65:. The receptor is known to consist of two hydrogen bond donors, where the C3 and 17β-side chain of the ligands connect, as well as three hydrophobic groups distributed over the steroidal structure. The best receptor inhibitors comply with these factors. Azasteroids are a type of steroid derivatives which have nitrogen atoms replaced at various positions for one of the carbon atoms in the steroid ring system. Two 4-azasteroids, finasteride and dutasteride are marketed as 5-ARIs. Finasteride (Proscar or Propecia) was the first steroidal 5α-reductase inhibitor approved by the U.S.
136:
240:
148:
204:
171:
199:
Benzo(f)quinolonone are also tricyclic compounds, but derivatives of the 4-azasteroid structure. The compounds that have been designed can be divided into two categories, hexahydro derivatives and octahydro derivatives. The octahydro derivatives have been proven to be more potent. Compound LY 191704,
195:
Benzo(c)quinolizinones are tricyclic derivatives of 10-azasteroids. The D-ring has been removed and the C-ring substituted for an aromatic one. The first compounds developed were selective 5-alpha reductase type 1 inhibitors, but the most potent one inhibits both type 1 and 2. The fluorine atom is an
131:
Many steroidal 5-ARIs have been researched but only 3 are marketed. Two of them are 4-azasteroids and will be covered here. As mentioned above, the third one, epristeride is only marketed in China and will not be covered here. The basic SAR of 4-azasteroids is shown below. For competitive inhibiting
72:
Dutasteride (Avodart) was the second steroidal 5α-reductase approved after finasteride. It is a competitive inhibitor of all three 5α-reductase isoenzymes and it inhibits types 1 and 2 better than finasteride, leading to it causing further reduction in DHT, with >90% recuded DHT levels following
162:
Dutasteride, however, is a so-called dual inhibitor with both 5α-R1 and 5α-R2 inhibition. IC50 for 5α-R1 is 7 nM but 6 nM for 5α-R2. As mentioned above, it reduces DHT > 90% overall, or precisely 94.7% and for intraprostatic DHT the reduction is 97-99%. Dutasteride has also been found to inhibit
56:
The 5α-reductase isozymes possess a similar steroidal catalytic site. The only available information about the 5α-reductase isozymes is their primary sequence estimated from c-DNAs and that affects the design of the novel inhibitors. The crystal structure of the 5α-reductase isozymes is not known
191:
The common factor in nonsteroidal 5-ARI discovery is that the first compounds were all selective inhibitors to 5α-reductase type 1 only, but were then developed in order to get dual inhibition on both type 1 and 2, since inhibition of the type 2 isozyme is a more important factor in treating the
214:
Piperidones are also 4-azasteroid derivatives but both B- and D-ring have been removed. The original compounds designed were type 1 selective, especially the ones containing a chlorine atom connected to the aromatic ring. By inserting a styryl group to the piperidones type 2 inhibitory activity
87:
Various pharmaceutical and academic groups have conducted the synthesis of nonsteroidal compounds that inhibit human 5α-reductases due to the unwanted hormonal side effects of steroidal compounds. Nonsteroidal inhibitors can be categorized due to their structure. Many have been obtained from
222:
designed to resemble steroidal carboxylic acids such as episteride. As with the other nonsteroidal inhibitors, they have been designed by removing steroid ring systems. As with the piperidones, addition of a styryl group provides good dual inhibition on isozyme 1 and 2, but the nonsteroidal
1196:
416:
Chen, Grace
Shiahuy; Chang, Chih-Shiang; Kan, Wai Ming; Chang, Chih-Long; Wang, K. C.; Chern, Ji-Wang (2001-11-01). "Novel Lead Generation through Hypothetical Pharmacophore Three-Dimensional Database Searching: Discovery of Isoflavonoids as Nonsteroidal Inhibitors of Rat 5α-Reductase".
143:
Finasteride is considered similar to the transition state of reduced testosterone and is thus a slow-offset, irreversible inhibitor. The similarity to the transition state is a formation of an enzyme-NADP-dihydrofinasteride adduct by rearrangement on the A-ring of the compound.
158:
Finasteride mainly inhibits the 5α-R2 (IC50=69 nM) and 5α-R3 (IC50=17.4 nM) with little inhibition of 5α-R1 (IC50=360 nM). As mentioned above, finasteride reduces prostatic DHT levels on a 70-90% range but the detailed reduction of DHT is 70.8% and 85% of intraprostatic DHT.
