35:
334:
203:
663:, physiological concentrations of DOPAL in isolated mitochondria were highly potent in inducing a pathway associated with programmed cell death (or apoptosis), permeability transition. This suggests the cytotoxity of DOPAL and its role in the progression of Parkinson's disease, which has long been associated with mitochondrial abnormalities and neurotoxicity by way of dopaminergic compounds, while reducing the emphasis on other dopamine derivatives and metabolites.
506:
1134:
Legros H, Dingeval MG, Janin F, Costentin J, Bonnet JJ (March 2004). "Toxicity of a treatment associating dopamine and disulfiram for catecholaminergic neuroblastoma SH-SY5Y cells: relationships with 3,4-dihydroxyphenylacetaldehyde formation".
1196:
998:
Kristal, B.; Conway, A. D.; Brown, A. M.; Jain, J. C.; Ulluci, P. A.; Li, S. W.; Burke, W. J. (2001). "Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria".
1189:
519:
1182:
1558:
949:
Goldstein, David S.; Sullivan, Patti; Holmes, Courtney; Miller, Gary W.; Alter, Shawn; Strong, Randy; Mash, Deborah C.; Kopin, Irwin J.; Sharabi, Yehonatan (2013).
673:
of DOPAL and thereby increase DOPAL levels, can produce dopaminergic neurotoxicity or augment dopaminergic neurodegeneration. Examples of ALDH inhibitors include
34:
383:
2070:
2049:
2041:
2090:
2045:
1551:
2085:
1944:
1792:
1749:
1371:
1323:
718:
348:
844:"The Catecholaldehyde Hypothesis for the Pathogenesis of Catecholaminergic Neurodegeneration: What We Know and What We Do Not Know"
1954:
1934:
1612:
666:
1845:
1588:
1353:
258:
1939:
1544:
1348:
1285:
221:
1510:
1929:
1924:
1802:
526:
2021:
580:
291:
1381:
1338:
1995:
1860:
1840:
1772:
1583:
1515:
1443:
724:
632:
312:
1974:
1448:
1328:
554:
114:
1492:
596:
1607:
1567:
252:
951:"Determinants of buildup of the toxic dopamine metabolite <SCP>DOPAL</SCP> in Parkinson's disease"
1835:
1899:
1875:
1870:
1855:
1782:
1777:
1602:
678:
624:
612:
1716:
1593:
198:
2037:
2033:
2029:
1969:
1885:
1208:
1091:"Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons"
652:
648:
628:
1850:
1453:
107:
1964:
1894:
1520:
1343:
1830:
1767:
1666:
1525:
902:"The "Sick-but-not-Dead" Phenomenon Applied to Catecholamine Deficiency in Neurodegenerative Diseases"
752:. USA: National Center for Biotechnology Information. 24 June 2005. Identification and Related Records
608:
2025:
1979:
1865:
1290:
1144:
47:
329:
1880:
1787:
1656:
1487:
1425:
73:
1646:
745:
2080:
2075:
482:
132:
1479:
1305:
1300:
1160:
1116:
1071:
1015:
980:
931:
875:
813:
656:
640:
588:
568:
561:
1205:
1152:
1106:
1098:
1061:
1051:
1007:
970:
962:
921:
913:
865:
855:
803:
795:
411:
180:
2005:
2000:
1174:
300:
1959:
1949:
1919:
1701:
1686:
1333:
83:
333:
202:
1148:
926:
901:
808:
783:
631:
in this regard than dopamine itself and other metabolites of dopamine. According to the
160:
1315:
1295:
1249:
1111:
1090:
1066:
1039:
975:
950:
870:
843:
660:
600:
497:
1156:
1102:
1011:
2064:
1759:
1724:
1671:
1620:
1233:
1216:
694:
592:
487:
462:
191:
1904:
1575:
1376:
644:
280:
1909:
1807:
1729:
1676:
1626:
1502:
1363:
1253:
576:
799:
1744:
1616:
1435:
1420:
1399:
1263:
1056:
674:
670:
572:
550:
439:
211:
171:
1822:
1536:
1471:
1467:
1412:
1403:
1225:
604:
1164:
1120:
1075:
1019:
984:
935:
917:
879:
817:
705:. DOPAL itself is also known to inhibit ALDH at high concentrations (>5
61:
DOPAL; 2-(3,4-Dihydroxyphenyl)acetaldehyde; Dopaldehyde; Dopamine aldehyde
1812:
1739:
1734:
1691:
1661:
1641:
1272:
1245:
1237:
860:
698:
690:
557:
1636:
1631:
686:
682:
452:
267:
966:
1241:
636:
17:
496:
Except where otherwise noted, data are given for materials in their
1914:
1706:
1681:
241:
1797:
1696:
1040:"Impaired dopamine metabolism in Parkinson's disease pathogenesis"
702:
151:
113:
106:
96:
1711:
232:
1540:
1178:
357:
InChI=1S/C8H8O3/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,4-5,10-11H,3H2
367:
InChI=1/C8H8O3/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,4-5,10-11H,3H2
317:
1033:
1031:
1029:
1089:
Doorn JA, Florang VR, Schamp JH, Vanle BC (January 2014).
