58:
467:
312:
1406:
84:
855:
1537:
851:
1548:
45:
68:
1962:, alone or in combination with other ingredients. In the latter case, often, the intended function of the DMSO is as a solvent, to carry the other ingredients across the skin. Also in horses, DMSO is used intravenously, again alone or in combination with other drugs. It is used alone for the treatment of increased intracranial pressure and/or cerebral edema in horses.
993:
856:
3827:
1468:
to remove both DMSO and water. Reactions conducted in DMSO are often diluted with water to precipitate or phase-separate products. The relatively high freezing point of DMSO, 18.5 °C (65.3 °F), means that at, or just below, room temperature it is a solid, which can limit its utility in some
2193:
DMSO can decompose at the boiling temperature of 189 °C at normal pressure, possibly leading to an explosion. The decomposition is catalyzed by acids and bases and therefore can be relevant at even lower temperatures. A strong to explosive reaction also takes place in combination with halogen
1918:
documentary in 1980 featuring an early proponent. However, DMSO is an ingredient in some products listed by the U.S. FDA as fake cancer cures and the FDA has had a running battle with distributors. One such distributor is
Mildred Miller, who promoted DMSO for a variety of disorders and was
2026:
DMSO can cause contaminants, toxins, and medicines to be absorbed through the skin, which may cause unexpected effects. DMSO is thought to increase the effects of blood thinners, steroids, heart medicines, sedatives, and other drugs. In some cases this could be harmful or dangerous.
1664:
DMSO is finding increased use in manufacturing processes to produce microelectronic devices. It is widely used to strip photoresist in TFT-LCD 'flat panel' displays and advanced packaging applications (such as wafer-level packaging / solder bump patterning). DMSO is an effective
2092:
Early clinical trials with DMSO were stopped because of questions about its safety, especially its ability to harm the eye. The most commonly reported side effects include headaches and burning and itching on contact with the skin. Strong allergic reactions have been reported.
854:
1459:
Because of its high boiling point, 189 °C (372 °F), DMSO evaporates slowly at normal atmospheric pressure. Samples dissolved in DMSO cannot as easily be recovered compared to other solvents, as it is very difficult to remove all traces of DMSO by conventional
2306:
Matthews WS, Bares JE, Bartmess JE, Bordwell FG, Cornforth FJ, Drucker GE, Margolin Z, McCallum RJ, McCollum GJ, Vanier NR (1975). "Equilibrium acidities of carbon acids. VI. Establishment of an absolute scale of acidities in dimethyl sulfoxide solution".
2428:
von
Demselben (1867). "Ueber die Einwirkung von Saltpetersäure auf Schwefelmethyl und Schwefeläthyl" [On the effect of nitric acid on methyl sulfide and ethyl sulfide]. In Erlenmeyer, E.; Rieckher, T.; Volhard, J.; Liebig, J.; Wöhler, F. (eds.).
1942:. There is insufficient evidence to support the hypothesis that DMSO has any effect, and most sources agree that its history of side effects when tested warrants caution when using it as a dietary supplement, for which it is marketed heavily with the
2115:. In 1980, the US Congress held hearings on claims that the FDA was slow in approving DMSO for other medical uses. In 2007, the US FDA granted "fast track" designation on clinical studies of DMSO's use in reducing brain tissue swelling following
2102:
reported that a manufacturer of the chemical warned that the death of an Irish woman after undergoing DMSO treatment for a sprained wrist may have been due to the treatment, although no autopsy was done, nor was a causal relationship established.
1722:, added to cell media to reduce ice formation and thereby prevent cell death during the freezing process. Approximately 10% may be used with a slow-freeze method, and the cells may be frozen at −80 °C (−112 °F) or stored in
1656:
effects can occur and, if DMSO control groups are not carefully planned, then solvent effects can falsely be attributed to the prospective drug. For example, even a very low dose of DMSO has a powerful protective effect against
4154:
1811:
disorders that were studied. The authors recommended DMSO for genitourinary inflammatory conditions not caused by infection or tumor in which symptoms were severe or patients failed to respond to conventional therapy.
3620:
3825:, George Kvakovszky; David Villarrubia II & Scott Stevenson et al., "Process for preparing low malodorous dimethyl sulfoxide", published 2009, assigned to Gaylord Chemical Company LLC
872:
1494:
spectroscopy, again due to its ability to dissolve a wide range of analytes, the simplicity of its own spectrum, and its suitability for high-temperature NMR spectroscopic studies. Disadvantages to the use of
1448:. It is also extensively used as an extractant in biochemistry and cell biology. Because DMSO is only weakly acidic, it tolerates relatively strong bases and as such has been extensively used in the study of
1119:, who reported his findings in 1867. Its modern use as an industrial solvent began through popularization by Thor Smedslund at the Stepan Chemical Company. Dimethyl sulfoxide is produced industrially from
57:
3559:
3912:
3779:
Glindemann D, Novak J, Witherspoon J (January 2006). "Dimethyl sulfoxide (DMSO) waste residues and municipal waste water odor by dimethyl sulfide (DMS): the North-East WPCP plant of
Philadelphia".
2081:
1846:
mixtures used to preserve organs, tissues, and cell suspensions. Without it, up to 90% of frozen cells will become inactive. It is particularly important in the freezing and long-term storage of
1761:, discovered it could penetrate the skin and other membranes without damaging them and could carry other compounds into a biological system. In medicine, DMSO is predominantly used as a topical
1712:). For example, 10% final concentration of DMSO in the PCR mixture with Phusion decreases primer annealing temperature (i.e. primer melting temperature) by 5.5–6.0 °C (9.9–10.8 °F).
4147:
3394:
1614:, and has a high boiling point (this improves the accuracy of test compound concentrations by reducing room temperature evaporation). One limitation with DMSO is that it can affect
1975:
4140:
782:
3681:
1006:
3905:
1292:
3628:
1336:
857:
2851:
Balakin KV, Savchuk NP, Tetko IV (2006). "In silico approaches to prediction of aqueous and DMSO solubility of drug-like compounds: trends, problems and solutions".
3898:
3552:
1896:. It is subject to renal and pulmonary excretion. A possible side effect of DMSO is therefore elevated blood dimethyl sulfide, which may cause a blood borne
516:
2072:
gloves, which are very commonly used in chemical laboratories, may protect from brief contact but have been found to degrade rapidly with exposure to DMSO.
1618:
growth and viability, with low DMSO concentrations sometimes stimulating cell growth, and high DMSO concentrations sometimes inhibiting or killing cells.
2755:"Bioprospecting for antibacterial drugs: a multidisciplinary perspective on natural product source material, bioassay selection and avoidable pitfalls"
3091:
879:
1464:. One technique to fully recover samples is removal of the organic solvent by evaporation followed by addition of water (to dissolve DMSO) and
2209:(DMS), the corresponding sulfide, also produced by marine phytoplankton and emitted to the oceanic atmosphere where it is oxidized to DMSO, SO
2162:(DMS) that has a strong disagreeable odor, similar to rotten cabbage. However, chemically pure DMSO is odorless because of the lack of C-S-C (
1701:. It is added to the PCR mix before reacting, where it interferes with the self-complementarity of the DNA, minimizing interfering reactions.
1078:
in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO is metabolised to compounds that leave a
3402:
3373:
3220:
3157:
3105:
Sawai M, Takase K, Teraoka H, Tsukada K (1990). "Reversible G1 arrest in the cell cycle of human lymphoid cell lines by dimethyl sulfoxide".
2598:
2369:
1754:
1096:
4184:
3293:
1792:. It is frequently compounded with antifungal medications, enabling them to penetrate not just skin but also toenails and fingernails.
3685:
1926:
The use of DMSO as an alternative treatment for cancer is of particular concern, as it has been shown to interfere with a variety of
3866:
3854:
481:
4426:
2974:
Kelava T, Cavar I, Čulo F (Oct 2010). "Influence of small doses of various drug vehicles on acetaminophen-induced liver injury".
3598:
3576:
3009:
Kvakovszky G, McKim AS, Moore J (2007). "A Review of
Microelectronic Manufacturing Applications Using DMSO-Based Chemistries".
1824:
1296:
3880:
2481:
1116:
1104:
975:
942:
818:
2724:
2685:
2654:
1013:
2622:
Cramer, R. E.; Bopp, T. T. (1977). "Graphical display of the enthalpies of adduct formation for Lewis acids and bases".
2108:
1998:
sulfides (which typically have foul odors), and similar odiferous sulfur compounds, the pure chemical DMSO is odorless.
1796:
1734:
424:
3011:
1157:
445:
67:
1518:
1491:
1456:
values (C-H, O-H, S-H and N-H acidities) for thousands of organic compounds have been determined in DMSO solution.
663:
4431:
3054:
Chakrabarti R, Schutt CE (August 2001). "The enhancement of PCR amplification by low molecular-weight sulfones".
