94:
812:
358:
valence electrons). Finally, in the column titled "Total
Valence electrons," write the number of valence electrons each atom has when unbonded. This information comes from the periodic table. For Group 1-8 elements (everything excluding transition metals, lanthanides, and actinides), the number of valence electrons is equal to their Group number. Now that the table has been made, calculating number of bonds and lone pairs is possible.
33:
987:
256:
A trick is to count up valence electrons, then count up the number of electrons needed to complete the octet rule (or with hydrogen just 2 electrons), then take the difference of these two numbers. The answer is the number of electrons that make up the bonds. The rest of the electrons just go to fill
745:
When comparing resonance structures for the same molecule, usually those with the fewest formal charges contribute more to the overall resonance hybrid. When formal charges are necessary, resonance structures that have negative charges on the more electronegative elements and positive charges on the
741:
of the oxygens forms the double bond. In this case, there are three possible resonance structures. Expressing resonance when drawing Lewis structures may be done either by drawing each of the possible resonance forms and placing double-headed arrows between them or by using dashed lines to represent
1025:
Despite their simplicity and development in the early twentieth century, when understanding of chemical bonding was still rudimentary, Lewis structures capture many of the key features of the electronic structure of a range of molecular systems, including those of relevance to chemical reactivity.
688:
The formal charge of an atom is computed as the difference between the number of valence electrons that a neutral atom would have and the number of electrons that belong to it in the Lewis structure. Electrons in covalent bonds are split equally between the atoms involved in the bond. The total of
357:
In the column titled "Molecular
Formula," write each individual atom of the molecule in individual rows. Then, in the column titled "Octect electrons," write the number of electrons each atom requires to achieve an octect (this will be 8 for every element except for Hydrogen, which can only hold 2
248:
Lewis structures for polyatomic ions may be drawn by the same method. When counting electrons, negative ions should have extra electrons placed in their Lewis structures; positive ions should have fewer electrons than an uncharged molecule. When the Lewis structure of an ion is written, the entire
806:
Tie up loose ends. Two Lewis structures must be drawn: Each structure has one of the two oxygen atoms double-bonded to the nitrogen atom. The second oxygen atom in each structure will be single-bonded to the nitrogen atom. Place brackets around each structure, and add the charge (−) to the upper
252:
A simpler method has been proposed for constructing Lewis structures, eliminating the need for electron counting: the atoms are drawn showing the valence electrons; bonds are then formed by pairing up valence electrons of the atoms involved in the bond-making process, and anions and cations are
174:
Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are
240:
Finally, each atom (other than hydrogen) that is surrounded by fewer than eight electrons (counting each bond as two) is processed as follows: For every two electrons needed, two dots are deleted from a neighboring atom and an additional line is drawn between the two atoms. This represents the
802:
Satisfy the octet rule. Both oxygen atoms currently have 8 electrons assigned to them. The nitrogen atom has only 6 electrons assigned to it. One of the lone pairs on an oxygen atom must form a double bond, but either atom will work equally well. Therefore, there is a resonance
1053:
There are simple and archetypal molecular systems for which a Lewis description, at least in unmodified form, is misleading or inaccurate. Notably, the naive drawing of Lewis structures for molecules known experimentally to contain unpaired electrons (e.g.,
1009:
of the lines. Hydrogen atoms bonded to carbon are not shown—they can be inferred by counting the number of bonds to a particular carbon atom—each carbon is assumed to have four bonds in total, so any bonds not shown are, by implication, to hydrogen atoms.
798:
Place lone pairs. The 14 remaining electrons should initially be placed as 7 lone pairs. Each oxygen may take a maximum of 3 lone pairs, giving each oxygen 8 electrons including the bonding pair. The seventh lone pair must be placed on the nitrogen
436:. "X" represents Hydrogen or Halogens. When Be is bonded with 2 other atoms, or when B and Al are bonded with 3 other atoms, they do not form full valence shells. Assume single bonds and use the actual bond number to calculate lone pairs.
974:
1001:. In condensed structural formulas, many or even all of the covalent bonds may be left out, with subscripts indicating the number of identical groups attached to a particular atom. Another shorthand structural diagram is the
722:, and the molecule exists as a resonance hybrid. Each of the different possibilities is superimposed on the others, and the molecule is considered to have a Lewis structure equivalent to some combination of these states.