1038:
Masato A, Plotegher N, Boassa D, Bubacco L (August 2019).
514:
784:"The catecholaldehyde hypothesis: where MAO fits in"
746:"3,4-dihydroxyphenylacetaldehyde - Compound Summary"
655:(ALDH). DOPAL is a metabolite of dopamine formed by
1988:
1821:
1758:
1574:
1501:
1466:
1434:
1411:
1398:
1362:
1314:
1271:
1262:
1224:
1215:
220:
1372:3,4-Dihydroxyphenylglycolaldehyde (DOPEGAL, DHMAL)
1324:3,4-Dihydroxyphenylglycolaldehyde (DOPEGAL, DHMAL)
1296:Hydroxytyrosol (3,4-dihydroxyphenylethanol; DOPET)
131:
1349:3,4-Dihydroxyphenylethylene glycol (DOPEG, DHPG)
895:
893:
891:
889:
777:
775:
773:
771:
769:
767:
279:
837:
835:
833:
831:
829:
827:
659:(MAO). In differentiated neuronal cells of the
82:
1552:
1511:3-Methoxy-4-hydroxyphenylacetaldehyde (HMPAL)
1354:3-Methoxy-4-hydroxyphenylglycol (MHPG, MOPEG)
1190:
8:
1559:
1545:
1537:
1408:
1268:
1221:
1197:
1183:
1175:
332:
201:
179:
26:
1382:3-Methoxy-4-hydroxymandelaldehyde (MHMAL)
1339:3-Methoxy-4-hydroxymandelaldehyde (MHMAL)
1110:
1065:
1055:
974:
925:
869:
859:
807:
299:
1281:3,4-Dihydroxyphenylacetaldehyde (DOPAL)
737:
388:
353:
328:
257:
1286:3,4-Dihydroxyphenylacetic acid (DOPAC)
467:351 °C (664 °F; 624 K)
192:
669:(ALDH inhibitors), which prevent the
360:Key: IADQVXRMSNIUEL-UHFFFAOYSA-N
159:
7:
1493:5-Methoxyindoleacetaldehyde (5-MIAL)
1483:-Acetylserotonin (NAS; normelatonin)
1444:5-Hydroxyindoleacetaldehyde (5-HIAL)
1449:5-Hydroxyindoleacetic acid (5-HIAA)
370:Key: IADQVXRMSNIUEL-UHFFFAOYAV
270:
240:
1516:4-Hydroxyphenylacetaldehyde (HPAL)
25:
2071:Aldehyde dehydrogenase inhibitors
1329:3,4-Dihydroxymandelic acid (DHMA)
1000:Free Radical Biology and Medicine
719:3,4-Dihydroxyphenylglycolaldehyde
667:Aldehyde dehydrogenase inhibitors
52:(3,4-Dihydroxyphenyl)acetaldehyde
651:. DOPAL is detoxified mainly by
504:
423:
33:
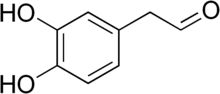
28:3,4-Dihydroxyphenylacetaldehyde
2050:Monoamine metabolism modulators
539:3,4-Dihydroxyphenylacetaldehyde
500:(at 25 °C , 100 kPa).