1686:
1587:
1445:
1370:
865:
375:
319:
3653:
1704:
DMSO in a PCR is applicable for supercoiled plasmids (to relax before amplification) or DNA templates with high
1388:. The relative donor strength of DMSO toward a series of acids, versus other Lewis bases, can be illustrated by
1816:
1804:
1730:
1238:
369:
1300:
1234:. For this reason, the basicities of many weakly basic organic compounds have been examined in this solvent.
2533:
Tidwell TT (1990). "Oxidation of
Alcohols by Activated Dimethyl Sulfoxide and Related Reactions: An Update".
1351:
through sulfur. The fourth DMSO is bonded through oxygen. In general, the oxygen-bonded mode is more common.
3701:
3624:
2255:
2247:
2155:
2098:
1878:
1851:
1433:
3822:
3718:
3699:
Carley W (September 9, 1965). "DMSO may have caused death of woman, makers of 'Wonder' drug warn doctors".
3495:"Transient receptor potential channels encode volatile chemicals sensed by rat trigeminal ganglion neurons"
2334:
1627:
studies of test compounds. It has, for example, been employed as a co-solvent to assist absorption of the
307:
4071:
2135:
2116:
1874:
1640:
1615:
1332:
3965:
2753:
Cushnie TP, Cushnie B, Echeverría J, Fowsantear W, Thammawat S, Dodgson JL, Law S, Clow SM (June 2020).
2217:
2112:
1943:
1800:
1511:
1414:
1366:
1033:
954:
112:
31:
1405:
1354:
In carbon tetrachloride solutions DMSO functions as a Lewis base with a variety of Lewis acids such as
2385:
Thomas R, Shoemaker CB, Eriks K (1966). "The
Molecular and Crystal Structure of Dimethyl Sulfoxide, (H
2194:
compounds, metal nitrides, metal perchlorates, sodium hydride, periodic acid and fluorinating agents.
249:
61:
Stereo structural formula of dimethyl sulfoxide with an explicit electron pair and assorted dimensions
4132:
4101:
4066:
3788:
3506:
3466:
3020:
2899:
2402:
1909:
1847:
1742:
1441:
100:
3418:"Say no to DMSO: dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes"
1249:
is provided by the S(O)R group. The sodium derivative of DMSO formed in this way is referred to as
4421:
4096:
2888:"Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans"
2535:
2127:
2111:(NAS) published findings in favor of DMSO in 1972. In 1978, the US FDA approved DMSO for treating
1995:
1758:
698:
462:
148:
83:
3732:"Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system"
4163:
3984:
3660:
3183:
3036:
2782:
2702:
2012:
1983:
1979:
1885:
1855:
1653:
1461:
1308:
1086:
747:
732:
209:
189:
182:
3890:
2640:
The plots shown in this paper used older parameters. Improved E&C parameters are listed in
2361:
4416:
4316:
4111:
4039:
3942:
3862:
3850:
3804:
3761:
3534:
3447:
3345:
3328:
3275:
3216:
3171:
3163:
3153:
3122:
3073:
2991:
2927:
2868:
2828:
2774:
2604:
2594:
2515:
2477:
2430:
2365:
2354:
2170:) linkages. Deodorization of DMSO is achieved by removing the odorous impurities it contains.
2104:
1987:
1774:
1766:
1595:
1591:
1426:
1422:
1418:
1288:
1071:
927:
901:
3210:
2886:
Cai WJ, Huang JH, Zhang SQ, Wu B, Kapahi P, Zhang XM, Shen ZY (2011). Blagosklonny MV (ed.).
3947:
3796:
3751:
3743:
3730:
Hanslick JL, Lau K, Noguchi KK, Olney JW, Zorumski CF, Mennerick S, Farber NB (April 2009).
3524:
3514:
3437:
3429:
3337:
3301:
3265:
3255:
3145:
3114:
3065:
3028:
2983:
2917:
2907:
2860:
2818:
2766:
2694:
2631:
2571:
2544:
2507:
2498:
2469:
2410:
2316:
2234:
2228:
2206:
2159:
1991:
1893:
1889:
1843:
1603:
1264:
1128:
1120:
1037:
948:
682:
628:
544:
3684:. Commonwealth of Australia: Department of Health and Ageing. 23 April 2003. Archived from
1795:
DMSO has been examined for the treatment of numerous conditions and ailments, but the U.S.
433:
4215:
4106:
3979:
3195:
2187:
2183:
2043:
1870:(70% EMEM + 30% fetal bovine serum + antibiotic mixture) is used. As part of an
1723:
1715:
It is well known as a reversible cell cycle arrester at phase G1 of human lymphoid cells.
1709:
1674:
1481:
1470:
1304:
1067:
907:
338:
269:
3792:
3510:
3024:
2903:
2406:
1536:
466:
311:
229:
158:
4220:
3957:
3849:]. Ecomed Sicherheit (in German). Landsberg/Lech: Verlagsgruppe Hüthig Jehle Rehm.
3756:
3731:
3529:
3494:
3442:
3417:
3270:
3243:
3056:
2922:
2887:
2147:
2069:
2054:
1920:
1839:
1719:
1666:
1649:
1599:
1576:
1503:
1276:
1268:
1242:
1185:
1092:
984:
653:
3641:
3069:
2562:
Calligaris M (2004). "Structure and bonding in metal sulfoxide complexes: An update".
4410:
4331:
3960:
3341:
3118:
3040:
2786:
2179:
2123:
2084:
Drug, and a company has been prosecuted for adding it to products as a preservative.
1808:
1785:
1738:
1611:
1465:
1272:
1251:
1153:
1124:
1064:
643:
617:
607:
300:
3606:
3584:
3416:
Hall MD, Telma KA, Chang KE, Lee TD, Madigan JP, Lloyd JR, et al. (July 2014).
3323:
3244:"Onychomycosis treated with a dilute povidone-iodine/dimethyl sulfoxide preparation"
2807:"Investigation of 3 industry-wide applied storage conditions for compound libraries"
2706:
1547:
4390:
4336:
4296:
4210:
4197:
4175:
3999:
3989:
3925:
2039:
1927:
1854:, which are often frozen in a mixture of 10% DMSO, a freezing medium, and 30%
1828:
1670:
1181:
1149:
1145:
803:
798:
793:
788:
718:
3493:
Lübbert M, Kyereme J, Schöbel N, Beltrán L, Wetzel CH, Hatt H (October 21, 2013).
3433:
3369:
44:
3519:
2912:
2720:
2284:
17:
4369:
4306:
4281:
4271:
4230:
4116:
4034:
4024:
4019:
4004:
3974:
3149:
3140:
1939:
1935:
1835:
1782:
1770:
1658:
1607:
1580:
1409:
Distillation of DMSO requires a partial vacuum to achieve a lower boiling point.
891:
397:
2864:
2770:
2662:
4395:
4346:
4326:
4311:
4240:
4167:
4086:
4029:
4014:
4009:
3747:
2575:
2446:
2414:
2241:
2122:
DMSO exposure to developing mouse brains can produce brain degeneration. This
2061:
1914:
1871:
1705:
1698:
639:
572:
260:
3167:
3144:. Methods in Molecular Biology. Vol. 368. Humana Press. pp. 39–57.
2823:
2806:
2608:
2473:
4360:
4341:
4301:
4291:
4276:
4245:
4205:
4121:
3994:
3934:
2683:
Bordwell FG (1988). "Equilibrium acidities in dimethyl sulfoxide solution".
2641:
2238:
1971:
1931:
1897:
1863:
1799:(FDA) has approved its use only for the symptomatic relief of patients with
1762:
1690:
1477:
1449:
1389:
1385:
1348:
1312:
1246:
1100:
1060:
921:
709:
3808:
3765:
3538:
3451:
3279:
3175:
3092:"Guidelines for PCR Optimization with Phusion High-Fidelity DNA Polymerase"
3077:
2995:
2931:
2872:
2832:
2805:
Ilouga PE, Winkler D, Kirchhoff C, Schierholz B, Wölcke J (November 2007).
2778:
3126:
2519:
1773:. Because DMSO increases the rate of absorption of some compounds through
1291:, DMSO is used as a mild oxidant. It forms the basis of several selective
1115:
Dimethyl sulfoxide was first synthesized in 1866 by the
Russian scientist
4321:
4286:
4266:
4250:
4081:
4044:
3349:
3324:"Dimethyl Sulfoxide in Treatment of Inflammatory Genitourinary Disorders"
3260:
2548:
2151:
2047:
1955:
1635:
1628:
1583:
1572:
1260:
1075:
878:
871:
864:
837:
280:
3719:
https://www.fda.gov/ForIndustry/ImportProgram/ImportAlerts/ucm162294.htm
2698:
2511:
2320:
1311:
is conceptually similar. These all involve formation of an intermediate
289:
4171:
3921:
2163:
1631:
1623:
1437:
1256:
960:
593:
384:
320:
3800:
3032:
2635:
2360:(56th ed.). St. Louis, Missouri: Wolters Kluwer Health. p.