791:
Count valence electrons. Nitrogen has 5 valence electrons; each oxygen has 6, for a total of (6 × 2) + 5 = 17. The ion has a charge of −1, which indicates an extra electron, so the total number of electrons is
562:
737:), for instance, must form a double bond between nitrogen and one of the oxygens to satisfy the octet rule for nitrogen. However, because the molecule is symmetrical, it does not matter
488:
of the atom, with exceptions. In general, the formal charge of an atom can be calculated using the following formula, assuming non-standard definitions for the markup used:
241:
conversion of a lone pair of electrons into a bonding pair, which adds two electrons to the former atom's valence shell while leaving the latter's electron count unchanged.
234:
electrons remain to be placed. These are temporarily drawn as dots, one per electron, to a maximum of eight per atom (two in the case of hydrogen), minus two for each bond.
1512:
1358:
244:
In the preceding steps, if there are not enough electrons to fill the valence shells of all atoms, preference is given to those atoms whose electronegativity is higher.
760:
The resonance structure should not be interpreted to indicate that the molecule switches between forms, but that the molecule acts as the average of multiple forms.
682:
653:
624:
595:
827:
1062:) leads to incorrect inferences of bond orders, bond lengths, and/or magnetic properties. A simple Lewis model also does not account for the phenomenon of
689:
the formal charges on an ion should be equal to the charge on the ion, and the total of the formal charges on a neutral molecule should be equal to zero.
703:
For some molecules and ions, it is difficult to determine which lone pairs should be moved to form double or triple bonds, and two or more different
476:
structures by determining the apparent electronic charge of each atom within, based upon its electron dot structure, assuming exclusive covalency or
374:
Lone pairs are not directly calculated, instead one calculates the number of remaining electrons. This is done as follows: TVe - 2(number of bonds)
50:
1005:(also known as a bond-line formula or carbon skeleton diagram). In a skeletal formula, carbon atoms are not signified by the symbol C but by the
387:
This system works in nearly all cases, however there are 3 instances where it will not work. These exceptions are outlined in the table below.
186:
and beyond usually react by gaining, losing, or sharing electrons until they have achieved a valence shell electron configuration with a full
1750:
1505:
1351:
742:
the partial bonds (although the latter is a good representation of the resonance hybrid which is not, formally speaking, a Lewis structure).
715:. This is sometimes the case when multiple atoms of the same type surround the central atom, and is especially common for polyatomic ions.
707:
structures may be written for the same molecule or ion. In such cases it is usual to write all of them with two-way arrows in between
54:
1543:
1479:
1311:
1178:
1152:
1498:
1344:
1050:
to adequately describe their bonding, making Lewis structures comparatively less important (although they are still common).
211:
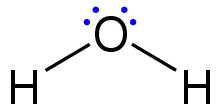
Once the total number of valence electrons has been determined, they are placed into the structure according to these steps:
1781:
795:
Connect the atoms by single bonds. Each oxygen must be bonded to the nitrogen, which uses four electrons—two in each bond.
757:, which is the correct description according to quantum chemical calculations instead of the common expanded octet model.
494:
1026:
Thus, they continue to enjoy widespread use by chemists and chemistry educators. This is especially true in the field of
1030:, where the traditional valence-bond model of bonding still dominates, and mechanisms are often understood in terms of
1806:
249:
structure is placed in brackets, and the charge is written as a superscript on the upper right, outside the brackets.
1811:
1816:
1771:
1755:
1038:, which are shorthand versions of Lewis structures. Due to the greater variety of bonding schemes encountered in
237:
Electrons are distributed first to the outer atoms and then to the others, until there are no more to be placed.
1730:
1087:
1043:
413:
Round calculated bond number down to the nearest whole number. (e.g. 4.5 bonds would round down to 4 bonds)
1013:
Other diagrams may be more complex than Lewis structures, showing bonds in 3D using various forms such as
1690:
1685:
1014:
1006:
750:
719:
473:
263:
There is a way to construct Lewis
Structures reliably via the use of a table similar to the one below:
788:
Nitrogen is the least electronegative atom of the two, so it is the central atom by multiple criteria.
260:
Another simple and general procedure to write Lewis structures and resonance forms has been proposed.
1656:
1443:
1278:
1242:
1114:
204:
The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of
43:
66:
1776:
1670:
1448:
1039:
754:
698:
152:
98:
93:
1326:
1403:
1214:
1109:
1104:
481:
179:
215:
Initially, one line (representing a single bond) is drawn between each pair of connected atoms.