183:. There is no detailed data about the cause of hepatotoxicity in 4-MA regarding SAR, but a conclusion may be drawn that the R2 group is the cause as there are other 4-azasteroid compounds containing the same R1 group as 4-MA, or CH3, without showing hepatotoxicity.
937:
132:
functions there are two functions considered crucial, 4-en-3-one function and a lipophilic 17β-side chain with one or more oxygen atoms. The main problems for 4-azasteroids is the rapid conversion into inactive 4,5-dihydro form, which is done by the enzyme.
882:
838:
57:
because the nature of the 5α-reductase enzyme is so unstable during purification. The first 5-ARIs were designed by modifying the structure of natural substrates, including the substitution of one carbon atom of the rings of the steroids by a
178:
Finasteride is an unsaturated analogue of another 4-azasteroid, or 4-MA. 4-MA is known to have dual inhibiting features with good inhibition on 5α-R1 (IC50=1.7 nM) and 5α-R2 (IC50=1.9 nM). However, 4-MA was never marketed as it showed
463:
Yamana, Kazutoshi; Labrie, Fernand; Luu-The, Van (2010-08-01). "Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride".
927:
52:
family and has an important role in biological actions towards steroid metabolism. If the steroid 5α-reductase is overexpressed it causes overproduction of DHT that can lead to androgenic disorders in humans.
982:
977:
907:
992:
261:(EMA) has concluded that the extract of the natural product Saw palmetto can be used to treat symptoms of benign prostatic hyperplasia (BPH) as research has shown its 5-ARI effects. An extract of
912:
200:
later named bexlosteride, is the most potent octahydro derivative designed. It is a selective inhibitor to the type 1 isozyme, especially because of the chlorine atom and the amino-methyl group.
114:
falls into the category of benzo(f)quinolonones, and is probably the derivative that has come closest to being marketed. It functions as a 5-ARI1 inhibitor which inhibits testosterone stimulated
997:
942:
828:
108:
Nonsteroidal inhibitors are thought to act as competitive inhibitors on the 5α-reductase isozymes, except for epristeride analogues (carboxylic acids), which are noncompetitive inhibitors.
1006:
957:
862:
79:
is the third marketed steroidal 5-ARI. It is a noncompetitive, specific inhibitor. It potency is not as significant as finasteride or dutasteride and thus it is only marketed in China.
872:
987:
1044:
922:
897:
947:
371:
Karnsomwan, Wiranpat; Rungrotmongkol, Thanyada; De-Eknamkul, Wanchai; Chamni, Supakarn (2016-06-01). "In silico structural prediction of human steroid 5α-reductase type II".
902:
877:
867:
687:
69:(USFDA). It inhibits the function of two of the isoenzymes (type II and III). In human it decreases the prostatic DHT level by 70–90% and reduces the prostatic size.
972:
701:
967:
1011:
932:
917:
852:
843:
823:
163:
5α-R3, in vitro, with IC50=0.33 nM. The 2,5-difluorophenyl side chain on the D-ring of the compound shows significant lipophilic features and as increased
2090:
833:
287:
1037:
892:
887:
227:
2080:
2047:
962:
748:
623:
524:
2085:
1030:
952:
325:
Aggarwal, Saurabh; Thareja, Suresh; Verma, Abhilasha; Bhardwaj, Tilak Raj; Kumar, Manoj (2010). "An overview on 5α-reductase inhibitors".
2116:
2095:
1305:
292:
278:. It is also used under the brand name Permixon in Europe as a pharmaceutical drug for the treatment of benign prostatic hyperplasia.
1370:
1350:
1070:
1325:
1561:
652:
Kulig, Katarzyna., Malawska, Barbara (2006). "Trends in the
Development of New Drugs for Treatment of Benign Hyperplasia".
1823:
1635:
1992:
1584:
373:
66:
2064:
2000:
1525:
1279:
1104:
796:
1950:
1184:
1776:
1765:
1159:
1134:
741:
258:
17:
1355:
1180:
167:
enhances the potency of the compounds binding at pocket site, its potency is much greater than of finasteride.
2121:
2052:
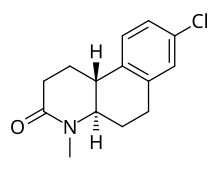
1942:
1271:
1226:
1216:
1176:
1164:
786:
1360:
1266:
1172:
1168:
1109:
1984:
1645:
681:
1996:
1804:
1695:
1290:
1275:
1251:
1144:
1129:
734:
268:
118:
cell growth but without testosterone the compound shows no effect and was therefore never marketed.