2178:
Dimethyl sulfoxide can produce an explosive reaction when exposed to
1970:
The perceived garlic taste upon skin contact with DMSO may be due to
1362:
1355:
1328:
1189:
1079:
1055:
1052:
1041:
759:
240:
2987:
2754:
2496:
Epstein WW, Sweat FW (March 1967). "Dimethyl
Sulfoxide Oxidations".
983:
Except where otherwise noted, data are given for materials in their
358:
3471:
2167:
2065:
2035:
1959:
1807:
relief to the majority of the 213 patients with inflammatory
1404:
1192:
769:
408:
220:
188:
181:
171:
2451:
Excuse me sir, would you like to buy a kilo of isopropyl bromide?
1912:. Its popularity as an alternative cure is stated to stem from a
4061:
2220:(MSM), a related chemical often marketed as a dietary supplement
2031:
1867:
1859:
1820:
1789:
1778:
1327:
Related to its ability to dissolve many salts, DMSO is a common
1152:
and the oxygen is nucleophilic toward hard electrophiles. With
349:
4136:
3894:
1099:
consistent with other three-coordinate S(IV) compounds, with a
2591:
Lewis basicity and affinity scales : data and measurement
2464:
Roy, Kathrin-Maria (15 June 2000), "Sulfones and
Sulfoxides",
1765:, a vehicle for topical application of pharmaceuticals, as an
1694:
1453:
2949:
2593:. Chichester, West Sussex, U.K.: John Wiley. pp. 50–51.
2150:
can cause odor problems in municipal effluents: waste water
1417:
and is less toxic than other members of this class, such as
450:
66:
56:
2468:, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA,
2134:
mL/kg, a level exceeded in children exposed to DMSO during
1085:
In terms of chemical structure, the molecule has idealized
1788:
systems. Its effect may be enhanced with the addition of
1510:
O resonance in the H-NMR spectrum. It is often mixed with
1223:
The methyl groups of DMSO are only weakly acidic, with a
1082:-like taste in the mouth after DMSO is absorbed by skin.
849:
2107:
using DMSO was halted and did not begin again until the
2034:, substances dissolved in DMSO may quickly be absorbed.
1842:
and is still an important constituent of cryoprotectant
1753:
Use of DMSO in medicine dates from around 1963, when an
1502:
are its high viscosity, which broadens signals, and its
1858:. In the cryogenic freezing of heteroploid cell lines (
1602:
of test compounds (important when working with a large
1590:
programs. This is because it is able to dissolve both
1001:
3885:
3599:"Material Safety Data Sheet: Ethyl alcohol 200 Proof"
1440:
for chemical reactions involving salts, most notably
1347:). In this complex, three DMSO ligands are bonded to
1877:
the DMSO is re-infused along with the patient's own
4378:
4359:
4259:
4229:
4196:
4183:
4053:
3956:
3933:
3682:"Brisbane drug company convicted of counterfeiting"
2182:; at a low temperature, this reaction produces the
2353:
1954:DMSO is commonly used in veterinary medicine as a
1063:most widely used commercially. It is an important
3558:. Gaylord Chemical Company, L.L.C. 21 July 2016.
3322:Shirley SW, Stewart BH, Mirelman S (March 1978).
1990:disulfides (which have odors resembling garlic),
1866:, etc.) a mixture of 10% DMSO with 90%
1337:dichlorotetrakis(dimethyl sulfoxide)ruthenium(II)
1237:Deprotonation of DMSO requires strong bases like
658:0.556 millibars or 0.0556 kPa at 20 °C
3577:"Material Safety Data Sheet: Dimethyl Sulfoxide"
3395:"187 Fake Cancer "Cures" Consumers Should Avoid"
2589:Laurence, Christian; Gal, Jean-François (2010).
1255:. It is a base, e.g., for the deprotonation of
396:
27:Organosulfur chemical compound used as a solvent
2969:
2967:
2846:
2844:
2842:
2800:
2798:
2796:
2748:
2746:
2744:
2742:
2038:selection is important when working with DMSO.
853:
157:
3553:"Safety Data Sheet: Dimethyl Sulfoxide (DMSO)"
2466:Ullmann's Encyclopedia of Industrial Chemistry
2435:(in German). Meyer ; Winter. p. 148.
1831:, the therapeutic occlusion of blood vessels.
1669:, being safer than many of the others such as
1661:(acetaminophen)-induced liver injury in mice.
4148:
3906:
8:
3142:Cryopreservation and Freeze-Drying Protocols
1384:. The donor properties are discussed in the
2019:, oral, rat, 14,500 mg/kg; ethanol: LD
1803:. A 1978 study concluded that DMSO brought
1570:DMSO is used to dissolve test compounds in
30:"DMSO" redirects here. For other uses, see
4193:
4155:
4141:
4133:
3913:
3899:
3891:
3841:Roth, Lutz; Weller, Ursula (August 2000).
3714:
3712:
3248:International Medical Case Reports Journal
465:
310:
268:
36:
3755:
3528:
3518:
3441:
3269:
3259:
2943:
2941:
2921:
2911:
2822:
2237:(also DMS), the corresponding sulfate: a
432:
3363:
3361:
3359:
3242:Capriotti K, Capriotti JA (2015-10-08).
2285:"Dimethyl Sulfoxide (DMSO) -- Technical"
2225:Related compounds with methyl on oxygen
1729:In cell culture, DMSO is used to induce
1528:for lower viscosity and melting points.
3881:International Chemical Safety Card 0459
2267:
1827:liquid embolic agent, which is used in
521:
486:
461:
374:
288:
3565:from the original on 13 February 2019.
3191:
3181:
2721:"Bordwell pKa Table (Acidity in DMSO)"
1755:Oregon Health & Science University
1648:studies, DMSO has some limitations in
622:189 °C (372 °F; 462 K)
301:
3886:Dimethyl Sulfoxide Information Center
3374:National Council Against Health Fraud
2950:"Biological actions of drug solvents"
1436:(HMPA). DMSO is frequently used as a
1097:trigonal pyramidal molecular geometry
896:89 °C (192 °F; 362 K)
493:Key: IAZDPXIOMUYVGZ-UHFFFAOYSA-N
248:
228:
71:Spacefill model of dimethyl sulfoxide
7:
3781:Environmental Science and Technology
2216:Dimethyl sulfone, commonly known as
1279:. It is also a potent nucleophile.
802:
792:
612:19 °C (66 °F; 292 K)
2727:from the original on 9 October 2008
2030:Because DMSO easily penetrates the
2011:DMSO is a non-toxic solvent with a
1598:compounds, can be used to maintain
1369:, metalloporphyrins, and the dimer
1293:sulfonium-based oxidation reactions
503:Key: IAZDPXIOMUYVGZ-UHFFFAOYAR
387:
357:
1506:, which leads to an overwhelming H
1245:. Stabilization of the resultant
25:
2811:Journal of Biomolecular Screening
2258:, also known as the "Toxic Woman"
136:Methyl sulfoxide (2:1), Dermasorb
3843:Gefährliche Chemische Reaktionen
3642:Rubber Chemical Resistance Chart
2080:In Australia, it is listed as a
2023:, oral, rat, 7,060 mg/kg).
1819:, DMSO is used as a solvent for
1546:
1535:
1059:. This colorless liquid is the
991:
556:
82:
43:
3823:US application 2009005601A1
3465:Saling, Joseph (20 June 2022).
3215:. Kluwer Academic. p. 20.
2453:. Pierce Chemical. p. 145.
1757:Medical School team, headed by
987:(at 25 °C , 100 kPa).
490:InChI=1S/C2H6OS/c1-4(2)3/h1-2H3
3368:Jarvis WT (24 November 2001).
2948:Kelava T, Cavar I (Nov 2011).
2564:Coordination Chemistry Reviews
1490:), it is a useful solvent for
1335:. Illustrative is the complex
1127:, by oxidation with oxygen or
976:Dimethyl sulfoxide (data page)
943:Sodium methylsulfinylmethylide
819:Occupational safety and health
565:
562:
550:
500:InChI=1/C2H6OS/c1-4(2)3/h1-2H3
89:A sample of dimethyl sulfoxide
1:
3605:. 21 May 2013. Archived from
3583:. 21 May 2013. Archived from
3434:10.1158/0008-5472.CAN-14-0247
3070:10.1016/S0378-1119(01)00621-7
2686:Accounts of Chemical Research
2624:Journal of Chemical Education
2015:higher than ethanol (DMSO: LD
1735:P19 embryonic carcinoma cells
1621:DMSO is used as a vehicle in
1556:DMSO is used as a solvent in
1144:The sulfur center in DMSO is
3924:, including antispasmodics (
3847:Dangerous Chemical Reactions
3520:10.1371/journal.pone.0077998
3342:10.1016/0090-4295(78)90118-8
3212:The century of space science
3119:10.1016/0014-4827(90)90108-m
2913:10.1371/journal.pone.0028835
2203:Varying oxidation of sulfur
2109:National Academy of Sciences
1797:Food and Drug Administration
1319:SX where X is a heteroatom)
1140:Reactions with electrophiles
3150:10.1007/978-1-59745-362-2_3
2853:Current Medicinal Chemistry
2250:, another methylating agent
2231:, the corresponding sulfite
1718:DMSO may also be used as a
1158:trimethylsulfoxonium iodide
52:
4448:
2865:10.2174/092986706775197917
2771:10.1007/s11095-020-02849-1
2356:Drug Facts and Comparisons
1919:consequently convicted of
1446:nucleophilic substitutions
1297:Pfitzner–Moffatt oxidation
29:
3748:10.1016/j.nbd.2008.11.006
2576:10.1016/j.ccr.2004.02.005
2415:10.1107/S0365110X66002263
2158:(anoxic) conditions into
1687:polymerase chain reaction
1588:high-throughput screening
1469:chemical processes (e.g.
