127:
851:
41:
32:
1261:
770:
Yet another coordinatization of the diamond cubic involves the removal of some of the edges from a three-dimensional grid graph. In this coordinatization, which has a distorted geometry from the standard diamond cubic structure but has the same topological structure, the vertices of the diamond cubic
624:
between them in the diamond structure. The four nearest neighbors of each point may be obtained, in this coordinate system, by adding one to each of the four coordinates, or by subtracting one from each of the four coordinates, accordingly as the coordinate sum is zero or one. These four-dimensional
615:
Alternatively, each point of the diamond cubic structure may be given by four-dimensional integer coordinates whose sum is either zero or one. Two points are adjacent in the diamond structure if and only if their four-dimensional coordinates differ by one in a single coordinate. The total difference
20:
537:
382:
415:
782:
that takes the point (0,0,0) into the point (3,3,3), for instance. However, it is still a highly symmetric structure: any incident pair of a vertex and edge can be transformed into any other incident pair by a
103:, which have a similar structure, with one kind of atom (such as silicon in cristobalite) at the positions of carbon atoms in diamond but with another kind of atom (such as oxygen) halfway between those (see
1212:
Blank, V.; Popov, M.; Pivovarov, G.; Lvova, N. et al. (1998). "Ultrahard and superhard phases of fullerite C60: comparison with diamond on hardness and wear". Diamond and
Related Materials 7 (2–5): 427.
757:
284:
420:
588:
apart in the integer lattice; the edges of the diamond structure lie along the body diagonals of the integer grid cubes. This structure may be scaled to a cubical unit cell that is some number
239:
of the diamond cubic structure (the proportion of space that would be filled by spheres that are centered on the vertices of the structure and are as large as possible without overlapping) is
296:
584:
1088:
912:
628:
869:
systems that follow the diamond cubic geometry have a high capacity to withstand compression, by minimizing the unbraced length of individual
1179:
1116:
1065:
1017:
992:
967:
942:
1312:
104:
1041:
771:
are represented by all possible 3d grid points and the edges of the diamond cubic are represented by a subset of the 3d grid edges.
1038:
Combinatorial Image
Analysis: 13th International Workshop, IWCIA 2009, Playa Del Carmen, Mexico, November 24–27, 2009, Proceedings
1287:
242:
983:
Askeland, Donald R.; Phulé, Pradeep
Prabhakar (2006), "Example 3-15: Determining the Packing Factor for Diamond Cubic Silicon",
532:{\displaystyle {\begin{aligned}x=y&=z\ ({\text{mod }}\ 2),\\x+y+z&=0{\text{ or }}1\ ({\text{mod }}\ 4).\end{aligned}}}
290:. Zincblende structures have higher packing factors than 0.34 depending on the relative sizes of their two component atoms.
69:
is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was
392:
Mathematically, the points of the diamond cubic structure can be given coordinates as a subset of a three-dimensional
293:
The first-, second-, third-, fourth-, and fifth-nearest-neighbor distances in units of the cubic lattice constant are
1090:
Graph
Drawing: 16th International Symposium, GD 2008, Heraklion, Crete, Greece, September 21–24, 2008, Revised Papers
1036:
Nagy, Benedek; Strand, Robin (2009), "Neighborhood sequences in the diamond grid – algorithms with four neighbors",
791:. Moreover, the diamond crystal as a network in space has a strong isotropic property. Namely, for any two vertices
377:{\displaystyle {\tfrac {\sqrt {3}}{4}},{\tfrac {\sqrt {2}}{2}},{\tfrac {\sqrt {11}}{4}},1,{\tfrac {\sqrt {19}}{4}},}
396:
by using a cubic unit cell four units across. With these coordinates, the points of the structure have coordinates