259:3,4-dihydroxyphenylacetaldehyde
782:Goldstein DS (February 2020).
429:
417:
1:
2042:Monoamine reuptake inhibitors
2022:Receptor/signaling modulators
1157:10.1016/S0161-813X(03)00148-7
1103:10.1016/S1353-8020(13)70019-1
1012:10.1016/s0891-5849(01)00484-1
900:Goldstein DS (October 2020).
1454:5-Hydroxytryptophol (5-HTOL)
603:. There is also spontaneous
1426:5-Hydroxytryptophan (5-HTP)
1344:Vanillylmandelic acid (VMA)
1097:. 20 Suppl 1 (1): S73–S75.
725:5-Hydroxyindoleacetaldehyde
633:catecholaldehyde hypothesis
2107:
2091:Phenolic human metabolites
2046:Monoamine releasing agents
1488:5-Methoxytryptamine (5-MT)
842:Goldstein DS (June 2021).
800:10.1007/s00702-019-02106-9
619:Dopaminergic neurotoxicity
555:monoamine neurotransmitter
478:Related 2-phenyl aldehydes
2086:Monoaminergic neurotoxins
2014:
1568:Monoaminergic neurotoxins
1095:Parkinsonism Relat Disord
1057:10.1186/s13024-019-0332-6
955:Journal of Neurochemistry
494:
471:
404:
379:
344:
66:
58:
46:
41:
32:
1521:Indoleacetaldehyde (IAL)
1301:3-Methoxytyramine (3-MT)
788:J Neural Transm (Vienna)
679:dopaminergic neurotoxins
635:, DOPAL plays a role in
1306:Homovanillic acid (HVA)
1209:metabolic intermediates
625:dopaminergic neurotoxin
623:DOPAL is known to be a
613:reactive oxygen species
1526:Phenacetaldehyde (PAL)
918:10.1055/s-0040-1713874
653:aldehyde dehydrogenase
639:-related dopaminergic
597:dopamine β-hydroxylase
1945:MDMA (midomafetamine)
1808:Oxidopamine (6-OHDA)
1730:Oxidopamine (6-OHDA)
1720:-Methylnorsalsolinol
1291:Homovanillyl alcohol
861:10.3390/ijms22115999
48:Preferred IUPAC name
1930:MDA (tenamfetamine)
1861:4-CAB (α-ethyl-PCA)
1803:MDA (tenamfetamine)
1149:2004NeuTx..25..365L
649:Parkinson's disease
447: g·mol
396:OC1=CC=C(CC=O)C=C1O
29:
1598:-Cysteinyldopamine
627:. It is much more
585:-methyltransferase
569:metabolic pathways
527:Infobox references
483:Phenylacetaldehyde
472:Related compounds
27:
2058:
2057:
2053:
1890:-Dimethyldopamine
1881:α-Me-DA (3,4-DHA)
1534:
1533:
1462:
1461:
1394:
1393:
1390:
1389:
967:10.1111/jnc.12345
657:monoamine oxidase
641:neurodegeneration
609:dopamine quinones
607:of dopamine into
589:3-methoxytyramine
562:monoamine oxidase
547:dopamine aldehyde
545:), also known as
535:Chemical compound
533:
532:
313:CompTox Dashboard
115:Interactive image
108:Interactive image
16:(Redirected from
2098:
2016:
1667:Dopamine quinone
1561:
1554:
1547:
1538:
1409:
1269:
1222:
1206:Neurotransmitter
1199:
1192:
1185:
1176:
1169:
1168:
1131:
1125:
1124:
1114:
1086:
1080:
1079:
1069:
1059:
1044:Mol Neurodegener
1035:
1024:
1023:
995:
989:
988:
978:
946:
940:
939:
929:
897:
884:
883:
873:
863:
839:
822:
821:
811:
779:
762:
761:
759:
757:
750:PubChem Compound
742:
708:
677:and other known
517:
511:
508:
507:
446:
431:
425:
419:
412:Chemical formula
391:Oc1ccc(CC=O)cc1O
337:
336:
321:
319:
303:
283:
272:
261:
244:
224:
205:
194:
183:
163:
135:
117:
110:
86:
37:
30:
21:
2106:
2105:
2101:
2100:
2099:
2097:
2096:
2095:
2061:
2060:
2059:
2054:
2010:
1984:
1960:Norfenfluramine
1950:Methamphetamine
1817:
1754:
1707:MPP (cyperquat)
1702:Methamphetamine
1613:ALDH inhibitors
1570:
1565:
1535:
1530:
1497:
1458:
1430:
1386:
1358:
1334:Normetanephrine
1310:
1258:
1242:DOPA (levodopa)
1211:
1203:
1173:
1172:
1137:Neurotoxicology
1133:
1132:
1128:
1088:
1087:
1083:
1037:
1036:
1027:
997:
996:
992:
948:
947:
943:
899:
898:
887:
841:
840:
825:
781:
780:
765:
755:
753:
744:
743:
739:
734:
715:
706:
621:
593:β-hydroxylation
536:
529:
524:
523:
522: ?)