981:
974:
969:
913:
816:
811:
775:
725:
537:
512:
477:
141:
131:
117:(Methanesulfinyl)methane
111:
99:
94:
81:
51:
42:
2824:10.1177/1087057106295507
2474:10.1002/14356007.a25_487
2339:pubchem.ncbi.nlm.nih.gov
2287:. Atofina Chemicals, inc
2068:gloves are recommended.
1879:hematopoietic stem cells
1852:hematopoietic stem cells
1838:DMSO has been used as a
1817:interventional radiology
1452:. A set of non-aqueous
1239:lithium diisopropylamide
1111:Synthesis and production
970:Supplementary data page
105:(Methanesulfinyl)methane
4427:Foul-smelling chemicals
3736:Neurobiology of Disease
3702:The Wall Street Journal
3654:"Chemical hygiene plan"
3625:American Cancer Society
3209:Johannes Geiss (2001).
2976:Can J Physiol Pharmacol
2759:Pharmaceutical Research
2248:Methyl methanesulfonate
2099:The Wall Street Journal
1908:DMSO is marketed as an
1884:DMSO is metabolized by
1434:hexamethylphosphoramide
1101:nonbonded electron pair
4072:Salicylhydroxamic acid
3467:"DMSO: Uses and Risks"
3298:www.accessdata.fda.gov
2352:Novak KM, ed. (2002).
2136:bone marrow transplant
2117:traumatic brain injury
2096:On September 9, 1965,
1875:bone marrow transplant
1821:ethylene vinyl alcohol
1644:. As with its use in
1641:Caenorhabditis elegans
1410:
1333:coordination chemistry
1123:, a by-product of the
860:
122:Dimethyl(oxido)sulfur
72:
62:
3609:on 19 September 2018.
3587:on 19 September 2018.
2954:Periodicum Biologorum
2432:Annalen der Pharmacie
2218:methylsulfonylmethane
2154:transform DMSO under
2126:could be detected at
2113:interstitial cystitis
1801:interstitial cystitis
1781:, it is used in some
1743:skeletal muscle cells
1442:Finkelstein reactions
1430:-methyl-2-pyrrolidone
1415:polar aprotic solvent
1408:
1367:trimethyltin chloride
1323:Ligand and Lewis base
1103:on the approximately
1034:organosulfur compound
859:
134:Methylsulfinylmethane
113:Systematic IUPAC name
70:
60:
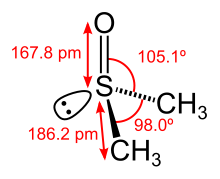
32:DMSO (disambiguation)
4102:Pentosan polysulfate
4067:Acetohydroxamic acid
3294:"Import Alert 62-06"
3261:10.2147/IMCRJ.S90775
2549:10.1055/s-1990-27036
2335:"Dimethyl sulfoxide"
1910:alternative medicine
1904:Alternative medicine
1848:embryonic stem cells
1691:secondary structures
1586:programs, including
1070:that dissolves both
842:(fire diamond)
101:Preferred IUPAC name
4097:Magnesium hydroxide
3793:2006EnST...40..202G
3511:2013PLoSO...877998L
3025:2007ECSTr..11b.227K
2904:2011PLoSO...628835C
2699:10.1021/ar00156a004
2512:10.1021/cr60247a001
2447:Gergel, Max G.
2407:1966AcCry..21...12T
2321:10.1021/ja00857a010
2146:DMSO disposed into
1950:Veterinary medicine
1301:Corey–Kim oxidation
753:Trigonal pyramidal
629:Solubility in water
580: g·mol
210:Beilstein Reference
39:
38:Dimethyl sulfoxide
4386:Dimethyl sulfoxide
4092:Dimethyl sulfoxide
3661:Cornell University
2765:(7): Article 125.
2013:median lethal dose
1980:trigeminal ganglia
1886:disproportionation
1856:fetal bovine serum
1775:biological tissues
1629:flavonol glycoside
1612:cell culture media
1462:rotary evaporation
1411:
1309:Kornblum oxidation
1072:polar and nonpolar
1026:Dimethyl sulfoxide
1014:Infobox references
914:Related compounds
861:
588:Colourless liquid
376:Dimethyl+sulfoxide
73:
63:
37:
4404:
4403:
4355:
4354:
4317:Meclofenamic acid
4185:Anti-inflammatory
4130:
4129:
4112:Phenyl salicylate
4054:Other urologicals
4040:Trospium chloride
3943:Ammonium chloride
3801:10.1021/es051312a
3428:(14): 3913–3922.
3222:978-0-7923-7195-3
3159:978-1-58829-377-0
3033:10.1149/1.2779383
2636:10.1021/ed054p612
2600:978-0-470-74957-9
2371:978-1-57439-110-7
2315:(24): 7006–7014.
2274:DMSO (medication)
2105:Clinical research
1930:drugs, including
1767:anti-inflammatory
1689:(PCR) to inhibit
1423:dimethylacetamide
1419:dimethylformamide
1289:organic synthesis
1265:phosphonium salts
1180:This salt can be
1117:Alexander Zaytsev
1074:compounds and is
1022:Chemical compound
1020:
1019:
935:Related compounds
928:Diethyl sulfoxide
902:Safety data sheet
446:CompTox Dashboard
190:Interactive image
183:Interactive image
125:
120:
77:
76:
18:Dimethylsulfoxide
16:(Redirected from
4439:
4432:Methyl compounds
4194:
4157:
4150:
4143:
4134:
3948:Calcium chloride
3915:
3908:
3901:
3892:
3869:
3860:
3838:
3832:
3831:
3830:
3826:
3819:
3813:
3812:
3776:
3770:
3769:
3759:
3727:
3721:
3716:
3707:
3706:
3705:. New York City.
3696:
3690:
3689:
3678:
3672:
3671:
3669:
3668:
3658:
3650:
3644:
3639:
3633:
3632:
3631:on 27 July 2010.
3627:. Archived from
3617:
3611:
3610:
3595:
3589:
3588:
3573:
3567:
3566:
3564:
3557:
3549:
3543:
3542:
3532:
3522:
3490:
3484:
3483:
3481:
3479:
3462:
3456:
3455:
3445:
3413:
3407:
3406:
3405:on 23 July 2017.
3401:. Archived from
3391:
3385:
3384:
3382:
3380:
3365:
3354:
3353:
3319:
3313:
3312:
3310:
3309:
3300:. Archived from
3290:
3284:
3283:
3273:
3263:
3239:
3233:
3232:
3230:
3229:
3206:
3200:
3199:
3193:
3189:
3187:
3179:
3137:
3131:
3130:
3102:
3096:
3095:
3088:
3082:
3081:
3064:(1–2): 293–298.
3051:
3045:
3044:
3012:ECS Transactions
3006:
3000:
2999:
2971:
2962:
2961:
2945:
2936:
2935:
2925:
2915:
2883:
2877:
2876:
2848:
2837:
2836:
2826:
2802:
2791:
2790:
2750:
2737:
2736:
2734:
2732:
2717:
2711:
2710:
2680:
2674:
2673:
2671:
2670:
2661:. Archived from
2659:exactantigen.com
2651:
2645:
2639:
2619:
2613:
2612:
2586:
2580:
2579:
2570:(3–4): 351–375.
