornforth JW, Cornforth MRH, Mathew KK.
1270:Journal of the American Chemical Society
1249:Asymmetric Synthesis of Natural Products
531:effect can be observed. This locks the
118:chiral information is introduced in the
29:
1223:
245:) when the carbonyl is positioned in a
84:Asymmetric induction was introduced by
1606:Evans, D.A.; Duffy, J.L.; Dart, M.J.
951:Felkin–Anh and polar Felkin–Anh model
874:case, the selectivity is much lower.
866:alkene the steric hindrance between R
723:) of the remaining two substituents.
619:illustrates the Cornforth and Felkin
7:
1585:Reetz. M.T.; Kesseler, K.; Jung, A.
795:Acyclic alkenes asymmetric induction
92:. Several types of induction exist.
1136:is determined by the structure and
979:Addition of achiral allylmetals to
153:Cram's rule of asymmetric induction
238:from the least hindered side (see
25:
2044:NMR spectroscopy of stereoisomers
799:Chiral acyclic alkenes also show
661:Carbonyl 1,3 asymmetric induction
257:atom, which are the two smallest
138:Carbonyl 1,2 asymmetric induction
2082:Diastereomeric recrystallization
1525:Cherest M, Felkin H, Prudent N.
1101:reduction of a 10-membered ring
858:) rather than the large group (R
834:
787:
776:
757:
726:
690:
653:
608:
564:
546:
487:
472:
462:
398:
367:
176:
134:is economically most desirable.
1490:Reetz MT, Hullmann M, Seitz T.
815:, based on theoretical work by
273:as the most bulky group in the
234:in this reaction attacking the
1:
1311:10.1016/S0040-4039(00)89719-1
1105:intermediate en route to the
687:adduct as the major product.
116:external asymmetric induction
96:Internal asymmetric induction
2077:Chiral column chromatography
1190:range of achiral aldehydes.
108:relayed asymmetric induction
1565:Leitereg, T.J.; Cram, D.J.
1492:Angew. Chem. Int. Ed. Engl.
1412:Anh, N. T.; Eisenstein, O.
280:The second reaction is the
162:adjacent asymmetric center.
2165:
2039:Chiral derivatizing agents
1920:enantioselective synthesis
842:In the example shown, the
588:electron withdrawing group
422:electron-withdrawing group
1208:Macrocyclic stereocontrol
1072:macrocyclic stereocontrol
925:When considering how two
293:lithium aluminium hydride
223:(see for explanation the
1965:Supramolecular chirality
1333:Anh, N. T.; Eisenstein,
1063:addition, and catalytic
286:1,2-diphenyl-1-propanone
1657:Oxford University Press
932:computational modelling
803:upon reactions such as
524:or Zn is introduced, a
495:Anti–Felkin selectivity
206:1,2-diphenyl-1-propanol
186:2-phenylpropionaldehyde
65:feature present in the
1544:Reetz, M.T.; Jung, A.
1198:
1177:
1026:tert-butanesulfinamide
898:
890:
882:
375:The Felkin rules are:
261:creating a minimum of
39:
2103:Chiral pool synthesis
2017:Diastereomeric excess
1469:Cram DJ, Elhafez FA.
1196:
1175:
1142:nucleophilic addition
1013:nucleophilic addition
896:
888:
880:
650:, is pictured below:
329:nucleophilic addition
104:chiral pool synthesis
88:based on his work on
33:
2113:Asymmetric catalysis
2098:Asymmetric induction
1448:Mengel A., Reiser O.
1301:Volume 9, Issue 18,
1144:reaction to achiral
801:diastereoselectivity
679:to the substituent R
648:transition structure
623:that places the EWG
603:transition structure
339:conformation in the
216:, predominantly the
146:
132:asymmetric synthesis
86:Hermann Emil Fischer
79:asymmetric synthesis
43:Asymmetric induction
2011:Enantiomeric excess
1831:10.1021/ja00341a055
1796:10.1021/ja00503a048
1747:(49): 15851–15855.
1299:Tetrahedron Letters
1282:10.1021/ja01143a007
1011:. In this case the
944:nucleophilic attack
701:Non-chelation model
319:(1968) named after
249:formation with the
2108:Chiral auxiliaries
2072:Kinetic resolution
1970:Inherent chirality
1955:-symmetric ligands
1305:, Pages 2199-2204
1199:
1178:
1099:Sodium borohydride
1083:-methylmorpholine
1019:Chiral auxiliaries
957:Burgi-Dunitz angle
916:chemical syntheses
899:
891:
883:
481:Bürgi–Dunitz angle
383:are reactant-like.