2029:
1988:
1892:
1838:
1820:
1680:
1655:
1650:
1632:
1621:
1575:
1295:
21:
557:"The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases"
1934:
1896:
1365:
1300:
1256:
1246:
1221:
1149:
1124:
497:
398:
350:
275:
219:
615:
2004:
1828:
1685:
1675:
1613:
1119:
1085:
669:
619:
588:
520:
489:
481:
442:
434:
390:
342:
1946:
1877:
1200:
857:
781:
661:
607:
578:
568:
473:
426:
382:
334:
272:
135:
1970:
1535:
1445:
1330:
226:
2058:
1954:
1916:
1904:
1863:
1700:
1509:
1501:
1493:
1465:
1450:
1395:
1211:
791:
771:
583:
556:
263:
244:
180:
49:
2110:
1435:
608:
164:
501:
402:
354:
1960:
1848:
1755:
1740:
1730:
1725:
1715:
1670:
1665:
1604:
1571:
1557:
1543:
1455:
1420:
1385:
1375:
1340:
1285:
1236:
1231:
1057:
338:
208:
111:
88:
azasteroid inhibitors by taking away one or more rings from the steroid structure.
37:
239:
1980:
1964:
1912:
1908:
1900:
1885:
1858:
1799:
1794:
1789:
1784:
1750:
1710:
1565:
1505:
1489:
1415:
1410:
1380:
1345:
1335:
1320:
1315:
1241:
1188:
776:
757:
152:
76:
665:
1974:
1930:
1853:
1833:
1745:
1720:
1690:
1497:
1480:
1440:
1425:
1405:
1154:
1139:
1022:
386:
62:
58:
485:
438:
394:
1938:
1920:
1843:
1735:
1660:
1640:
1551:
1547:
1485:
1400:
1114:
673:
592:
493:
477:
446:
346:
1306:
Trenbolone hexahydrobenzylcarbonate (trenbolone cyclohexylmethylcarbonate)
573:
203:
170:
2024:
1926:
1515:
1192:
1065:
1053:
147:
1475:
1093:
33:
430:
238:
225:
202:
169:
146:
134:
115:
91:
Four main categories of nonsteroidal 5-ARIs have been described:
1026:
730:
726:
702:"Herbal medicine: Summary for the Public. Saw Palmetto Fruit"
610:
Prostate Cancer: Basic
Mechanisms and Therapeutic Approaches
48:
Steroid 5α-reductase is a membrane-associated enzyme in an
938:
Nucleoside and nucleotide reverse-transcriptase inhibitors
519:. Google Books: Kluwer Academic Publishers. p. 398.
555:
Azzouni F, Godoy A, Li Y, Mohler J, et al. (2012).
517:
16:
This article is about the discovery and development of
883:
Dual serotonin and norepinephrine reuptake inhibitors
1585:
1212:
Androstanolone (stanolone, dihydrotestosterone, DHT)
466:
Hormone
Molecular Biology and Clinical Investigation
230:
Carboxylic acid derivative and piperidone derivative
2014:
1876:
1813:
1775:
1764:
1612:
1603:
1534:
1451:
Normethandrone (methylestrenolone, normethisterone)
1227:
Drostanolone propionate (dromostanolone propionate)
1084:
1071:
1064:
809:
764:
614:. Singapore: World Scientific Publishing. pp.
1341:Metandienone (methandienone, methandrostenolone)
928:Non-nucleoside reverse-transcriptase inhibitors
1393:17α-Alkylated dihydrotestosterone derivatives:
223:carboxylic acids are mostly type 1 selective.
32:These are two types of 5-ARIs, categorized as
1433:17α-Alkylated 19-nortestosterone derivatives:
1038:
742:
8:
1252:Metenolone enanthate (methenolone enanthate)
686:: CS1 maint: multiple names: authors list (
1576:
1772:
1609:
1081:
1045:
1031:
1023:
749:
735:
727:
515:Borchardt, Ronald T.; et al. (2006).