1745:
1538:
1416:
1393:
1388:
1307:
1174:
1148:
1047:
1027:
811:
199:
1421:
1373:
1286:
1250:
1206:
1035:
1002:
998:
205:
183:
156:
660:
631:
602:
573:
70:
1469:
1435:
1074:(benzene) experiences special stabilization beyond normal delocalization effects, while C
969:{\displaystyle {\begin{matrix}{\ce {CH3-CH2-CH2-CH3}}\\{\ce {CH3CH2CH2CH3}}\end{matrix}}}
480:
bonding. It has uses in determining possible electron re-configuration when referring to
62:
1282:
1246:
1231:
Miburo, Barnabe B. (1993), "Simplified Lewis
Structure Drawing for Non-science Majors",
208:
on each individual atom. Non-valence electrons are not represented in Lewis structures.
1638:
1622:
1617:
1533:
1269:
1233:
1167:
807:
right outside the brackets. Draw a double-headed arrow between the two resonance forms.
485:
447:
Assume single bonds, use the minimum number of bonds necessary to create the molecule.
1800:
1711:
1651:
1646:
1627:
1521:
1031:
465:
459:
380:, the number of remaining electrons would be: 32 - 2(4) = 32 - 8 = 24. Therefore, CCl
128:
17:
1218:
997:
Chemical structures may be written in more compact forms, particularly when showing
444:
Bond calculation will provide too few bonds for the number of atoms in the molecule.
1453:
1099:
190:
of (8) electrons, hydrogen (H) can only form bonds which share just two electrons.
749:
Single bonds can also be moved in the same way to create resonance structures for
32:
1612:
1607:
1602:
1063:
168:
90:
Diagrams for the bonding between atoms of a molecule and lone pairs of electrons
1594:
1578:
1568:
986:
187:
1066:. For instance, Lewis structures do not offer an explanation for why cyclic C
1716:
684:
represents the total number of electrons in bonds the atom has with another.
477:
140:
718:
When this situation occurs, the molecule's Lewis structure is said to be a
1490:
1336:
1583:
1046:, many of the molecules encountered require the use of fully delocalized
626:
represents the number of valence electrons in a free atom of the element.
469:
410:
Sum of TVe will be an odd number. Bond number will not be a whole number.
148:
144:
136:
1210:
1132:
1573:
769:
147:
that may exist in the molecule. A Lewis structure can be drawn for any
1290:
1254:
253:
formed by adding or removing electrons to/from the appropriate atoms.
1474:
991:
979:
1194:
1090:
provides the most straightforward explanation for these phenomena.
985:
92:
468:
is used in the description, comparison, and assessment of likely
421:
Does not break the system, must instead memorize when it occurs.
367:, bond number would be: (40 - 32) ÷ 2 = 8 ÷2 = 4. Therefore, CCl
132:
1494:
1340:
1267:
Lever, A. B. P. (1972), "Lewis
Structures and the Octet Rule",
384:
has 24 remaining electrons, which means it has 12 lone pairs.
26:
1331:
978:
Two varieties of condensed structural formula, both showing
810:
958:
945:
932:
919:
900:
882:
864:
846:
407:
Free
Radicals (molecules with unpaired valence electrons)
655:
represents the number of unshared electrons on the atom.
441:
Expanded Octet (only occurs for elements in Groups 3-8)
58:
832:
830:
663:
634:
605:
576:
497:
557:{\displaystyle C_{f}=N_{v}-U_{e}-{\frac {B_{n}}{2}}}
361:
Bond number is calculated as follows: (Oe - TVe) ÷ 2
1764:
1738:
1729:
1704:
1678:
1669:
1636:
1592:
1561:
1554:
1462:
1434:
1402:
1381:
1372:
1166:
968:
676:
647:
618:
589:
556:
222:is the total number of electrons to be placed and
1314:, pp. 49–53 – Explanation of formal charge usage.
218:Each bond consists of a pair of electrons, so if
1082:(cyclobutadiene) actually experiences a special
127:) – are diagrams that show the
1506:
1352:
8:
1100:Valence shell electron pair repulsion theory
484:, and often results in the same sign as the
746:less electronegative elements are favored.
167:by adding lines between atoms to represent
163:Lewis structures extend the concept of the
1735:
1675:
1558:
1513:
1499:
1491:
1378:
1359:
1345:
1337:
226:is the number of single bonds just drawn,
957:
952:
944:
939:
931:
926:
918:
913:
908:
899:
894:
885:
881:
876:
867:
863:
858:
849:
845:
840:
835:
831:
829:
668:
662:
639:
633:
610:
604:
581:
575:
543:
537:
528:
515:
502:
496:
389:
265:
159:, who introduced it in his 1916 article
1327:Lewis Dot Diagrams of Selected Elements
1173:(2nd ed.), Pearson Prentice–Hall,
1125:
155:. The Lewis structure was named after
7:
1332:Lewis structures for all compounds
194:Construction and electron counting
25:
1544:Introduction to quantum mechanics
1302:Miessler, G. L. and Tarr, D. A.,
1165:G.L. Miessler; D.A. Tarr (2003),
1133:IUPAC definition of Lewis formula
31:
402:How to Fix the Lewis Structure
1306:(2nd ed., Prentice Hall 1998)
464:In terms of Lewis structures,
285:Total Valence electrons (TVe)
107: – also called
1:
257:all the other atoms' octets.