323:also predicts the
267:gauche orientation
225:Fischer projection
212:) as a mixture of
112:chiral auxiliaries
40:
2131:
2130:
2067:Recrystallization
2059:Chiral resolution
1753:10.1021/ja065782w
1670:978-0-19-850346-0
1651:Organic Chemistry
1628:J .Am. Chem. Soc.
1608:Tetrahedron Lett.
1567:J. Am. Chem. Soc.
1546:J. Am. Chem. Soc.
1527:Tetrahedron Lett.
1471:J. Am. Chem. Soc.
1414:Tetrahedron Lett.
1375:J. Am. Chem. Soc.
1355:J. Am. Chem. Soc.
1335:O. Nouv. J. Chim.
1276:(23): 5828–5835.
1134:stereoselectivity
1132:out of many, the
1126:organic synthesis
1009:Newman projection
927:functional groups
719:to the bulkier (R
683:, leading to the
644:molecular orbital
617:Newman projection
479:Incorporation of
381:transition states
361:stereoselectivity
282:organic reduction
275:anti conformation
240:Newman projection
130:. This method of
51:chemical reaction
36:Gibbs free energy
16:(Redirected from
2156:
2034:Optical rotation
1979:Chiral molecules
1944:Planar chirality
1912:
1905:
1898:
1889:
1870:
1867:
1861:
1858:
1852:
1849:
1843:
1842:
1814:
1808:
1807:
1790:(9): 2493–2495.
1779:
1773:
1772:
1735:
1729:
1726:
1720:
1717:
1711:
1708:
1702:
1699:
1693:
1690:
1684:
1681:
1675:
1674:
1654:
1644:
1638:
1624:
1618:
1604:
1598:
1587:Tetrahedron Lett
1583:
1577:
1563:
1557:
1542:
1536:
1523:
1517:
1507:
1501:
1488:
1482:
1481:(23); 5828–5835.
1467:
1461:
1446:
1440:
1430:Top. Curr. Chem.
1426:
1420:
1410:
1404:
1390:
1384:
1371:
1365:
1351:
1345:
1331:
1322:
1319:
1313:
1292:
1286:
1285:
1265:
1259:
1246:
1240:
1228:
1091:osmium tetroxide
1037:Cyclic molecules
838:
791:
780:
761:
730:
706:Cram–Reetz model
694:
657:
627:to the incoming
621:transition state
612:
586:relative to the
568:
550:
491:
476:
466:
451:Odile Eisenstein
447:Felkin–Anh model
441:Felkin–Anh model
402:
387:Torsional strain
371:
341:transition state
263:steric hindrance
198:Grignard reagent
180:
166:steric hindrance
120:transition state
47:enantioinduction
21:
18:Cram's rule
2164:
2163:
2159:
2158:
2157:
2155:
2154:
2153:
2144:Stereochemistry
2134:
2133:
2132:
2127:
2118:Organocatalysis
2086:
2053:
2022:
2006:Racemic mixture
1974:
1960:Axial chirality
1954:
1927:Chirality types
1922:
1916:
1878:
1873:
1868:
1864:
1859:
1855:
1850:
1846:
1816:
1815:
1811:
1781:
1780:
1776:
1737:
1736:
1732:
1727:
1723:
1718:
1714:
1709:
1705:
1700:
1696:
1691:
1687:
1682:
1678:
1671:
1646:
1645:
1641:
1625:
1621:
1605:
1601:
1584:
1580:
1564:
1560:
1543:
1539:
1524:
1520:
1508:
1504:
1489:
1485:
1468:
1464:
1460:(5), 1191–1224.
1447:
1443:
1427:
1423:
1411:
1407:
1391:
1387:
1372:
1368:
1352:
1348:
1332:
1325:
1320:
1316:
1293:
1289:
1267:
1266:
1262:
1251:, Ari Koskinen
1247:
1243:
1229:
1225:
1221:
1204:
1122:
1110:eucannabinolide
1061:dimethylcuprate
1034:
1021:
977:
953:
940:
904:
869:
861:
857:
853:
797:
767:
736:
722:
708:
703:
682:
672:
670:Chelation model
663:
638:
634:
555:stereoselective
523:
497:
457:
443:
424:. For instance
325:stereochemistry
313:
149:
140:
59:diastereoisomer
28:
23:
22:
15:
12:
11:
5:
2162:
2160:
2152:
2151:
2146:
2136:
2135:
2129:
2128:
2126:
2125:
2120:
2115:
2110:
2105:
2100:
2094:
2092:
2088:
2087:
2085:
2084:
2079:
2074:
2069:
2063:
2061:
2055:
2054:
2052:
2051:
2046:
2041:
2036:
2030:
2028:
2024:
2023:
2021:
2020:
2014:
2008:
2003:
1998:
1993:
1988:
1982:
1980:
1976:
1975:
1973:
1972:
1967:
1962:
1957:
1952:
1946:
1941:
1936:
1930:
1928:
1924:
1923:
1917:
1915:
1914:
1907:
1900:
1892:
1886:
1885:
1877:
1876:External links
1874:
1872:
1871:
1862:
1853:
1844:
1825:(3): 625–627.