1302:
1292:
48:
1317:
1307:
130:
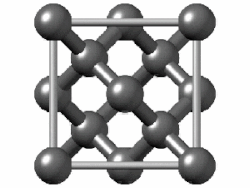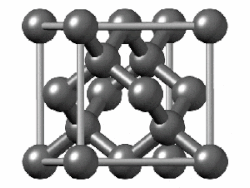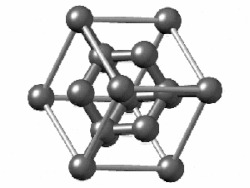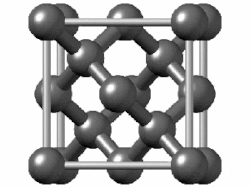
Visualisation of a diamond cubic unit cell: 1. Components of a unit cell, 2. One unit cell, 3. A lattice of
1214:
779:
209:
153:. The lattice describes the repeat pattern; for diamond cubic crystals this lattice is "decorated" with a
165:
93:
859:
784:
236:
1045:
894:
844:
287:
225:
147:
1224:
Lorimer, A. "The
Diamond Cubic Truss", Interior World: Design & Detail, vol.121, 2013, pp. 80–81
1010:
Concise
Dictionary of Materials Science: Structure and Characterization of Polycrystalline Materials
874:
189:
1122:
1094:
617:
565:
542:
1140:
Parhami, B.; Kwai, Ding-Ming (2001), "A unified formulation of honeycomb and diamond networks",
1175:
1112:
1061:
1013:
988:
963:
938:
221:
185:
126:
66:
24:
1265:
774:
The diamond cubic is sometimes called the "diamond lattice" but it is not, mathematically, a
1167:
1149:
1104:
1053:
556:
All of the other points in the structure may be obtained by adding multiples of four to the
213:
1087:(2009), "Isometric Diamond Subgraphs", in Tollis, Ioannis G.; Patrignani, Maurizio (eds.),
286:
significantly smaller (indicating a less dense structure) than the packing factors for the
228:, where each atom has nearest neighbors of an unlike element. Zincblende's space group is F
900:
788:
775:
393:
217:
150:
115:
59:
1093:, Lecture Notes in Computer Science, vol. 5417, Springer-Verlag, pp. 384–389,
1049:
19:
1297:
1084:
850:
232:
3m, but many of its structural properties are quite similar to the diamond structure.
1281:
840:
621:
560:
coordinates of these eight points. Adjacent points in this structure are at distance
161:
82:
1126:
40:
1271:
873:. The diamond cubic geometry has also been considered for the purpose of providing
764:
100:
74:
52:
1108:
625:
coordinates may be transformed into three-dimensional coordinates by the formula
99:
in any proportion. There are also crystals, such as the high-temperature form of
1233:
R. Kraft. Construction
Arrangement, USA, United States Patents, US3139959, 1964
1057:
935:
Diamond films: chemical vapor deposition for oriented and heteroepitaxial growth
906:
882:
820:
158:
143:
44:
188:
in each dimension. The diamond lattice can be viewed as a pair of intersecting
1245:
1234:
31:
1172:
Topological
Crystallography -With a View Towards Discrete Geometric Analysis-
89:
1260:
1244:
Gilman, J. Tetrahedral Truss, USA, United States
Patents, US4446666, 1981
878:
760:
836:
85:
70:
1194:
Sunada, Toshikazu (2008), "Crystals that nature might miss creating",
1153:
1274:
to construct self-avoiding random walks on the diamond cubic lattice
616:
in coordinate values between any two points (their four-dimensional
1099:
870:
866:
855:
795:
of the crystal net, and for any ordering of the edges adjacent to
125:
96:
819:-edge. Another (hypothetical) crystal with this property is the
958:
Wiberg, Egon; Wiberg, Nils; Holleman, Arnold
Frederick (2001),
78:
51:
of the diamond lattice showing the 3-fold symmetry along the
752:{\displaystyle (a,b,c,d)\to (a+b-c-d,\ a-b+c-d,\ -a+b+c-d).}
279:{\displaystyle {\tfrac {\pi {\sqrt {3}}}{16}}\approx 0.34,}
885:, have been found to be more effective for this purpose.
118:
in the technical sense of this word used in mathematics.
763:
subset of the four-dimensional integer lattice, it is a
592:
of units across by multiplying all coordinates by
358:
335:
318:
301:
247:
208:
of the width of the unit cell in each dimension. Many
1142:
IEEE Transactions on Parallel and Distributed Systems
1044:, vol. 5852, Springer-Verlag, pp. 109–121,
631:
568:
418:
299:
245:
933:Kobashi, Koji (2005), "2.1 Structure of diamond",
751:
578:
531:
376:
278:
847:) is attributed to the diamond cubic structure.