121:Lewis electron dot structures
710:
151:bonded molecule, as well as
42:may incorporate text from a
1195:"The Atom and the Molecule"
708:
71:the project page about this
1833:
696:
457:
197:
175:placed next to the atoms.
161:The Atom and the Molecule.
1529:
1731:Molecular orbital theory
1088:Molecular orbital theory
1044:organometallic chemistry
418:Valence Shell Deficiency
399:How it Breaks the System
1382:Non-structural formulas
1367:Molecular visualization
117:electron dot structures
1015:space-filling diagrams
994:
990:A skeletal diagram of
970:
819:Alternative formations
815:
678:
649:
620:
591:
558:
376:For the example of CCl
363:For the example of CCl
275:Molecular Formula (CCl
153:coordination compounds
101:
1193:Lewis, G. N. (1916),
1021:Usage and limitations
989:
971:
814:
751:hypervalent molecules
679:
677:{\displaystyle B_{n}}
650:
648:{\displaystyle U_{e}}
621:
619:{\displaystyle N_{v}}
597:is the formal charge.
592:
590:{\displaystyle C_{f}}
559:
97:Lewis structure of a
96:
18:Dot and cross diagram
1444:Ball-and-stick model
1115:Natural bond orbital
1032:curve-arrow notation
828:
661:
632:
603:
574:
495:
171:in a chemical bond.
165:electron dot diagram
113:Lewis dot structures
67:copyright violations
44:large language model
1671:Valence bond theory
1449:Space-filling model
1404:Structural formulas
1304:Inorganic Chemistry
1283:1972JChEd..49..819L
1247:1998JChEd..75..317M
1211:10.1021/ja02261a002
1169:Inorganic Chemistry
1145:Chemical Principles
1143:Zumdahl, S. (2005)
960:
947:
934:
921:
902:
884:
866:
848:
768:The formula of the
755:sulfur hexafluoride
720:resonance structure
699:Resonance structure
482:reaction mechanisms
392:
282:Octet electron (Oe)
271:
180:main group elements
1807:1916 introductions
1147:Houghton-Mifflin (
1110:Structural formula
1105:Molecular geometry
1048:molecular orbitals
1034:superimposed upon
995:
966:
964:
948:
935:
922:
909:
890:
872:
854:
836:
816:
674:
645:
616:
587:
554:
390:
266:
109:Lewis dot formulas
102:
1812:Chemical formulas
1794:
1793:
1790:
1789:
1765:Constituent units
1746:Molecular orbital
1725:
1724:
1705:Constituent units
1665:
1664:
1539:Quantum mechanics
1488:
1487:
1430:
1429:
1417:Condensed formula
1394:Molecular formula
1389:Empirical formula
1374:Chemical formulas
1291:10.1021/ed049p819
1255:10.1021/ed075p317
1199:J. Am. Chem. Soc.