1809:
1774:
1730:
1721:
1712:
1703:
1694:
1685:
1676:
1669:
1639:
1619:
1599:
1578:
1558:
1537:
1518:
1502:
1483:
1462:
1441:
1421:
1405:
1385:
1366:
1346:
1323:
1314:
1287:
1260:
1241:
1222:
1220:
1217:
1216:
1215:
1213:Cieplak effect
1210:
1203:
1200:
1187:Dieter Seebach
1121:
1118:
1050:W. Clark Still
1033:
1030:
1020:
1017:
976:
973:
963:forming bond.
952:
949:
939:
936:
903:
900:
867:
859:
855:
851:
840:
839:
796:
793:
766:
763:
735:
732:
720:
707:
704:
702:
699:
680:
671:
668:
662:
659:
636:
632:
521:
520:such as Al-iPr
496:
493:
455:
442:
439:
438:
437:
414:
411:
407:
406:
403:
395:
394:
384:
373:
372:
312:
309:
253:group and the
236:carbonyl group
182:
181:
157:Donald J. Cram
148:
145:
139:
136:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2161:
2150:
2147:
2145:
2142:
2141:
2139:
2124:
2121:
2119:
2116:
2114:
2111:
2109:
2106:
2104:
2101:
2099:
2096:
2095:
2093:
2089:
2083:
2080:
2078:
2075:
2073:
2070:
2068:
2065:
2064:
2062:
2060:
2056:
2050:
2047:
2045:
2042:
2040:
2037:
2035:
2032:
2031:
2029:
2025:
2018:
2015:
2012:
2009:
2007:
2004:
2002:
2001:Meso compound
1999:
1997:
1994:
1992:
1989:
1987:
1984:
1983:
1981:
1977:
1971:
1968:
1966:
1963:
1961:
1958:
1956:
1951:
1947:
1945:
1942:
1940:
1937:
1935:
1932:
1931:
1929:
1925:
1921:
1913:
1908:
1906:
1901:
1899:
1894:
1893:
1890:
1883:
1880:
1879:
1875:
1866:
1863:
1857:
1854:
1848:
1845:
1840:
1836:
1832:
1828:
1824:
1820:
1813:
1810:
1805:
1801:
1797:
1793:
1789:
1785:
1778:
1775:
1770:
1766:
1762:
1758:
1754:
1750:
1746:
1742:
1734:
1731:
1725:
1722:
1716:
1713:
1707:
1704:
1698:
1695:
1689:
1686:
1680:
1677:
1672:
1666:
1662:
1658:
1653:
1652:
1643:
1640:
1636:
1632:
1629:
1623:
1620:
1616:
1612:
1609:
1603:
1600:
1596:
1592:
1588:
1582:
1579:
1575:
1571:
1568:
1562:
1559:
1555:
1551:
1547:
1541:
1538:
1534:
1531:
1528:
1522:
1519:
1515:
1512:
1506:
1503:
1499:
1496:
1493:
1487:
1484:
1480:
1476:
1472:
1466:
1463:
1459:
1455:
1451:
1445:
1442:
1438:
1434:
1431:
1425:
1422:
1418:
1415:
1409:
1406:
1402:
1398:
1395:
1389:
1386:
1382:
1379:
1376:
1370:
1367:
1363:
1359:
1356:
1350:
1347:
1343:
1339:
1336:
1330:
1328:
1324:
1318:
1315:
1312:
1308:
1304:
1300:
1296:
1291:
1288:
1283:
1279:
1275:
1271:
1264:
1261:
1258:
1257:0-471-93848-3
1254:
1250:
1245:
1242:
1239:
1235:
1232:
1227:
1224:
1218:
1214:
1211:
1209:
1206:
1205:
1201:
1195:
1191:
1188:
1183:
1174:
1170:
1168:
1164:
1160:
1156:
1151:
1147:
1143:
1139:
1135:
1131:
1127:
1119:
1117:
1115:
1111:
1108:
1107:sesquiterpene
1104:
1100:
1096:
1092:
1088:
1086:
1082:
1077:
1073:
1068:
1066:
1065:hydrogenation
1062:
1058:
1055:
1051:
1046:
1042:
1038:
1031:
1029:
1027:
1018:
1016:
1014:
1010:
1006:
1000:
998:
994:
990:
986:
982:
974:
972:
969:
964:
960:
958:
950:
948:
945:
937:
935:
933:
928:
923:
921:
917:
913:
909:
901:
895:
887:
879:
875:
873:
865:
849:
845:
837:
833:
832:
831:
829:
827:
823:
818:
814:
810:
806:
802:
794:
792:
790:
785:
781:
779:
774:
772:
764:
762:
760:
755:
753:
749:
745:
741:
733:
731:
729:
724:
718:
714:
705:
700:
698:
695:
693:
688:
686:
678:
669:
667:
660:
658:
656:
651:
649:
645:
640:
630:
626:
622:
618:
613:
611:
606:
604:
600:
599:dipole moment
596:
593:
589:
585:
581:
576:
574:
569:
567:
562:
560:
556:
551:
549:
544:
542:
538:
534:
530:
527:
519:
515:
510:
506:
502:
494:
492:
490:
485:
482:
477:
475:
470:
467:
465:
460:
452:
448:
440:
435:
431:
427:
423:
419:
415:
412:
409:
408:
404:
401:
397:
396:
392:
388:
385:
382:
378:
377:
376:
370:
366:
365:
364:
362:
358:
354:
350:
346:
342:
338:
334:
331:reactions to
330:
326:
322:
318:
310:
308:
306:
302:
298:
294:
290:
287:
283:
278:
276:
272:
268:
264:
260:
256:
252:
248:
244:
241:
237:
233:
228:
226:
222:
219:
215:
214:diastereomers
211:
207:
203:
199:
195:
191:
187:
179:
175:
174:
173:
171:
167:
163:
158:
154:
144:
137:
135:
133:
129:
128:chiral ligand
125:
121:
117:
113:
109:
105:
101:
100:covalent bond
97:
93:
91:
90:carbohydrates
87:
82:
80:
76:
72:
68:
64:
60:
56:
52:
48:
44:
37:
32:
19:
2123:Biocatalysis
2097:
1996:Diastereomer
1986:Stereoisomer
1949:
1939:Stereocenter
1918:Concepts in
1865:
1856:
1847:
1822:
1818:
1812:
1787:
1783:
1777:
1744:
1740:
1733:
1724:
1715:
1706:
1697:
1688:
1679:
1650:
1642:
1634:
1630:
1627:
1622:
1614:
1610:
1607:
1602:
1594:
1590:
1586:
1581:
1573:
1569:
1566:
1561:
1553:
1549:
1545:
1540:
1532:
1529:
1526:
1521:
1513:
1511:J. Chem.Soc.
1510:
1505:
1497:
1494:
1491:
1486:
1478:
1474:
1470:
1465:
1457:
1453:
1449:
1444:
1436:
1432:
1429:
1424:
1416:
1413:
1408:
1400:
1396:
1393:
1388:
1380:
1377:
1374:
1369:
1361:
1357:
1354:
1349:
1341:
1337:
1334:
1317:
1302:
1294:
1290:
1273:
1269:
1263:
1248:
1244:
1226:
1179:
1130:stereoisomer
1123:
1084:
1080:
1069:
1045:erythromycin
1035:
1022:
1001:
978:
965:
961:
954:
941:
924:
920:stereocentre
905:
871:
863:
848:steric clash
843:
841:
828:double bonds
825:
821:
817:Kendall Houk
813:Houk's model
812:
809:electrophile
798:
786:
782:
775:
770:
768:
756:
751:
747:
743:
739:
737:
725:
716:
712:
709:
696:
689:
684:
676:
673:
664:
652:
641:
624:
614:
607:
594:
583:
577:
570:
563:
552:
545:
498:
486:
478:
471:
468:
461:
446:
444:
418:polar effect
374:
317:Felkin model
316:
314:
311:Felkin model
300:
288:
285:
279:
259:substituents
242:
229:
209:
205:
202:bromobenzene
189:
185:
183:
169:
160:
155:named after
152:
150:
141:
115:
107:
95:
94:
83:
46:
42:
41:
1428:Anh, N. T.