513:
509:
505:
501:
485:
479:
444:
434:
428:
422:
414:
400:
397:
392:
387:
386:
375:
372:
371:
368:
362:
361:
358:
352:
351:
340:
322:
315:
306:
286:
273:
247:
227:
214:
186:
166:
138:
120:
100:
89:
76:
62:
54:
53:
23:
22:
15:
12:
11:
5:
2104:
2102:
2094:
2093:
2088:
2083:
2078:
2073:
2063:
2062:
2056:
2055:
2015:
2012:
2011:
2009:
2008:
2003:
1998:
1992:
1990:
1986:
1985:
1983:
1982:
1977:
1972:
1967:
1962:
1957:
1952:
1947:
1942:
1937:
1932:
1927:
1922:
1917:
1912:
1907:
1902:
1897:
1892:
1883:
1878:
1873:
1868:
1863:
1858:
1853:
1848:
1843:
1838:
1833:
1827:
1825:
1819:
1818:
1816:
1815:
1810:
1805:
1800:
1795:
1790:
1785:
1780:
1775:
1770:
1764:
1762:
1756:
1755:
1753:
1752:
1747:
1742:
1737:
1732:
1727:
1722:
1714:
1709:
1704:
1699:
1694:
1689:
1684:
1679:
1674:
1669:
1664:
1659:
1654:
1649:
1644:
1639:
1634:
1629:
1624:
1610:
1608:6-OHDA quinone
1605:
1600:
1591:
1586:
1580:
1578:
1572:
1571:
1566:
1564:
1563:
1556:
1549:
1541:
1532:
1531:
1529:
1528:
1523:
1518:
1513:
1507:
1505:
1499:
1498:
1496:
1495:
1490:
1485:
1476:
1474:
1464:
1463:
1460:
1459:
1457:
1456:
1451:
1446:
1440:
1438:
1432:
1431:
1429:
1428:
1423:
1417:
1415:
1406:
1396:
1395:
1392:
1391:
1388:
1387:
1385:
1384:
1379:
1374:
1368:
1366:
1360:
1359:
1357:
1356:
1351:
1346:
1341:
1336:
1331:
1326:
1320:
1318:
1316:Norepinephrine
1312:
1311:
1309:
1308:
1303:
1298:
1293:
1288:
1283:
1277:
1275:
1266:
1260:
1259:
1257:
1256:
1250:Norepinephrine
1230:
1228:
1219:
1217:Catecholamines
1213:
1212:
1204:
1202:
1201:
1194:
1187:
1179:
1171:
1170:
1143:(3): 365–375.
1126:
1081:
1025:
1006:(8): 924–931.
990:
961:(5): 591–603.
941:
912:(5): 502–514.
885:
823:
794:(2): 169–177.