903: – Scientific study of crystal structures
803:, there is a net-preserving congruence taking
288:face-centered and body-centered cubic lattices
8:
36:3D ball-and-stick model of a diamond lattice
799:and any ordering of the edges adjacent to
1098:
897: – Materials made only out of carbon
835:The compressive strength and hardness of
827:crystal, (10,3)-a, or the diamond twin).
630:
569:
567:
508:
494:
448:
419:
417:
357:
334:
317:
300:
298:
253:
246:
244:
985:The Science and Engineering of Materials
849:
39:
29:
18:
925:
913:Triakis truncated tetrahedral honeycomb
877:though structures composed of skeletal
1031:
1029:
759:Because the diamond structure forms a
138:Diamond's cubic structure is in the Fd
1079:
1077:
839:and various other materials, such as
146:(space group 227), which follows the
77:also adopt this structure, including
7:
105:Category:Minerals in space group 227
23:Rotating model of the diamond cubic
620:) gives the number of edges in the
549:(0,0,0), (0,2,2), (2,0,2), (2,2,0),
915: – Space-filling tessellation
552:(3,3,3), (3,1,1), (1,3,1), (1,1,3)
545:4) that satisfy these conditions:
14:
1042:Lecture Notes in Computer Science
843:, (which has the closely related
192:lattices, with each separated by
1259:
987:, Cengage Learning, p. 82,
962:, Academic Press, p. 1300,
815:-edge to the similarly ordered
909: – Periodic spatial graph
743:
662:
659:
656:
632:
519:
505:
459:
445:
1:
1109:10.1007/978-3-642-00219-9_37
1313:Minerals in space group 227
1058:10.1007/978-3-642-10210-3_9
854:Example of a diamond cubic
579:{\displaystyle {\sqrt {3}}}
1336:
1008:Novikov, Vladimir (2003),
122:Crystallographic structure
114:, this structure is not a
110:Although often called the
412:satisfying the equations
16:Type of crystal structure
1012:, CRC Press, p. 9,
541:There are eight points (
49:stereographic projection
1288:Crystal structure types
937:, Elsevier, p. 9,
210:compound semiconductors
862:
780:translational symmetry
753:
580:
533:
388:Mathematical structure
378:
280:
135:
55:
37:
27:
858:system for resisting
853:
831:Mechanical properties
754:
581:
534:
379:
281:
237:atomic packing factor
129:
43:
35:
22:
1268:at Wikimedia Commons
895:Allotropes of carbon
845:zincblende structure
629:
566:
416:
297:
243:
226:zincblende structure
224:adopt the analogous
184:of the width of the
73:, other elements in
1050:2009LNCS.5852..109N
960:Inorganic chemistry
875:structural rigidity
761:distance-preserving
190:face-centered cubic
148:face-centered cubic
1196:Notices of the AMS
863:
823:(also called the K
749:
618:Manhattan distance
576:
529:
527:
374:
369:
346:
329:
312:
276:
265:
136:
56:
38:
28:
1264:Media related to
1181:978-4-431-54176-9
1168:Sunada, Toshikazu
1154:10.1109/71.899940
1118:978-3-642-00219-9
1067:978-3-642-10210-3
1019:978-0-8493-0970-0
994:978-0-534-55396-8
969:978-0-12-352651-9
944:978-0-08-044723-0
718:
691:
574:
515:
511:
504:
497:
455:
451:
444:
368:
364:
345:
341:
328:
324:
311:
307:
264:
258:
222:indium antimonide
94:silicon–germanium