1394:Tetrahedron
1236:definition
1182:H. C. Brown
1041:macrocycles
975:Cram’s rule
805:epoxidation
734:Evans model
629:nucleophile
592:nucleophile
580:nucleophile
541:nucleophile
505:nucleophile
432:group by a
426:haloketones
321:Hugh Felkin
232:nucleophile
147:Cram's rule
2138:Categories
1991:Enantiomer
1739:Acetate".
1659:. p.
1535:2199–2204.
1450:Chem. Rev.
1219:References
1114:macrocycle
1089:(NMO) and
1057:alkylation
918:where one
912:electronic
537:Lewis base
518:Lewis acid
514:Lewis base
501:conformers
434:cyclohexyl
357:tert-butyl
122:through a
55:enantiomer
2149:Asymmetry
2091:Reactions
1934:Chirality
1839:0002-7863
1804:0002-7863
1761:0002-7863
1234:Gold Book
1150:chirality
1146:aldehydes
1138:chirality
981:aldehydes
850:between R
824:than for
582:will add
559:chelation
529:chelation
526:bidentate
391:staggered
353:isopropyl
303:). Now a
247:staggered
67:substrate
2027:Analysis
1769:17147397
1516:112–127.
1500:477–480.
1202:See also
1163:titanium
1028:imines.
997:titanium
985:α-carbon
968:Grignard
573:titanium
535:and the
533:carbonyl
509:carbonyl
337:eclipsed
333:carbonyl
299:isomer (
255:hydrogen
170:scheme 1
143:others.
124:catalyst
75:catalyst
1637:, 4322.
1617:, 8537.
1576:, 4011.
1556:, 4833.
1403:, 1563.
1383:, 5065.
1364:, 6146.
1169:, etc.
1167:silicon
1054:enolate
601:of the
305:hydride
297:erythro
265:, in a
194:racemic
159:states
71:reagent
53:of one
1837:
1802:
1767:
1759:
1667:
1597:, 729.
1439:, 146.
1419:, 155.
1255:
1148:, the
1087:-oxide
1076:olefin
908:steric
430:phenyl
359:, the
345:methyl
271:phenyl
251:methyl
221:isomer
63:chiral
45:(also
1530:1968,
1514:1959,
1495:1987.
1378:1973,
1344:, 61.
1231:IUPAC
1155:boron
1103:enone
1095:enone
1043:like
1005:ethyl
989:boron
864:trans
826:trans
771:anti-
748:anti-
744:anti-
740:anti-
717:anti-
713:anti-
685:anti-
677:anti-
635:and R
625:anti-
615:This
584:anti-
553:This
349:ethyl
291:with
218:threo
114:. In
106:. In
2019:(de)
2013:(ee)
1835:ISSN
1800:ISSN
1765:PMID
1757:ISSN
1665:ISBN
1631:1996
1611:1994
1591:1984
1570:1968
1550:1983
1475:1952
1454:1999
1433:1980
1417:1976
1397:1974
1358:1973
1338:1977
1303:1968
1253:ISBN
1238:Link
995:and
910:and
752:anti
595:anti
445:The
379:The
315:The
269:and
151:The
1827:doi
1823:105
1792:doi
1788:101
1749:doi
1745:128
1661:895
1635:118
1554:105
1533:18,
1498:26,
1307:doi
1278:doi
1159:tin
1124:In
993:tin
872:cis
844:cis
822:cis
355:to
351:to
347:to
327:of
284:of
227:).
204:to
200:of
172:).
126:of
57:or
2140::
1833:.
1821:.
1798:.
1786:.
1763:.
1755:.
1743:.
1663:.
1655:.
1633:,
1615:35
1613:,
1595:25
1593:,
1589:.
1574:90
1572:,
1552:,
1548:,
1479:74
1477:;
1473:;
1458:99
1456:,
1452:,
1437:88
1435:,
1401:30
1399:,
1381:95
1362:95
1360:,
1340:,
1326:^
1274:74
1272:.
1165:,
1161:,
1157:,
1059:,
999:.
991:,
830:.
639:.
605:.
416:A
301:2a
277:.
192:,
81:.
73:,
69:,
34:A
1953:2
1950:C
1911:e
1904:t
1897:v
1841:.
1829::
1806:.
1794::
1771:.
1751::
1673:.
1342:1
1309::
1284:.
1280::
1085:N
1081:N
868:S
860:L
856:M
852:S
721:M
681:β
637:L
633:S
522:2
456:N
454:S
289:2
243:A
210:2
208:(
190:1
188:(
168:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.