2559:
2553:
2552:
2530:
2524:
2523:
2499:Chemical Reviews
2493:
2487:
2486:
2461:
2455:
2454:
2443:
2437:
2436:
2425:
2419:
2418:
2395:Acta Crystallogr
2382:
2376:
2375:
2359:
2349:
2343:
2342:
2331:
2325:
2324:
2309:J. Am. Chem. Soc
2303:
2297:
2296:
2294:
2292:
2281:
2275:
2272:
2235:Dimethyl sulfate
2229:Dimethyl sulfite
2207:Dimethyl sulfide
2174:Explosion hazard
2160:dimethyl sulfide
2133:
2060:
2053:
1944:usual disclaimer
1894:dimethyl sulfone
1890:dimethyl sulfide
1685:DMSO is used in
1604:chemical library
1550:
1539:
1233:
1211:)O + NaI + H
1199:I + NaH → (CH
1129:nitrogen dioxide
1121:dimethyl sulfide
1058:
1004:
998:
995:
994:
955:Dimethyl sulfone
949:Dimethyl sulfide
881:
874:
867:
852:
806:
796:
768:
717:
683:Refractive index
601:
579:
567:
564:
558:
552:
545:Chemical formula
470:
469:
454:
452:
436:
400:
389:
378:
361:
339:Gmelin Reference
322:
314:
303:
292:
272:
252:
232:
192:
185:
161:
123:
118:
86:
53:
47:
40:
21:
4447:
4446:
4442:
4441:
4440:
4438:
4437:
4436:
4407:
4406:
4405:
4400:
4374:
4351:
4255:
4233:
4225:
4216:Oxyphenbutazone
4188:
4186:
4179:
4161:
4131:
4126:
4107:Phenazopyridine
4049:
3980:Desfesoterodine
3966:antimuscarinics
3963:
3952:
3929:
3919:
3877:
3872:
3857:
3840:
3839:
3835:
3828:
3821:
3820:
3816:
3778:
3777:
3773:
3729:
3728:
3724:
3717:
3710:
3698:
3697:
3693:
3680:
3679:
3675:
3666:
3664:
3656:
3652:
3651:
3647:
3640:
3636:
3619:
3618:
3614:
3597:
3596:
3592:
3575:
3574:
3570:
3562:
3555:
3551:
3550:
3546:
3492:
3491:
3487:
3477:
3475:
3464:
3463:
3459:
3422:Cancer Research
3415:
3414:
3410:
3393:
3392:
3388:
3378:
3376:
3367:
3366:
3357:
3321:
3320:
3316:
3307:
3305:
3292:
3291:
3287:
3241:
3240:
3236:
3227:
3225:
3223:
3208:
3207:
3203:
3190:
3180:
3160:
3139:
3138:
3134:
3104:
3103:
3099:
3090:
3089:
3085:
3053:
3052:
3048:
3008:
3007:
3003:
2988:10.1139/Y10-065
2973:
2972:
2965:
2947:
2946:
2939:
2885:
2884:
2880:
2850:
2849:
2840:
2804:
2803:
2794:
2752:
2751:
2740:
2730:
2728:
2719:
2718:
2714:
2693:(12): 456–463.
2682:
2681:
2677:
2668:
2666:
2653:
2652:
2648:
2621:
2620:
2616:
2601:
2588:
2587:
2583:
2561:
2560:
2556:
2543:(10): 857–870.
2532:
2531:
2527:
2495:
2494:
2490:
2484:
2463:
2462:
2458:
2445:
2444:
2440:
2427:
2426:
2422:
2392:
2388:
2384:
2383:
2379:
2372:
2351:
2350:
2346:
2333:
2332:
2328:
2305:
2304:
2300:
2290:
2288:
2283:
2282:
2278:
2273:
2269:
2265:
2212:
2200:
2188:Swern oxidation
2176:
2144:
2131:
2090:
2088:Clinical safety
2082:Schedule 4 (S4)
2078:
2058:
2051:
2044:fluoroelastomer
2022:
2018:
2009:
2004:
1968:
1952:
1906:
1751:
1731:differentiation
1724:liquid nitrogen
1710:thermostability
1683:
1675:dichloromethane
1610:with water and
1600:stock solutions
1568:
1567:
1566:
1565:
1553:
1552:
1551:
1542:
1541:
1540:
1526:
1522:
1515:
1509:
1501:
1488:
1473:with cooling).
1471:crystallization
1466:cryodesiccation
1403:
1398:
1382:
1378:
1374:
1359:
1346:
1342:
1325:
1318:
1305:Swern oxidation
1285:
1277:diaminocarbenes
1269:Wittig reagents
1259:to form sodium
1231:
1224:
1221:
1214:
1210:
1206:
1202:
1175:
1171:
1167:
1142:
1137:
1113:
1090:
1068:aprotic solvent
1051:
1050:
1045:
1023:
1016:
1011:
1010:
1009: ?)
1000:
996:
992:
988:
965:
936:
924:
886:
885:
884:
883:
876:
869:
862:
858:
850:
829:
785:
766:
762:
750:
748:Molecular shape
741:
735:
715:
702:
697:
693:
691:
672:
631:
599:
577:
561:
555:
547:
533:
530:
525:
520:
519:
508:
505:
504:
501:
495:
494:
491:
485:
484:
473:
455:
448:
439:
419:
403:
390:
364:
341:
332:
295:
275:
255:
235:
212:
195:
175:
164:
151:
137:
135:
127:
126:
121:
107:
106:
90:
87:
35:
28:
23:
22:
15:
12:
11:
5:
4445:
4443:
4435:
4434:
4429:
4424:
4419:
4409:
4408:
4402:
4401:
4399:
4398:
4393:
4388:
4382:
4380:
4376:
4375:
4373:
4372:
4366:
4364:
4357:
4356:
4353:
4352:
4350:
4349:
4344:
4339:
4334:
4329:
4324:
4319:
4314:
4309:
4304:
4299:
4294:
4289:
4284:
4279:
4274:
4269:
4263:
4261:
4257:
4256:
4254:
4253:
4248:
4243:
4237:
4235:
4227:
4226:
4224:
4223:
4221:Phenylbutazone
4218:
4213:
4208:
4202:
4200:
4191:
4181:
4180:
4162:
4160:
4159:
4152:
4145:
4137:
4128:
4127:
4125:
4124:
4119:
4114:
4109:
4104:
4099:
4094:
4089:
4084:
4075:
4074:
4069:
4057:
4055:
4051:
4050:
4048:
4047:
4042:
4037:
4032:
4027:
4022:
4017:
4012:
4007:
4002:
3997:
3992:
3987:
3982:
3977:
3971:
3969:
3961:antispasmodics
3954:
3953:
3951:
3950:
3945:
3939:
3937:
3931:
3930:
3920:
3918:
3917:
3910:
3903:
3895:
3889:
3888:
3883:
3876:
3875:External links
3873:
3871:
3870:
3855:
3833:
3814:
3787:(1): 202–207.
3771:
3722:
3708:
3691:
3688:on 2012-03-21.
3673:
3663:. October 1999
3645:
3634:
3612:
3603:ScienceLab.com
3590:
3581:ScienceLab.com
3568:
3544:
3505:(10): e77998.
3485:
3457:
3408:
3386:
3355:
3336:(3): 215–220.
3314:
3285:
3234:
3221:
3201:
3192:|journal=
3158:
3132:
3097:
3083:
3046:
3019:(2): 227–234.
3001:
2982:(10): 980–87.
2963:
2937:
2898:(12): e28835.
2878:
2859:(2): 223–241.
2838:
2792:
2738:
2712:
2675:
2646:
2614:
2599:
2581:
2554:
2525:
2506:(3): 247–260.
2488:
2482:
2456:
2449:(March 1977).