288:Discovery and development of antiandrogens
1614:
1296:Oxabolone cipionate (oxabolone cypionate)
1140:Prasterone (dehydroepiandrosterone, DHEA)
1086:
582:
572:
1247:Metenolone acetate (methenolone acetate)
1313:17α-Alkylated testosterone derivatives:
888:Selective serotonin reuptake inhibitors
304:
1326:Chlorodehydromethyltestosterone (CDMT)
679:
28:Development of 5α-reductase inhibitors
1466:Norvinisterone (vinylnortestosterone)
1145:Prasterone enanthate (DHEA enanthate)
550:
548:
546:
544:
542:
540:
538:
536:
7:
1590:Tooltip human chorionic gonadotropin
1473:17α-Ethynyltestosterone derivatives:
647:
645:
643:
641:
639:
637:
635:
458:
456:
366:
364:
320:
318:
316:
314:
312:
310:
308:
83:Nonsteroidal 5α-reductase inhibitors
2096:List of androgens/anabolic steroids
1076:Tooltip anabolic–androgenic steroid
2025:Androvax (androstenedione albumin)
1463:17α-Vinyltestosterone derivatives:
1346:Methandriol (methylandrostenediol)
978:Bcr-Abl tyrosine-kinase inhibitors
218:Nonsteroidal carboxylic acids are
14:
2091:Progestogens and antiprogestogens
1490:ethisterone (ethynyltestosterone)
1150:Prasterone sulfate (DHEA sulfate)
993:Neurokinin 1 receptor antagonists
868:Dipeptidyl peptidase-4 inhibitors
196:important part of the structure.
61:such as nitrogen thereby forming
44:Steroidal 5α-reductase inhibitors
1371:Methyltestosterone 3-hexyl ether
1351:Methandriol bisenanthoyl acetate
1209:Dihydrotestosterone derivatives:
983:Cannabinoid receptor antagonists
271:, is a 5-ARI that is sold as an
122:Structure–activity relationships
1264:19-Nortestosterone derivatives:
812:and development of drug classes
293:List of 5α-reductase inhibitors
73:1 year of oral administration.
2030:Ovandrotone albumin (Fecundin)
1502:norethisterone (norethindrone)
419:Journal of Medicinal Chemistry
339:10.1016/j.steroids.2009.10.005
1:
829:Angiotensin receptor blockers
2081:Androgen receptor modulators
1993:hydroxyprogesterone caproate
1456:Propetandrol (propethandrol)
1386:Tiomesterone (thiomesterone)
374:Medicinal Chemistry Research
67:Food and Drug Administration
2086:Estrogens and antiestrogens
2022:Androstenedione immunogens:
2001:medroxyprogesterone acetate
1581:Tooltip luteinizing hormone
1526:Medroxyprogesterone acetate
1280:nandrolone phenylpropionate
1185:testosterone ester mixtures
1105:Androstenediol dipropionate
1007:Melatonin receptor agonists
958:Thalidomide and its analogs
913:Memantine and related drugs
863:Cyclooxygenase 2 inhibitors
654:Current Medicinal Chemistry
2138:
1951:ethinylestradiol sulfonate
873:Direct thrombin inhibitors
810:Case studies of discovery
666:10.2174/092986706779010315
606:Chang, Chawnshang (2005).
139:Basic SAR of 4-azasteroids
2042:
1619:Tooltip Androgen receptor
1523:Progesterone derivatives:
1135:Cloxotestosterone acetate
1102:Testosterone derivatives:
1091:Tooltip Androgen receptor
988:CCR5 receptor antagonists
387:10.1007/s00044-016-1541-y
259:European Medicines Agency
1406:Mebolazine (dimethazine)
1356:Methandriol dipropionate
1181:testosterone undecanoate
923:Neuraminidase inhibitors
20:(5-ARIs), also known as
2117:5α-Reductase inhibitors
1756:Topilutamide (fluridil)
1177:testosterone propionate
898:HIV-protease inhibitors
819:5α-Reductase inhibitors
18:5α-reductase inhibitors
1361:Methandriol propionate
1267:Bolandiol dipropionate
1173:testosterone enanthate
1169:testosterone cypionate
1110:Boldenone undecylenate
948:Proton pump inhibitors
478:10.1515/hmbci.2010.035
249:
231:
211:
175:
155:
140:
95:Benzo(c)quinolizinones
1985:chlormadinone acetate
1646:Chlormadinone acetate
1217:Androstanolone esters
242:
229:
206:
173:
150:
138:
1997:gestonorone caproate
1805:Saw palmetto extract
1696:Potassium canrenoate
1291:Norclostebol acetate
1276:nandrolone decanoate
1130:Clostebol propionate
903:Integrase inhibitors
878:Direct Xa inhibitors
269:saw palmetto extract
253:Saw palmetto extract
1989:cyproterone acetate
1897:prolactin releasers
1839:Cyproterone acetate
1821:Abiraterone acetate
1681:Nomegestrol acetate
1656:Delmadinone acetate
1651:Cyproterone acetate
1633:Abiraterone acetate
1165:Testosterone esters
574:10.1155/2012/530121
220:tricyclic compounds
98:Benzo(f)quinolonone
22:dihydrotestosterone
2069:Never to phase III
1935:diethylstilbestrol
1562:GnRH (gonadorelin)
1366:Methyltestosterone
1301:Trenbolone acetate
1257:Stenbolone acetate
1222:Bolazine capronate
1125:Clostebol caproate
973:Tubulin inhibitors
276:dietary supplement
250:
232:
212:
176:
156:
141:
2104:
2103:
2038:
2037:
2005:megestrol acetate
1878:Antigonadotropins
1872:
1871:
1829:Aminoglutethimide
1686:Osaterone acetate
1676:Megestrol acetate
1599:
1598:
1272:Nandrolone esters
1120:Clostebol acetate
1020:
1019:
968:TRPV1 antagonists
908:Lipase inhibitors
709:www.ema.europa.eu
660:(28): 3395–2416.