763:
736:
735:
733:
730:
729:
728:
722:
714:
711:
620:
617:
601:norepinephrine
534:
531:
530:
525:
503:
502:
498:standard state
495:
492:
491:
480:
477:
474:
473:
469:
468:
465:
459:
458:
455:
449:
448:
442:
436:
435:
432:
426:
420:
415:
410:
407:
406:
402:
401:
399:
398:
395:
393:
390:
382:
381:
380:
377:
376:
374:
373:
369:
366:
365:
363:
359:
356:
355:
347:
346:
345:
342:
341:
339:
338:
330:DTXSID10205680
325:
323:
311:
308:
307:
305:
304:
296:
294:
288:
287:
285:
284:
276:
274:
266:
263:
262:
255:
249:
248:
246:
245:
237:
235:
229:
228:
226:
225:
217:
215:
210:
207:
206:
196:
188:
187:
185:
184:
176:
174:
168:
167:
165:
164:
156:
154:
148:
147:
144:
143:Abbreviations
140:
139:
137:
136:
128:
126:
122:
121:
119:
118:
111:
103:
101:
94:
91:
90:
88:
87:
79:
77:
72:
69:
68:
64:
63:
60:
56:
55:
51:
50:
44:
43:
39:
38:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2103:
2092:
2089:
2087:
2084:
2082:
2079:
2077:
2074:
2072:
2069:
2068:
2066:
2052:
2051:
2047:
2043:
2039:
2038:Serotonergics
2035:
2034:Melatonergics
2031:
2030:Dopaminergics
2027:
2023:
2020:
2013:
2007:
2004:
2002:
1999:
1997:
1994:
1993:
1991:
1987:
1981:
1978:
1976:
1973:
1971:
1968:
1966:
1963:
1961:
1958:
1956:
1953:
1951:
1948:
1946:
1943:
1941:
1938:
1936:
1933:
1931:
1928:
1926:
1923:
1921:
1918:
1916:
1913:
1911:
1908:
1906:
1903:
1901:
1898:
1896:
1893:
1891:
1889:
1884:
1882:
1879:
1877:
1874:
1872:
1869:
1867:
1864:
1862:
1859:
1857:
1854:
1852:
1849:
1847:
1844:
1842:
1839:
1837:
1834:
1832:
1831:2'-Amino-MPTP
1829:
1828:
1826:
1824:
1820:
1814:
1811:
1809:
1806:
1804:
1801:
1799:
1796:
1794:
1791:
1789:
1788:6-Hydroxydopa
1786:
1784:
1781:
1779:
1776:
1774:
1771:
1769:
1768:2'-Amino-MPTP
1766:
1765:
1763:
1761:
1760:Noradrenergic
1757:
1751:
1748:
1746:
1743:
1741:
1738:
1736:
1733:
1731:
1728:
1726:
1725:Norsalsolinol
1723:
1721:
1719:
1715:
1713:
1710:
1708:
1705:
1703:
1700:
1698:
1695:
1693:
1690:
1688:
1685:
1683:
1680:
1678:
1675:
1673:
1672:Fenpropathrin
1670:
1668:
1665:
1663:
1660:
1658:
1657:DOPAL quinone
1655:
1653:
1650:
1648:
1645:
1643:
1640:
1638:
1635:
1633:
1630:
1628:
1625:
1622:
1621:methylmercury
1618:
1614:
1611:
1609:
1606:
1604:
1601:
1599:
1597:
1592:
1590:
1587:
1585:
1582:
1581:
1579:
1577:
1573:
1569:
1562:
1557:
1555:
1550:
1548:
1543:
1542:
1539:
1527:
1524:
1522:
1519:
1517:
1514:
1512:
1509:
1508:
1506:
1504:
1500:
1494:
1491:
1489:
1486:
1484:
1482:
1478:
1477:
1475:
1473:
1469:
1465:
1455:
1452:
1450:
1447:
1445:
1442:
1441:
1439:
1437:
1433:
1427:
1424:
1422:
1419:
1418:
1416:
1414:
1410:
1407:
1405:
1401:
1397:
1383:
1380:
1378:
1375:
1373:
1370:
1369:
1367:
1365:
1361:
1355:
1352:
1350:
1347:
1345:
1342:
1340:
1337:
1335:
1332:
1330:
1327:
1325:
1322:
1321:
1319:
1317:
1313:
1307:
1304:
1302:
1299:
1297:
1294:
1292:
1289:
1287:
1284:
1282:
1279:
1278:
1276:
1274:
1270:
1267:
1265:
1261:
1255:
1251:
1247:
1243:
1239:
1235:
1234:Phenylalanine
1232:
1231:
1229:
1227:
1223:
1220:
1218:
1214:
1210:
1207:
1200:
1195:
1193:
1188:
1186:
1181:
1180:
1177:
1166:
1162:
1158:
1154:
1150:
1146:
1142:
1138:
1130:
1127:
1122:
1118:
1113:
1108:
1104:
1100:
1096:
1092:
1085:
1082:
1077:
1073:
1068:
1063:
1058:
1053:
1049:
1045:
1041:
1034:
1032:
1030:
1026:
1021:
1017:
1013:
1009:
1005:
1001:
994:
991:
986:
982:
977:
972:
968:
964:
960:
956:
952:
945:
942:
937:
933:
928:
923:
919:
915:
911:
907:
903:
896:
894:
892:
890:
886:
881:
877:
872:
867:
862:
857:
853:
849:
848:Int J Mol Sci
845:
838:
836:
834:
832:
830:
828:
824:
819:
815:
810:
805:
801:
797:
793:
789:
785:
778:
776:
774:
772:
770:
768:
764:
751:
747:
741:
738:
731:
726:
723:
720:
717:
716:
712:
710:
704:
700:
696:
695:methylmercury
692:
688:
684:
680:
676:
672:
668:
664:
662:
658:
654:
650:
646:
642:
638:
634:
630:
626:
618:
616:
614:
610:
606:
602:
598:
594:
590:
586:
584:
578:
574:
570:
565:
563:
559:
556:
552:
548:
544:
540:
528:
521:
516:
499:
493:
490:
489:
488:Phenylglyoxal
484:
481:
476:
475:
470:
466:
464:
463:Boiling point
461:
460:
456:
454:
451:
450:
443:
441:
438:
437:
416:
413:
409:
408:
403:
394:
389:
385:
378:
364:
354:
350:
343:
335:
331:
327:
326:
324:
314:
310:
309:
302:
298:
297:
295:
293:
290:
289:
282:
278:
277:
275:
269:
265:
264:
260:
256:
254:
251:
250:
243:
239:
238:
236:
234:
231:
230:
223:
219:
218:
216:
213:
209:
208:
204:
200:
197:
195:
193:ECHA InfoCard
190:
189:
182:
178:
177:
175:
173:
170:
169:
162:
158:
157:
155:
153:
150:
149:
145:
142:
141:
134:
130:
129:
127:
124:
123:
116:
112:
109:
105:
104:
102:
98:
93:
92:
85:
81:
80:
78:
75:
71:
70:
65:
57:
49:
45:
40:
36:
31:
19:
2018:
2017:
1905:Fenfluramine
1887:
1823:Serotonergic
1717:
1651:
1647:DOPA quinone
1595:
1576:Dopaminergic
1503:Trace amines
1480:
1377:Metanephrine
1280:
1140:
1136:
1129:
1094:
1084:
1047:
1043:
1003:
999:
993:
958:
954:
944:
909:
906:Semin Neurol
905:
854:(11): 5999.
851:
847:
791:
787:
754:. Retrieved
749:
740:
665:
645:pathogenesis
622:
587:(COMT) into
582:
571:of dopamine
566:
546:
542:
538:
537:
486:
67:Identifiers
59:Other names
2026:Adrenergics
1910:Haloperidol
1677:Haloperidol
1627:Amphetamine
1364:Epinephrine
1254:Epinephrine
643:and in the
599:(DBH) into
577:methylation
457:1.306 g/mL
405:Properties
199:100.237.172
161:CHEBI:27978
2065:Categories
1846:2,4,5-THMA
1745:Salsolinol
1617:disulfiram
1589:2,4,5-THMA
1436:Catabolism
1421:Tryptophan
1400:Tryptophan
1264:Catabolism
756:13 October
732:References
681:including
675:disulfiram
671:catabolism
573:metabolism
560:formed by
551:metabolite
440:Molar mass
301:F2E9Q24TSL
212:IUPHAR/BPS
172:ChemSpider
95:3D model (
74:CAS Number
2081:Catechols
2076:Aldehydes
2019:See also:
1841:2,4,5-THA
1773:2,4,5-THA
1584:2,4,5-THA
1472:Melatonin
1468:Serotonin
1413:Anabolism
1404:Serotonin
1226:Anabolism
1050:(1): 35.