2438:
2420:
2390:
2386:
2377:
2370:
2344:
2326:
2298:
2276:
2266:
2264:
2261:
2260:
2259:
2256:Gloria Ramirez
2253:
2252:
2251:
2245:
2232:
2223:
2222:
2221:
2214:
2210:
2199:
2196:
2180:acyl chlorides
2175:
2172:
2143:
2140:
2089:
2086:
2077:
2074:
2050:, or thick (15
2020:
2016:
2008:
2005:
2003:
2000:
1974:activation of
1967:
1964:
1951:
1948:
1921:Medicare fraud
1905:
1902:
1840:cryoprotectant
1750:
1747:
1739:cardiomyocytes
1720:cryoprotectant
1682:
1679:
1667:paint stripper
1606:), is readily
1577:drug discovery
1555:
1554:
1545:
1544:
1543:
1534:
1533:
1532:
1531:
1530:
1524:
1520:
1513:
1507:
1504:hygroscopicity
1499:
1486:
1402:
1399:
1397:
1394:
1380:
1376:
1372:
1357:
1344:
1340:
1324:
1321:
1316:
1295:including the
1284:
1281:
1275:salts to form
1243:sodium hydride
1229:
1220:
1217:
1216:
1215:
1212:
1208:
1204:
1200:
1186:sodium hydride
1178:
1177:
1173:
1169:
1165:
1141:
1138:
1136:
1133:
1112:
1109:
1088:
1048:
1043:
1021:
1018:
1017:
1012:
990:
989:
985:standard state
982:
979:
978:
972:
971:
967:
966:
964:
963:
958:
952:
946:
939:
937:
934:
931:
930:
925:
919:
916:
915:
911:
910:
905:
898:
897:
894:
888:
887:
877:
870:
863:
848:
847:
846:
845:
843:
834:
833:
830:
827:
824:
823:
814:
813:
809:
808:
786:
781:
778:
777:
773:
772:
763:
758:
755:
754:
751:
746:
743:
742:
739:
736:
731:
728:
727:
723:
722:
721:at 20 °C
712:
706:
705:
700:
694:
689:
681:
678:
677:
674:
670:
660:
659:
656:
654:Vapor pressure
650:
649:
646:
636:
635:
632:
627:
624:
623:
620:
614:
613:
610:
604:
603:
596:
590:
589:
586:
582:
581:
575:
569:
568:
559:
553:
548:
543:
540:
539:
535:
534:
532:
531:
528:
526:
523:
515:
514:
513:
510:
509:
507:
506:
502:
499:
498:
496:
492:
489:
488:
480:
479:
478:
475:
474:
472:
471:
458:
456:
444:
441:
440:
438:
437:
429:
427:
421:
420:
418:
417:
413:
411:
405:
404:
402:
401:
393:
391:
383:
380:
379:
372:
366:
365:
363:
362:
354:
352:
346:
345:
342:
337:
334:
333:
331:
330:
326:
324:
316:
315:
305:
297:
296:
294:
293:
285:
283:
277:
276:
274:
273:
265:
263:
257:
256:
254:
253:
245:
243:
237:
236:
234:
233:
225:
223:
217:
216:
213:
208:
205:
204:
201:
200:Abbreviations
197:
196:
194:
193:
186:
178:
176:
169:
166:
165:
163:
162:
154:
152:
147:
144:
143:
139:
138:
133:
129:
128:
119:(substitutive)
116:
115:
109:
108:
104:
103:
97:
96:
92:
91:
88:
79:
78:
75:
74:
64:
49:
48:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
4444:
4433:
4430:
4428:
4425:
4423:
4420:
4418:
4415:
4414:
4412:
4397:
4394:
4392:
4389:
4387:
4384:
4383:
4381:
4377:
4371:
4368:
4367:
4365:
4362:
4358:
4348:
4345:
4343:
4340:
4338:
4335:
4333:
4332:Niflumic acid
4330:
4328:
4325:
4323:
4320:
4318:
4315:
4313:
4310:
4308:
4305:
4303:
4300:
4298:
4295:
4293:
4290:
4288:
4285:
4283:
4280:
4278:
4275:
4273:
4270:
4268:
4265:
4264:
4262:
4258:
4252:
4249:
4247:
4244:
4242:
4239:
4238:
4236:
4232:
4228:
4222:
4219:
4217:
4214:
4212:
4209:
4207:
4204:
4203:
4201:
4199:
4198:Pyrazolidines
4195:
4192:
4190:
4187:preparations,
4182:
4177:
4173:
4172:muscular pain
4169:
4166:products for
4165:
4158:
4153:
4151:
4146:
4144:
4139:
4138:
4135:
4123:
4120:
4118:
4115:
4113:
4110:
4108:
4105:
4103:
4100:
4098:
4095:
4093:
4090:
4088:
4085:
4083:
4080:
4077:
4076:
4073:
4070:
4068:
4065:
4063:
4059:
4058:
4056:
4052:
4046:
4043:
4041:
4038:
4036:
4033:
4031:
4028:
4026:
4023:
4021:
4018:
4016:
4013:
4011:
4008:
4006:
4003:
4001:
3998:
3996:
3993:
3991:
3988:
3986:
3983:
3981:
3978:
3976:
3973:
3972:
3970:
3967:
3962:
3959:
3955:
3949:
3946:
3944:
3941:
3940:
3938:
3936:
3932:
3927:
3923:
3916:
3911:
3909:
3904:
3902:
3897:
3896:
3893:
3887:
3884:
3882:
3879:
3878:
3874:
3868:
3867:3-609-48040-8
3864:
3858:
3856:3-609-73090-0
3852:
3848:
3844:
3837:
3834:
3824:
3818:
3815:
3810:
3806:
3802:
3798:
3794:
3790:
3786:
3782:
3775:
3772:
3767:
3763:
3758:
3753:
3749:
3745:
3741:
3737:
3733:
3726:
3723:
3720:
3715:
3713:
3709:
3704:
3703:
3695:
3692:
3687:
3683:
3677:
3674:
3662:
3655:
3649:
3646:
3643:
3638:
3635:
3630:
3626:
3622:
3616:
3613:
3608:
3604:
3600:
3594:
3591:
3586:
3582:
3578:
3572:
3569:
3561:
3554:
3548:
3545:
3540:
3536:
3531:
3526:
3521:
3516:
3512:
3508:
3504:
3500:
3496:
3489:
3486:
3474:
3473:
3468:
3461:
3458:
3453:
3449:
3444:
3439:
3435:
3431:
3427:
3423:
3419:
3412:
3409:
3404:
3400:
3396:
3390:
3387:
3375:
3371:
3364:
3362:
3360:
3356:
3351:
3347:
3343:
3339:
3335:
3331:
3330:
3325:
3318:
3315:
3304:on 2017-02-04
3303:
3299:
3295:
3289:
3286:
3281:
3277:
3272:
3267:
3262:
3257:
3253:
3249:
3245:
3238:
3235:
3224:
3218:
3214:
3213:
3205:
3202:
3197:
3185:
3177:
3173:
3169:
3165:
3161:
3155:
3151:
3147:
3143:
3136:
3133:
3128:
3124:
3120:
3116:
3112:
3108:
3107:Exp. Cell Res
3101:
3098:
3093:
3087:
3084:
3079:
3075:
3071:
3067:
3063:
3059:
3058:
3050:
3047:
3042:
3038:
3034:
3030:
3026:
3022:
3018:
3014:
3013:
3005:
3002:
2997:
2993:
2989:
2985:
2981:
2977:
2970:
2968:
2964:
2960:(3): 311–320.
2959:
2955:
2951:
2944:
2942:
2938:
2933:
2929:
2924:
2919:
2914:
2909:
2905:
2901:
2897:
2893:
2889:
2882:
2879:
2874:
2870:
2866:
2862:
2858:
2854:
2847:
2845:
2843:
2839:
2834:
2830:
2825:
2820:
2816:
2812:
2808:
2801:
2799:
2797:
2793:
2788:
2784:
2780:
2776:
2772:
2768:
2764:
2760:
2756:
2749:
2747:
2745:
2743:
2739:
2726:
2722:
2716:
2713:
2708:
2704:
2700:
2696:
2692:
2688:
2687:
2679:
2676:
2665:on 2009-10-05
2664:
2660:
2656:
2650:
2647:
2643:
2637:
2633:
2629:
2625:
2618:
2615:
2610:
2606:
2602:
2596:
2592:
2585:
2582:
2577:
2573:
2569:
2565:
2558:
2555:
2550:
2546:
2542:
2538:
2537:
2529:
2526:
2521:
2517:
2513:
2509:
2505:
2501:
2500:
2492:
2489:
2485:
2479:
2475:
2471:
2467:
2460:
2457:
2452:
2448:
2442:
2439:
2434:
2433:
2424:
2421:
2416:
2412:
2408:
2404:
2400:
2396:
2381:
2378:
2373:
2367:
2363:
2358:
2357:
2348:
2345:
2340:
2336:
2330:
2327:
2322:
2318:
2314:
2310:
2302:
2299:
2286:
2280:
2277:
2271:
2268:
2262:
2257:
2254:
2249:
2246:
2243:
2240:
2236:
2233:
2230:
2227:
2226:
2224:
2219:
2215:
2208:
2205:
2204:
2202:
2201:
2197:
2195:
2191:
2189:
2185:
2181:
2173:
2171:
2169:
2166:) and C-S-H (
2165:
2161:
2157:
2153:
2149:
2141:
2139:
2137:
2130:as low as 0.3
2129:
2125:
2124:neurotoxicity
2120:
2118:
2114:
2110:
2106:
2101:
2100:
2094:
2087:
2085:
2083:
2075:
2073:
2071:
2067:
2063:
2056:
2049:
2045:
2041:
2037:
2033:
2028:
2024:
2014:
2006:
2001:
1999:
1997:
1993:
1989:
1985:
1981:
1978:receptors in
1977:
1973:
1965:
1963:
1961:
1957:
1949:
1947:
1945:
1941:
1937:
1933:
1929:
1924:
1922:
1917:
1916:
1911:
1903:
1901:
1899:
1895:
1891:
1887:
1882:
1880:
1876:
1873:
1869:
1865:
1861:
1857:
1853:
1849:
1845:
1844:vitrification
1841:
1837:
1832:
1830:
1826:
1822:
1818:
1813:
1810:
1809:genitourinary
1806:
1802:
1798:
1793:
1791:
1787:
1786:drug delivery
1784:
1780:
1776:
1772:
1768:
1764:
1760:
1759:Stanley Jacob
1756:
1748:
1746:
1744:
1740:
1736:
1732:
1727:
1725:
1721:
1716:
1713:
1711:
1708:(to decrease
1707:
1702:
1700:
1696:
1692:
1688:
1680:
1678:
1676:
1672:
1668:
1662:
1660:
1655:
1651:
1650:animal models
1647:
1643:
1642:
1637:
1633:
1630:
1626:
1625:
1619:
1617:
1613:
1609:
1605:
1601:
1597:
1593:
1589:
1585:
1582:
1578:
1575:
1574:
1564:drug testing.