625:978-981-256-067-4
526:978-0-306-47384-5
431:10.1021/jm010433s
425:(23): 3759–3763.
174:Structure of 4-MA
2129:
1971:GnRH antagonists
1947:ethinylestradiol
1943:estradiol esters
1773:
1620:
1616:
1610:
1591:
1587:
1582:
1578:
1536:Progonadotropins
1201:Testoviron Depot
1092:
1088:
1082:
1077:
1073:
1047:
1040:
1033:
1024:
1012:Renin inhibitors
858:c-Met inhibitors
782:Drug development
751:
744:
737:
728:
721:
720:
718:
716:
706:
698:
692:
691:
685:
677:
649:
630:
629:
613:
603:
597:
596:
586:
576:
552:
531:
530:
512:
506:
505:
460:
451:
450:
413:
407:
406:
381:(6): 1049–1056.
368:
359:
358:
322:
273:over-the-counter
267:, also known as
235:Natural products
192:disease of BPH.
104:Carboxylic acids
24:(DHT) blockers.
2137:
2136:
2132:
2131:
2130:
2128:
2127:
2126:
2107:
2106:
2105:
2100:
2074:
2059:Clinical trials
2034:
2010:
1889:
1868:
1809:
1767:
1766:Steroidogenesis
1760:
1618:
1595:
1589:
1580:
1530:
1446:Norethandrolone
1331:Fluoxymesterone
1090:
1075:
1068:
1060:
1051:
1021:
1016:
1001:
943:PDE5 inhibitors
933:NS5A inhibitors
918:mTOR inhibitors
847:
811:
805:
765:Steps in design
760:
755:
725:
724:
714:
712:
704:
700:
699:
695:
678:
651:
650:
633:
626:
605:
604:
600:
554:
553:
534:
527:
514:
513:
509:
462:
461:
454:
415:
414:
410:
370:
369:
362:
324:
323:
306:
301:
284:
255:
248:(saw palmetto).
237:
189:
129:
124:
85:
46:
30:
12:
11:
5:
2135:
2133:
2125:
2124:
2122:Drug discovery
2119:
2109:
2108:
2102:
2101:
2099:
2098:
2093:
2088:
2083:
2078:
2073:
2072:
2071:
2070:
2067:
2056:
2050:
2044:
2043:
2040:
2039:
2036:
2035:
2033:
2032:
2027:
2018:
2016:
2012:
2011:
2009:
2008:
1978:
1968:
1958:
1955:paroxypropione
1924:
1917:chlorpromazine
1905:metoclopramide
1887:
1882:
1880:
1874:
1873:
1870:
1869:
1867:
1866:
1864:Spironolactone
1861:
1856:
1851:
1846:
1841:
1836:
1831:
1826:
1817:
1815:
1811:
1810:
1808:
1807:
1802:
1797:
1792:
1787:
1781:
1779:
1770:
1762:
1761:
1759:
1758:
1753:
1748:
1743:
1738:
1733:
1728:
1723:
1718:
1713:
1704:
1703:
1701:Spironolactone
1698:
1693:
1688:
1683:
1678:
1673:
1668:
1663:
1658:
1653:
1648:
1643:
1638:
1626:
1624:
1607:
1601:
1600:
1597:
1596:
1594:
1593:
1569:
1555:
1540:
1538:
1532:
1531:
1529:
1528:
1519:
1518:
1513:
1510:norgestrienone
1494:levonorgestrel
1483:
1478:
1469:
1468:
1459:
1458:
1453:
1448:
1443:
1438:
1429:
1428:
1423:
1418:
1413:
1408:
1403:
1398:
1396:Androisoxazole
1389:
1388:
1383:
1378:
1373:
1368:
1363:
1358:
1353:
1348:
1343:
1338:
1333:
1328:
1323:
1318:
1309:
1308:
1303:
1298:
1293:
1288:
1283:
1269:
1260:
1259:
1254:
1249:
1244:
1239:
1234:
1229:
1224:
1219:
1214:
1205:
1204:
1162:
1157:
1152:
1147:
1142:
1137:
1132:
1127:
1122:
1117:
1112:
1107:
1098:
1096:
1079:
1062:
1061:
1052:
1050:
1049:
1042:
1035:
1027:
1018:
1017:
1015:
1014:
1009:
1004:
999:
995:
990:
985:
980:
975:
970:
965:
960:
955:
950:
945:
940:
935:
930:
925:
920:
915:
910:
905:
900:
895:
890:
885:
880:
875:
870:
865:
860:
855:
853:Cephalosporins
850:
845:
841:
836:
831:
826:
824:ACE inhibitors
821:
815:
813:
807:
806:
804:
803:
802:
801:
800:
799:
789:
779:
774:
772:Drug discovery
768:
766:
762:
761:
756:
754:
753:
746:
739:
731:
723:
722:
711:. 5 April 2016
693:
631:
624:
598:
532:
525:
507:
452:
408:
360:
333:(2): 109–153.
303:
302:
300:
297:
296:
295:
290:
283:
280:
264:Serenoa repens
254:
251:
245:Serenoa repens
236:
233:
188:
185:
181:hepatotoxicity
128:
125:
123:
120:
106:
105:
102:
99:
96:
84:
81:
50:oxidoreductase
45:
42:
29:
26:
13:
10:
9:
6:
4:
3:
2:
2134:
2123:
2120:
2118:
2115:
2114:
2112:
2097:
2094:
2092:
2089:
2087:
2084:
2082:
2079:
2076:
2075:
2068:
2066:
2063:
2062:
2060:
2057:
2054:
2051:
2049:
2046:
2045:
2041:
2031:
2028:
2026:
2023:
2020:
2019:
2017:
2013:
2006:
2002:
1998:
1994:
1990:
1986:
1982:
1979:
1976:
1972:
1969:
1966:
1962:
1961:GnRH agonists
1959:
1956:
1952:
1948:
1944:
1940:
1936:
1932:
1928:
1925:
1922:
1918:
1914:
1910:
1906:
1902:
1898:
1894:
1891:
1884:
1883:
1881:
1879:
1875:
1865:
1862:
1860:
1857:
1855:
1852:
1850:
1847:
1845:
1842:
1840:
1837:
1835:
1832:
1830:
1827:
1825:
1822:
1819:
1818:
1816:
1812:
1806:
1803:
1801:
1798:
1796:
1793:
1791:
1788:
1786:
1783:
1782:
1780:
1778:
1774:
1771:
1769:
1763:
1757:
1754:
1752:
1749:
1747:
1744:
1742:
1739:
1737:
1734:
1732:
1729:
1727:
1724:
1722:
1719:
1717:
1714:
1712:
1709:
1708:Nonsteroidal:
1706:
1705:
1702:
1699:
1697:
1694:
1692:
1689:
1687:
1684:
1682:
1679:
1677:
1674:
1672:
1669:
1667:
1664:
1662:
1659:
1657:
1654:
1652:
1649:
1647:
1644:
1642:
1639:
1637:
1634:
1631:
1628:
1627:
1625:
1623:
1617:
1611:
1608:
1606:
1605:Antiandrogens
1602:
1588:
1579:
1573:
1572:Gonadotropins
1570:
1567:
1563:
1559:
1558:GnRH agonists
1556:
1553:
1549:
1545:
1544:Antiestrogens
1542:
1541:
1539:
1537:
1533:
1527:
1524:
1521:
1520:
1517:
1514:
1511:
1507:
1503:
1499:
1495:
1491:
1487:
1484:
1482:
1479:
1477:
1474:
1471:
1470:
1467:
1464:
1461:
1460:
1457:
1454:
1452:
1449:
1447:
1444:
1442:
1439:
1437:
1436:Ethylestrenol
1434:
1431:
1430:
1427:
1424:
1422:
1419:
1417:
1414:
1412:
1409:
1407:
1404:
1402:
1399:
1397:
1394:
1391:
1390:
1387:
1384:
1382:
1379:
1377:
1374:
1372:
1369:
1367:
1364:
1362:
1359:
1357:
1354:
1352:
1349:
1347:
1344:
1342:
1339:
1337:
1334:
1332:
1329:
1327:
1324:
1322:
1319:
1317:
1314:
1311:
1310:
1307:
1304:
1302:
1299:
1297:
1294:
1292:
1289:
1287:
1284:
1281:
1277:
1273:
1270:
1268:
1265:
1262:
1261:
1258:
1255:
1253:
1250:
1248:
1245:
1243:
1240:
1238:
1235:
1233:
1230:
1228:
1225:
1223:
1220:
1218:
1215:
1213:
1210:
1207:
1206:
1202:
1198:
1194:
1190:
1186:
1182:
1178:
1174:
1170:
1166:
1163:
1161:
1158:
1156:
1153:
1151:
1148:
1146:
1143:
1141:
1138:
1136:
1133:
1131:
1128:
1126:
1123:
1121:
1118:
1116:
1113:
1111:
1108:
1106:
1103:
1100:
1099:
1097:
1095:
1089:
1083:
1080:
1074:
1067:
1063:
1059:
1058:antiandrogens
1055:
1048:
1043:
1041:
1036:
1034:
1029:
1028:
1025:
1013:
1010:
1008:
1005:
1003:
996:
994:
991:
989:
986:
984:
981:
979:
976:
974:
971:
969:
966:
964:
961:
959:
956:
954:
951:
949:
946:
944:
941:
939:
936:
934:
931:
929:
926:
924:
921:
919:
916:
914:
911:
909:
906:
904:
901:
899:
896:
894:
891:
889:
886:
884:
881:
879:
876:
874:
871:
869:
866:
864:
861:
859:
856:
854:
851:
849:
842:
840:
839:Beta-blockers
837:
835:
834:Antiandrogens
832:
830:
827:
825:
822:
820:
817:
816:
814:
808:
798:
795:
794:
793:
790:
788:
785:
784:
783:
780:
778:
775:
773:
770:
769:
767:
763:
759:
752:
747:
745:
740:
738:
733:
732:
729:
710:
703:
697:
694:
689:
683:
675:
671:
667:
663:
659:
655:
648:
646:
644:
642:
640:
638:
636:
632:
627:
621:
617:
612:
611:
602:
599:
594:
590:
585:
580:
575:
570:
566:
562:
558:
551:
549:
547:
545:
543:
541:
539:
537:
533:
528:
522:
518:
511:
508:
503:
499:
495:
491:
487:
483:
479:
475:
471:
467:
459:
457:
453:
448:
444:
440:
436:
432:
428:
424:
420:
412:
409:
404:
400:
396:
392:
388:
384:
380:
376:
375:
367:
365:
361:
356:
352:
348:
344:
340:
336:
332:
328:
321:
319:
317:
315:
313:
311:
309:
305:
298:
294:
291:
289:
286:
285:
281:
279:
277:
274:
270:
266:
265:
260:
252:
247:
246:
241:
234:
228:
224:
221:
216:
210:
207:Structure of
205:
201:
197:
193:
186:
184:
182:
172:
168:
166:
165:lipophilicity
160:
154:
151:Structure of
149:
145:
137:
133:
127:4-Azasteroids
126:
121:
119:
117:
113:
109:
103:
100:
97:
94:
93:
92:
89:
82:
80:
78:
74:
70:
68:
64:
60:
54:
51:
43:
41:
39:
35:
27:
25:
23:
19:
2021:
1981:Progestogens
1849:Ketoconazole
1777:5α-Reductase
1741:Ketoconazole
1731:Enzalutamide
1726:Darolutamide
1716:Bicalutamide
1707:
1671:Medrogestone
1666:Drospirenone
1629:
1522:
1472:
1462:
1432:
1421:Oxymetholone
1392:
1376:Oxymesterone
1312:
1286:Norclostebol
1263:
1237:Mepitiostane
1232:Epitiostanol
1208:
1160:Testosterone
1101:
818:
715:28 September
713:. Retrieved
708:
696:
682:cite journal
657:
653:
609:
601:
564:
560:
516:
510:
472:(3): 293–9.