67:crystal structure
25:crystal structure
1325:
1263:
1247:
1242:
1236:
1231:
1225:
1222:
1216:
1210:
1204:
1203:
1191:
1185:
1184:
1164:
1158:
1156:
1137:
1131:
1129:
1102:
1081:
1072:
1070:
1033:
1024:
1022:
1005:
999:
997:
980:
974:
972:
955:
949:
947:
930:
818:
814:
810:
806:
802:
798:
794:
758:
756:
755:
750:
716:
689:
611:
610:
608:
607:
604:
601:
591:
587:
585:
583:
582:
577:
575:
570:
559:
538:
536:
535:
530:
528:
513:
512:
509:
502:
498:
495:
453:
452:
449:
442:
411:
383:
381:
380:
375:
370:
360:
359:
347:
337:
336:
330:
320:
319:
313:
303:
302:
285:
283:
282:
277:
266:
260:
259:
254:
248:
231:
214:gallium arsenide
207:
205:
204:
201:
198:
183:
181:
180:
177:
174:
141:
133:
34:
1335:
1334:
1328:
1327:
1326:
1324:
1323:
1322:
1303:Infinite graphs
1293:Crystallography
1278:
1277:
1256:
1251:
1250:
1243:
1239:
1232:
1228:
1223:
1219:
1211:
1207:
1193:
1192:
1188:
1182:
1166:
1165:
1161:
1139:
1138:
1134:
1119:
1085:Eppstein, David
1083:
1082:
1075:
1068:
1035:
1034:
1027:
1020:
1007:
1006:
1002:
995:
982:
981:
977:
970:
957:
956:
952:
945:
932:
931:
927:
922:
901:Crystallography
891:
833:
826:
816:
812:
808:
804:
800:
796:
792:
789:Euclidean space
627:
626:
605:
602:
597:
596:
594:
593:
589:
564:
563:
561:
557:
526:
525:
484:
466:
465:
432:
414:
413:
397:
394:integer lattice
390:
295:
294:
249:
241:
240:
229:
218:silicon carbide
202:
199:
196:
195:
193:
178:
175:
172:
171:
169:
168:, separated by
151:Bravais lattice
139:
131:
124:
112:diamond lattice
60:crystallography
30:
17:
12:
11:
5:
1333:
1332:
1329:
1321:
1320:
1318:Regular graphs
1315:
1310:
1308:Lattice points
1305:
1300:
1295:
1290:
1280:
1279:
1276:
1275:
1269:
1255:
1254:External links
1252:
1249:
1248:
1237:
1226:
1217:
1205:
1186:
1180:
1159:
1132:
1117:
1073:
1066:
1025:
1018:
1000:
993:
975:
968:
950:
943:
924:
923:
921:
918:
917:
916:
910:
904:
898:
890:
887:
881:, such as the
832:
829:
824:
778:: there is no
748:
745:
742:
739:
736:
733:
730:
727:
724:
721:
715:
712:
709:
706:
703:
700:
697:
694:
688:
685:
682:
679:
676:
673:
670:
667:
664:
661:
658:
655:
652:
649:
646:
643:
640:
637:
634:
573:
554:
553:
550:
524:
521:
518:
507:
501:
496: or
493:
490:
487:
485:
483:
480:
477:
474:
471:
468:
467:
464:
461:
458:
447:
441:
438:
435:
433:
431:
428:
425:
422:
421:
389:
386:
384:respectively.
373:
367:
363:
356:
353:
350:
344:
340:
333:
327:
323:
316:
310:
306:
275:
272:
269:
263:
257:
252:
166:primitive cell
164:atoms in each
123:
120:
83:semiconductors
15:
13:
10:
9:
6:
4:
3:
2:
1331:
1330:
1319:
1316:
1314:
1311:
1309:
1306:
1304:
1301:
1299:
1296:
1294:
1291:
1289:
1286:
1285:
1283:
1273:
1270:
1267:
1266:Diamond cubic
1262:
1258:
1257:
1253:
1246:
1241:
1238:
1235:
1230:
1227:
1221:
1218:
1215:
1209:
1206:
1201:
1197:
1190:
1187:
1183:
1177:
1173:
1169:
1163:
1160:
1155:
1151:
1147:
1143:
1136:
1133:
1128:
1124:
1120:
1114:
1110:
1106:
1101:
1096:
1092:
1091:
1086:
1080:
1078:
1074:
1069:
1063:
1059:
1055:
1051:
1047:
1043:
1039:
1032:
1030:
1026:
1021:
1015:
1011:
1004:
1001:
996:
990:
986:
979:
976:
971:
965:
961:
954:
951:
946:
940:
936:
929:
926:
919:
914:
911:
908:
905:
902:
899:
896:
893:
892:
888:
886:
884:
880:
876:
872:
868:
861:
857:
852:
848:
846:
842:
841:boron nitride
838:
830:
828:
822:
790:
786:
781:
777:
772:
768:
766:
762:
746:
740:
737:
734:
731:
728:
725:
722:
719:
713:
710:
707:
704:
701:
698:
695:
692:
686:
683:
680:
677:
674:
671:
668:
665:
653:
650:
647:
644:
641:
638:
635:
623:
622:shortest path
619:
613:
600:
571:
551:
548:
547:
546:
544:
539:
522:
516:
499:
491:
488:
486:
481:
478:
475:
472:
469:
462:
456:
439:
436:
434:
429:
426:
423:
409:
405:
401:
395:
387:
385:
371:
365:
361:
354:
351:
348:
342:
338:
331:
325:
321:
314:
308:
304:
291:
289:
273:
270:
267:
261:
255:
250:
238:
233:
227:
223:
219:
215:
211:
191:
187:
167:
163:
160:
159:tetrahedrally
156:
152:
149:
145:
128:
121:
119:
117:
113:
108:
106:
102:
98:
95:
91:
87:
84:
80:
76:
72:
68:
65:
64:diamond cubic
61:
54:
50:
46:
42:
33:
26:
21:
1240:
1229:
1220:
1208:
1199:
1195:
1189:
1174:, Springer,
1171:
1162:
1148:(1): 74–80,
1145:
1141:
1135:
1089:
1037:
1009:
1003:
984:
978:
959:
953:
934:
928:
864:
834:
773:
769:
765:partial cube
614:
598:
555:
540:
407:
403:
399:
391:
292:
234:
154:
137:
111:
109:
101:cristobalite
63:
57:
907:Laves graph
883:octet truss
865:Similarly,
860:compression
821:Laves graph
144:space group
45:Pole figure
1282:Categories
920:References
785:congruence
134:unit cells
1202:: 208–215
1100:0807.2218
879:triangles
811:and each
738:−
720:−
708:−
696:−
681:−
675:−
660:→
510:mod
450:mod
268:≈
251:π
186:unit cell
132:3 × 3 × 3
90:germanium
53:direction
1272:Software
1170:(2012),
1127:14066610
889:See also
212:such as
75:group 14
1046:Bibcode
837:diamond
776:lattice
609:
595:
586:
562:
558:x, y, z
206:
194:
182:
170:
157:of two
116:lattice
86:silicon
71:diamond
1178:
1125:
1115:
1064:
1016:
991:
966:
941:
871:struts
717:
690:
543:modulo
514:
503:
454:
443:
220:, and
162:bonded
97:alloys
92:, and
81:, the
62:, the
1298:Cubes
1123:S2CID
1095:arXiv
867:truss
856:truss
155:motif
79:α-tin
1176:ISBN
1113:ISBN
1062:ISBN
1014:ISBN
989:ISBN
964:ISBN
939:ISBN
793:x, y
271:0.34
235:The
216:, β-
88:and
1150:doi
1105:doi
1054:doi
807:to
787:of
107:).
58:In
47:in
1284::
1200:55
1198:,
1146:12
1144:,
1121:,
1111:,
1103:,
1076:^
1060:,
1052:,
1040:,
1028:^
767:.
612:.
406:,
402:,
362:19
339:11
262:16
142:m
1157:.
1152::
1130:.
1107::
1097::
1071:.
1056::
1048::
1023:.
998:.
973:.
948:.
825:4
817:y
813:x
809:y
805:x
801:y
797:x
747:.
744:)
741:d
735:c
732:+
729:b
726:+
723:a
714:,
711:d
705:c
702:+
699:b
693:a
687:,
684:d
678:c
672:b
669:+
666:a
663:(
657:)
654:d
651:,
648:c
645:,
642:b
639:,
636:a
633:(
606:4
603:/
599:a
590:a
572:3
523:.
520:)
517:4
506:(
500:1
492:0
489:=
482:z
479:+
476:y
473:+
470:x
463:,
460:)
457:2
446:(
440:z
437:=
430:y
427:=
424:x
410:)
408:z
404:y
400:x
398:(
372:,
366:4
355:,
352:1
349:,
343:4
332:,
326:2
322:2
315:,
309:4
305:3
274:,
256:3
230:4
203:4
200:/
197:1
179:4
176:/
173:1
140:3
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.