1563:
1559:
1549:
1538:
1529:
1527:
1516:
1505:
1498:
1493:
1489:
1485:
1479:
1474:
1472:
1467:
1463:
1457:
1455:
1451:
1447:
1443:
1439:
1435:
1431:
1429:
1424:
1420:
1416:
1407:
1400:
1395:
1393:
1391:
1387:
1383:
1368:
1364:
1360:
1352:
1350:
1338:
1334:
1330:
1322:
1320:
1314:
1310:
1306:
1302:
1298:
1294:
1290:
1282:
1280:
1278:
1274:
1273:formamidinium
1270:
1266:
1262:
1258:
1254:
1253:
1252:dimsyl sodium
1248:
1244:
1240:
1235:
1228:
1218:
1198:
1197:
1196:
1194:
1191:
1187:
1183:
1163:
1162:
1161:
1159:
1155:
1154:methyl iodide
1151:
1150:electrophiles
1147:
1139:
1134:
1132:
1130:
1126:
1125:Kraft process
1122:
1118:
1110:
1108:
1107:sulfur atom.
1106:
1102:
1098:
1094:
1091:
1083:
1081:
1077:
1073:
1069:
1066:
1062:
1057:
1054:
1046:
1039:
1035:
1031:
1027:
1015:
1008:
1003:
986:
980:
977:
973:
968:
962:
959:
956:
953:
950:
947:
944:
941:
940:
938:
933:
932:
929:
926:
923:
918:
917:
912:
909:
906:
903:
900:
899:
895:
893:
890:
889:
882:
875:
868:
844:
841:
840:
836:
835:
831:
826:
825:
821:
820:
815:
810:
805:
800:
795:
790:
787:
784:
780:
779:
776:Pharmacology
774:
771:
764:
761:
760:Dipole moment
757:
756:
752:
749:
745:
744:
737:
734:
730:
729:
724:
720:
713:
711:
708:
707:
703:
695:
688:
684:
680:
679:
675:
669:
665:
662:
661:
657:
655:
652:
651:
647:
645:
644:Diethyl ether
641:
638:
637:
633:
630:
626:
625:
621:
619:
618:Boiling point
616:
615:
611:
609:
608:Melting point
606:
605:
597:
595:
592:
591:
587:
584:
583:
576:
574:
571:
570:
549:
546:
542:
541:
536:
527:
522:
518:
511:
497:
487:
483:
476:
468:
464:
463:DTXSID2021735
460:
459:
457:
447:
443:
442:
435:
431:
430:
428:
426:
423:
422:
415:
414:
412:
410:
407:
406:
399:
395:
394:
392:
386:
382:
381:
377:
373:
371:
368:
367:
360:
356:
355:
353:
351:
348:
347:
343:
340:
336:
335:
328:
327:
325:
323:
318:
317:
313:
309:
306:
304:
302:ECHA InfoCard
299:
298:
291:
287:
286:
284:
282:
279:
278:
271:
267:
266:
264:
262:
259:
258:
251:
247:
246:
244:
242:
239:
238:
231:
227:
226:
224:
222:
219:
218:
214:
211:
207:
206:
202:
199:
198:
191:
187:
184:
180:
179:
177:
173:
168:
167:
160:
156:
155:
153:
150:
146:
145:
140:
130:
114:
110:
102:
98:
93:
85:
80:
69:
65:
59:
55:
54:
50:
46:
41:
33:
19:
4391:Idrocilamide
4385:
4337:Piketoprofen
4297:Flurbiprofen
4211:Mofebutazone
4189:non-steroids
4091:
4078:
4060:
4000:Imidafenacin
3990:Fesoterodine
3846:
3842:
3836:
3817:
3784:
3780:
3774:
3739:
3735:
3725:
3700:
3694:
3686:the original
3676:
3665:. Retrieved
3648:
3637:
3629:the original
3615:
3607:the original
3602:
3593:
3585:the original
3580:
3571:
3547:
3502:
3498:
3488:
3476:. Retrieved
3470:
3460:
3425:
3421:
3411:
3403:the original
3398:
3389:
3377:. Retrieved
3333:
3327:
3317:
3306:. Retrieved
3302:the original
3297:
3288:
3251:
3247:
3237:
3226:. Retrieved
3211:
3204:
3141:
3135:
3110:
3106:
3100:
3086:
3061:
3055:
3049:
3016:
3010:
3004:
2979:
2975:
2957:
2953:
2895:
2891:
2881:
2856:
2852:
2817:(1): 21–32.
2814:
2810:
2762:
2758:
2729:. Retrieved
2715:
2690:
2684:
2678:
2667:. Retrieved
2663:the original
2658:
2649:
2627:
2623:
2617:
2590:
2584:
2567:
2563:
2557:
2540:
2534:
2528:
2503:
2497:
2491:
2465:
2459:
2450:
2441:
2431:
2423:
2401:(1): 12–20.
2398:
2394:
2380:
2355:
2347:
2338:
2329:
2312:
2308:
2301:
2289:. Retrieved
2279:
2270:
2192:
2177:
2145:
2142:Odor problem
2121:
2097:
2095:
2091:
2079:
2040:Butyl rubber
2029:
2025:
2010:
1972:nonolfactory
1969:
1953:
1928:chemotherapy
1925:
1913:
1907:
1883:
1833:
1829:embolization
1814:
1794:
1777:, including
1752:
1728:
1717:
1714:
1703:
1695:DNA template
1684:
1671:nitromethane
1663:
1645:
1639:
1622:
1620:
1571:
1569:
1561:
1557:
1496:
1483:
1475:
1458:
1427:
1412:
1396:Applications
1353:
1326:
1286:
1250:
1236:
1226:
1222:
1188:to form the
1182:deprotonated
1179:
1148:toward soft
1146:nucleophilic
1143:
1114:
1084:
1029:
1025:
1024:
838:
828:Main hazards
817:
686:
667:
648:Not soluble
409:RTECS number
203:DMSO, Me2SO
142:Identifiers
132:Other names
4370:Zucapsaicin
4363:derivatives
4307:Indometacin
4282:Etofenamate
4272:Benzydamine
4234:derivatives
4231:Acetic acid
4117:Succinimide
4035:Tolterodine
4025:Solifenacin
4020:Propiverine
4005:Meladrazine
3975:Darifenacin
3964:(primarily
3922:Urologicals
3742:(1): 1–10.
3254:: 231–233.
3113:(1): 4–10.
2630:: 612–613.
2213:and sulfate
1940:oxaliplatin
1936:carboplatin
1836:cryobiology
1805:significant
1783:transdermal
1771:antioxidant
1699:DNA primers
1659:paracetamol
1654:Pleiotropic
1581:drug design
1105:tetrahedral
1095:. It has a
908:Oxford MSDS
892:Flash point
822:(OHS/OSH):
733:Point group
585:Appearance
538:Properties
308:100.000.604
230:CHEBI:28262
4422:Sulfoxides
4411:Categories
4396:Tolazoline
4347:Suxibuzone
4327:Nifenazone
4312:Ketoprofen
4241:Diclofenac
4087:Dapoxetine
4064:analogues:
4030:Terodiline
4015:Oxybutynin
4010:Mirabegron
3985:Emepronium
3935:Acidifiers
3667:2010-04-12
3308:2017-03-05
3228:2011-08-07
2669:2009-10-02
2483:3527306730
2263:References
2242:alkylating
2076:Regulation
1982:. Unlike
1915:60 Minutes
1872:autologous
1706:GC-content
1478:deuterated
1450:carbanions
1444:and other
1413:DMSO is a
1315:species (R
922:sulfoxides
726:Structure
640:Solubility
573:Molar mass
434:YOW8V9698H
261:ChemSpider
170:3D model (
149:CAS Number
124:(additive)
4361:Capsaicin
4342:Piroxicam
4302:Ibuprofen
4292:Feprazone
4277:Bufexamac
4246:Fentiazac
4206:Clofezone
4122:Tiopronin
3995:Flavoxate
3194:ignored (
3184:cite book
3168:1064-3745
3041:137979405
2787:219590658
2642:ECW model
2609:428031803
2536:Synthesis
2239:mutagenic
2168:mercaptan
1932:cisplatin
1900:symptom.