721:(DOPEGAL)
661:PC12 line
605:oxidation
581:catechol
84:5707-55-1
1989:Unsorted
1813:Xylamine
1740:Rotenone
1735:Paraquat
1692:Mancozeb
1662:Dopamine
1642:Dieldrin
1273:Dopamine
1246:Dopamine
1238:Tyrosine
1165:15019299
1121:24262193
1076:31488222
1020:11295535
985:23786406
936:32906170
927:10680399
880:34206133
818:31807952
809:10680281
727:(5-HIAL)
713:See also
699:rotenone
691:dieldrin
575:include
558:dopamine
1876:5,7-DHT
1871:5,6-DHT
1856:3,4-DCA
1836:2,4-DCA
1793:DOPEGAL
1783:5,7-DHT
1778:5,6-DHT
1637:Daidzin
1632:Benomyl
1615:(e.g.,
1603:5,6-DHT
1145:Bibcode
1112:3932615
1067:6728988
976:4096629
871:8199574
687:daidzin
683:benomyl
564:(MAO).
553:of the
549:, is a
520:what is
518: (
453:Density
445:152.149
268:PubChem
1996:5-HIAL
1163:
1119:
1109:
1074:
1064:
1018:
983:
973:
934:
924:
878:
868:
816:
806:
707:
701:, and
629:potent
567:Other
515:verify
512:
384:SMILES
281:119219
242:C04043
181:106504
146:DOPAL
133:B00668
125:3DMet
42:Names
2006:RHPTP
1866:5-IAI
1798:DSP-4
1750:Ziram
1697:Maneb
1652:DOPAL
709:μM).
703:ziram
637:aging
543:DOPAL
349:InChI
152:ChEBI
97:JSmol
18:DOPAL
2001:RHPP
1975:PCMA
1955:MMAI
1940:MDEA
1935:MDAI
1925:MBDB
1920:HPTP
1851:3-CA
1712:MPTP
1687:HPTP
1161:PMID
1117:PMID
1072:PMID
1016:PMID
981:PMID
932:PMID
876:PMID
814:PMID
758:2011
611:and
591:and
292:UNII
253:MeSH
233:KEGG
222:6632
1980:PIA
1970:PCA
1965:PBA
1915:HPP
1900:DCA
1895:αET
1682:HPP
1153:doi
1107:PMC
1099:doi
1062:PMC
1052:doi
1008:doi
971:PMC
963:doi
959:126
922:PMC
914:doi
866:PMC
856:doi
804:PMC
796:doi
792:127
647:of
595:by
579:by
318:EPA
271:CID
2067::
2048:•
2044:•
2040:•
2036:•
2032:•
2028:•
2024:•
1886:α,
1619:,
1594:5-
1252:→
1248:→
1244:→
1240:→
1236:→
1159:.
1151:.
1141:25
1139:.
1115:.
1105:.
1093:.
1070:.
1060:.
1048:14
1046:.
1042:.
1028:^
1014:.
1004:30
1002:.
979:.
969:.
957:.
953:.
930:.
920:.
910:40
908:.
904:.
888:^
874:.
864:.
852:22
850:.
846:.
826:^
812:.
802:.
790:.
786:.
766:^
748:.
697:,
693:,
689:,
685:,
615:.
1888:N
1718:N
1623:)
1596:S
1560:e
1553:t
1546:v
1481:N
1470:→
1402:→
1198:e
1191:t
1184:v
1167:.
1155::
1147::
1123:.
1101::
1078:.
1054::
1022:.
1010::
987:.
965::
938:.
916::
882:.
858::
820:.
798::
760:.
583:O
541:(
510:N
433:3
430:O
427:8
424:H
421:8
418:C
320:)
316:(
99:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.