469:
465:
422:
418:
411:
378:
372:
330:
326:
262:
256:
243:
217:
213:
209:bexlosteride
198:
194:
190:
187:Nonsteroidal
177:
161:
157:
142:
130:
112:Bexlosteride
110:
107:
90:
86:
75:
71:
55:
47:
38:nonsteroidal
31:
15:
2055:from market
1965:leuprorelin
1913:haloperidol
1909:risperidone
1901:domperidone
1893:antagonists
1859:Seviteronel
1800:Finasteride
1795:Epristeride
1790:Dutasteride
1785:Alfatradiol
1751:Seviteronel
1711:Apalutamide
1622:antagonists
1566:leuprorelin
1506:lynestrenol
1416:Oxandrolone
1411:Mestanolone
1381:Penmesterol
1336:Formebolone
1321:Calusterone
1316:Bolasterone
1242:Mesterolone
1189:Deposterona
1002:antagonists
787:Preclinical
777:Hit to lead
758:Drug design
215:increased.
153:finasteride
101:Piperidones
77:Epristeride
63:azasteroids
2111:Categories
1975:cetrorelix
1931:bifluranol
1854:Nilutamide
1834:Bifluranol
1824:+niraparib
1768:inhibitors
1746:Nilutamide
1721:Cimetidine
1691:Oxendolone
1636:+niraparib
1630:Steroidal:
1498:norgestrel
1486:Progestins
1481:Gestrinone
1441:Mibolerone
1426:Stanozolol
1155:Quinbolone
893:Gliflozins
567:: 530121.
299:References
59:heteroatom
2065:Phase III
2053:Withdrawn
1939:estradiol
1927:Estrogens
1921:sulpiride
1899:) (e.g.,
1844:Flutamide
1736:Flutamide
1661:Dienogest
1641:Canrenone
1552:clomifene
1548:tamoxifen
1401:Furazabol
1115:Clostebol
1066:Androgens
1054:Androgens
561:Adv. Urol
486:1868-1891
439:0022-2623
395:1054-2523
34:steroidal
2077:See also
1983:(incl.,
1890:receptor
1516:Tibolone
1197:Sustanon
1193:Omnadren
1094:agonists
963:Triptans
848:agonists
792:Clinical
674:17168713
593:22235201
502:28841145
494:25961201
447:11689062
403:12096090
355:44363501
347:19879888
327:Steroids
282:See also
40:5-ARIs.
1973:(e.g.,
1963:(e.g.,
1929:(e.g.,
1574:(e.g.,
1560:(e.g.,
1546:(e.g.,
1488:(e.g.,
1476:Danazol
1274:(e.g.,
1167:(e.g.,
1069:(incl.
953:Statins
584:3253436
2048:WHO-EM
2015:Others
1814:Others
797:Phases
672:
622:
591:
581:
523:
500:
492:
484:
445:
437:
401:
393:
353:
345:
705:(PDF)
498:S2CID
399:S2CID
351:S2CID
116:LNCaP
1056:and
998:5-HT
844:Beta
717:2017
688:link
670:PMID
620:ISBN
589:PMID
565:2012
521:ISBN
490:PMID
482:ISSN
443:PMID
435:ISSN
391:ISSN
343:PMID
257:The
36:and
1586:hCG
1072:AAS
662:doi
616:250
579:PMC
569:doi
474:doi
427:doi
383:doi
335:doi
2113::
2061::
2003:,
1999:,
1995:,
1991:,
1987:,
1953:,
1949:,
1945:,
1941:,
1937:,
1933:,
1919:,
1915:,
1911:,
1907:,
1903:,
1615:AR
1583:,
1577:LH
1564:,
1550:,
1508:,
1504:,
1500:,
1496:,
1492:,
1278:,
1203:))
1199:,
1195:,
1191:,
1183:,
1179:,
1175:,
1171:,
1087:AR
707:.
684:}}
680:{{
668:.
658:13
656:.
634:^
618:.
587:.
577:.
563:.
559:.
535:^
496:.
488:.
480:.
468:.
455:^
441:.
433:.
423:44
421:.
397:.
389:.
379:25
377:.
363:^
349:.
341:.
331:75
329:.
307:^
2007:)
1977:)
1967:)
1957:)
1923:)
1895:(
1888:2
1886:D
1592:)
1568:)
1554:)
1512:)
1282:)
1187:(
1078:)
1046:e
1039:t
1032:v
1000:3
846:2
750:e
743:t
736:v
719:.
690:)
676:.
664::
628:.
595:.
571::
529:.
504:.
476::
470:2
449:.
429::
405:.
385::
357:.
337::
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.