1898:halitosis
1769:, and an
1763:analgesic
1616:cell line
1584:screening
1390:C-B plots
1386:ECW model
1349:ruthenium
1313:sulfonium
1247:carbanion
1172:SO + CH
1156:it forms
1135:Reactions
1061:sulfoxide
1036:with the
832:Irritant
710:Viscosity
634:Miscible
416:PV6210000
329:200-664-3
321:EC Number
250:ChEMBL504
4417:Solvents
4322:Naproxen
4287:Felbinac
4267:Bendazac
4251:Tolmetin
4082:Collagen
4045:Vibegron
3861:CD-ROM:
3809:16433352
3766:19100327
3560:Archived
3539:24205061
3499:PLOS ONE
3452:24812268
3280:26491374
3176:18080461
3078:11675022
2996:20962895
2932:22216122
2892:PLOS ONE
2873:16472214
2833:17099243
2779:32529587
2731:23 April
2725:Archived
2707:26624076
2244:compound
2198:See also
2152:bacteria
2048:neoprene
2007:Toxicity
1984:dimethyl
1956:liniment
1749:Medicine
1726:safely.
1646:in vitro
1636:nematode
1608:miscible
1596:nonpolar
1573:in vitro
1558:in vitro
1303:and the
1267:to form
1261:enolates
1093:symmetry
1076:miscible
1032:) is an
920:Related
839:NFPA 704
812:Hazards
783:ATC code
281:DrugBank
4164:Topical
3958:Urinary
3789:Bibcode
3757:2682536
3530:3804614
3507:Bibcode
3478:19 July
3443:4153432
3379:19 July
3329:Urology
3271:4599634
3127:2298260
3021:Bibcode
2923:3244416
2900:Bibcode
2520:6042131
2403:Bibcode
2184:oxidant
2164:sulfide
2156:hypoxic
2070:Nitrile
1988:diallyl
1823:in the
1697:or the
1693:in the
1681:Biology
1634:in the
1632:Icariin
1624:in vivo
1562:in vivo
1476:In its
1438:solvent
1401:Solvent
1363:phenols
1283:Oxidant
1257:ketones
1219:Acidity
1176:I → I
1038:formula
1007:what is
1005: (
961:Acetone
801: (
799:M02AX03
797:)
791: (
789:G04BX13
664:Acidity
594:Density
529:CS(C)=O
524:CS(=O)C
385:PubChem
290:DB01093
215:506008
159:67-68-5
4079:Other:
3865:
3853:
3829:
3807:
3764:
3754:
3621:"DMSO"
3537:
3527:
3450:
3440:
3370:"DMSO"
3350:636125
3348:
3278:
3268:
3219:
3174:
3166:
3156:
3125:
3076:
3039:
2994:
2930:
2920:
2871:
2831:
2785:
2777:
2705:
2655:"DMSO"
2607:
2597:
2518:
2480:
2368:
2291:26 May
2148:sewers
2132:
2059:
2052:
2002:Safety
1960:horses
1938:, and
1480:form (
1432:, and
1343:(dmso)
1329:ligand
1307:. The
1271:, and
1190:sulfur
1080:garlic
1002:verify
999:
904:(SDS)
767:
716:
600:
598:1.1004
517:SMILES
359:D01043
241:ChEMBL
95:Names
4379:Other
4260:Other
4168:joint
3845:[
3657:(PDF)
3563:(PDF)
3556:(PDF)
3472:WebMD
3037:S2CID
2783:S2CID
2703:S2CID
2393:SO".
2128:doses
2066:latex
2057:/ 0.4
2036:Glove
1992:mono-
1976:TRPA1
1966:Taste
1737:into
1638:worm
1592:polar
1495:DMSO-
1482:DMSO-
1339:(RuCl
1193:ylide
1184:with
1160:, I:
1065:polar
714:1.996
704:= 48
696:1.479
602:g⋅cm
578:78.13
482:InChI
344:1556
221:ChEBI
172:JSmol
4170:and
4062:Urea
3926:G04B
3863:ISBN
3851:ISBN
3805:PMID
3762:PMID
3535:PMID
3480:2022
3448:PMID
3381:2022
3346:PMID
3276:PMID
3217:ISBN
3196:help
3172:PMID
3164:ISSN
3154:ISBN
3123:PMID
3074:PMID
3057:Gene
2992:PMID
2928:PMID
2869:PMID
2829:PMID
2775:PMID
2733:2019
2605:OCLC
2595:ISBN
2541:1990
2516:PMID
2478:ISBN
2366:ISBN
2362:2345
2293:2007
2186:for
2032:skin
1996:tri-
1994:and
1986:and
1958:for
1892:and
1868:EMEM
1864:VERO
1860:MDCK
1850:and
1825:Onyx
1790:EDTA
1779:skin
1741:and
1673:and
1594:and
1579:and
1560:and
1512:CDCl
1379:(CO)
1241:and
1232:= 35
1207:S(CH
1030:DMSO
765:3.96
425:UNII
370:MeSH
350:KEGG
4176:M02
3797:doi
3752:PMC
3744:doi
3525:PMC
3515:doi
3438:PMC
3430:doi
3399:FDA
3338:doi
3266:PMC
3256:doi
3146:doi
3115:doi
3111:187
3066:doi
3062:274
3029:doi
2984:doi
2958:113
2918:PMC
2908:doi
2861:doi
2819:doi
2767:doi
2695:doi
2632:doi
2572:doi
2568:248
2545:doi
2508:doi
2470:doi
2411:doi
2317:doi
2055:mil
1888:to
1834:In
1815:In
1733:of
1517:or
1492:NMR
1454:pKa
1331:in
1287:In
1164:(CH
804:WHO
794:WHO
676:35
642:in
451:EPA
398:679
388:CID
270:659
4413::
3803:.
3795:.
3785:40
3783:.
3760:.
3750:.
3740:34
3738:.
3734:.
3711:^
3659:.
3623:.
3601:.
3579:.
3533:.
3523:.
3513:.
3501:.
3497:.
3469:.
3446:.
3436:.
3426:74
3424:.
3420:.
3397:.
3372:.
3358:^
3344:.
3334:11
3332:.
3326:.
3296:.
3274:.
3264:.
3250:.
3246:.
3188::
3186:}}
3182:{{
3170:.
3162:.
3152:.
3121:.
3109:.
3072:.
3060:.
3035:.
3027:.
3017:11
3015:.
2990:.
2980:88
2978:.
2966:^
2956:.
2952:.
2940:^
2926:.
2916:.
2906:.
2894:.
2890:.
2867:.
2857:13
2855:.
2841:^
2827:.
2815:12
2813:.
2809:.
2795:^
2781:.
2773:.
2763:37
2761:.
2757:.
2741:^
2723:.
2701:.
2691:21
2689:.
2657:.
2628:54
2626:.
2603:.
2566:.
2539:.
2514:.
2504:67
2502:.
2476:,
2409:.
2399:21
2397:.
2389:C)
2364:.
2337:.
2313:97
2311:.
2190:.
2138:.
2119:.
2064:)
2062:mm
2046:,
2042:,
2021:50
2017:50
1946:.
1934:,
1923:.
1881:.
1862:,
1745:.
1677:.
1652:.
1523:Cl
1519:CD
1425:,
1421:,
1392:.
1375:Cl
1371:Rh
1365:,
1361:,
1299:,
1263:,
1195::
1131:.
1042:CH
807:)
719:cP
673:)
666:(p
4178:)
4174:(
4156:e
4149:t
4142:v
3968:)
3928:)
3914:e
3907:t
3900:v
3859:.
3811:.
3799::
3791::
3768:.
3746::
3670:.
3541:.
3517::
3509::
3503:8
3482:.
3454:.
3432::
3383:.
3352:.
3340::
3311:.
3282:.
3258::
3252:8
3231:.
3198:)
3178:.
3148::
3129:.
3117::
3094:.
3080:.
3068::
3043:.
3031::
3023::
2998:.
2986::
2934:.
2910::
2902::
2896:6
2875:.
2863::
2835:.
2821::
2789:.
2769::
2735:.
2709:.
2697::
2672:.
2644:.
2638:.
2634::
2611:.
2578:.
2574::
2551:.
2547::
2522:.
2510::
2472::
2417:.
2413::
2405::
2391:2
2387:3
2374:.
2341:.
2323:.
2319::
2295:.
2211:2
1525:2
1521:2
1514:3
1508:2
1500:6
1497:d
1487:6
1484:d
1428:N
1381:4
1377:2
1373:2
1358:2
1356:I
1345:4
1341:2
1317:2
1230:a
1227:K
1225:p
1213:2
1209:2
1205:2
1203:)
1201:3
1174:3
1170:2
1168:)
1166:3
1089:s
1087:C
1056:O
1053:S
1049:2
1047:)
1044:3
1040:(
1028:(
997:Y
957:,
951:,
945:,
880:0
873:2
866:1
770:D
740:s
738:C
701:r
699:ε
692:)
690:D
687:n
685:(
671:a
668:K
566:S
563:O
560:6
557:H
554:2
551:C
453:)
449:(
174:)
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.