1036:skeletal formulae
1028:organic chemistry
999:organic molecules
951:
938:
925:
912:
893:
875:
857:
839:
725:The nitrate ion (
552:
451:
450:
355:
354:
206:valence electrons
200:Electron counting
139:, as well as the
88:
87:
16:(Redirected from
1824:
1817:Chemical bonding
1736:
1676:
1657:Exchange-coupled
1559:
1522:Chemical bonding
1515:
1508:
1501:
1492:
1436:Molecular models
1422:Skeletal formula
1379:
1361:
1354:
1347:
1338:
1315:
1300:
1294:
1293:
1264:
1258:
1257:
1228:
1222:
1221:
1190:
1184:
1183:
1172:
1162:
1156:
1141:
1135:
1130:
1003:skeletal formula
975:
973:
972:
967:
965:
961:
959:
956:
949:
946:
943:
936:
933:
930:
923:
920:
917:
910:
903:
901:
898:
891:
889:
883:
880:
873:
871:
865:
862:
855:
853:
847:
844:
837:
783:
782:
781:
778:
736:
735:
734:
731:
714:
683:
681:
680:
675:
673:
672:
654:
652:
651:
646:
644:
643:
625:
623:
622:
617:
615:
614:
596:
594:
593:
588:
586:
585:
563:
561:
560:
555:
553:
548:
547:
538:
533:
532:
520:
519:
507:
506:
393:
272:
267:Lewis Dot of CCl
157:Gilbert N. Lewis
105:Lewis structures
83:
80:
74:
61:claims that are
35:
27:
21:
1832:
1831:
1827:
1826:
1825:
1823:
1822:
1821:
1797:
1796:
1795:
1786:
1760:
1721:
1700:
1696:Lewis structure
1661:
1632:
1588:
1550:
1525:
1519:
1489:
1484:
1470:Molecular graph
1458:
1426:
1412:Lewis structure
1398:
1368:
1365:
1323:
1318:
1301:
1297:
1266:
1265:
1261:
1230:
1229:
1225:
1192:
1191:
1187:
1181:
1164:
1163:
1159:
1142:
1138:
1131:
1127:
1123:
1096:
1084:destabilization
1081:
1077:
1073:
1069:
1061:
1057:
1023:
984:
983:
982:
976:
963:
962:
905:
904:
826:
825:
821:
779:
776:
775:
773:
766:
732:
729:
728:
726:
701:
695:
664:
659:
658:
635:
630:
629:
606:
601:
600:
577:
572:
571:
539:
524:
511:
498:
493:
492:
462:
456:
435:
431:
427:
383:
379:
375:
370:
366:
362:
278:
270:
202:
196:
91:
84:
78:
75:
53:information or
49:It may include
48:
36:
23:
22:
15:
12:
11:
5:
1830:
1828:
1820:
1819:
1814:
1809:
1799:
1798:
1792:
1791:
1788:
1787:
1785:
1784:
1782:Antibonding MO
1779:
1777:Non-bonding MO
1774:
1768:
1766:
1762:
1761:
1759:
1758:
1753:
1748:
1742:
1740:
1733:
1727:
1726:
1723:
1722:
1720:
1719:
1714:
1708:
1706:
1702:
1701:
1699:
1698:
1693:
1688:
1686:Hybrid orbital
1682:
1680:
1673:
1667:
1666:
1663:
1662:
1660:
1659:
1654:
1649:
1643:
1641:
1634:
1633:
1631:
1630:
1625:
1620:
1615:
1610:
1605:
1599:
1597:
1590:
1589:
1587:
1586:
1581:
1576:
1571:
1565:
1563:
1556:
1555:Types of bonds
1552:
1551:
1549:
1548:
1547:
1546:
1536:
1534:Atomic orbital
1530:
1527:
1526:
1520:
1518:
1517:
1510:
1503:
1495:
1486:
1485:
1483:
1482:
1477:
1472:
1466:
1464:
1460:
1459:
1457:
1456:
1451:
1446:
1440:
1438:
1432:
1431:
1428:
1427:
1425:
1424:
1419:
1414:
1408:
1406:
1400:
1399:
1397:
1396:
1391:
1385:
1383:
1376:
1370:
1369:
1366:
1364:
1363:
1356:
1349:
1341:
1335:
1334:
1329:
1322:
1321:External links
1319:
1317:
1316:
1295:
1270:J. Chem. Educ.
1259:
1234:J. Chem. Educ.
1223:
1185:
1179:
1157:
1136:
1124:
1122:
1119:
1118:
1117:
1112:
1107:
1102:
1095:
1092:
1079:
1075:
1071:
1067:
1059:
1055:
1022:
1019:
977:
955:
942:
929:
916:
907:
906:
897:
888:
879:
870:
861:
852:
843:
834:
833:
824:
823:
822:
820:
817:
809:
808:
804:
800:
796:
793:
789:
765:
762:
711:§ Example
697:Main article:
694:
691:
686:
685:
671:
667:
656:
642:
638:
627:
613:
609:
598:
584:
580:
565:
564:
551:
546:
542:
536:
531:
527:
523:
518:
514:
510:
505:
501:
486:partial charge
458:Main article:
455:
452:
449:
448:
445:
442:
438:
437:
433:
429:
425:
422:
419:
415:
414:
411:
408:
404:
403:
400:
397:
381:
377:
368:
364:
353:
352:
349:
346:
342:
341:
338:
335:
331:
330:
327:
324:
320:
319:
316:
313:
309:
308:
305:
302:
298:
297:
294:
291:
287:
286:
283:
280:
276:
268:
246:
245:
242:
238:
235:
216:
198:Main article:
195:
192:
99:water molecule
89:
86:
85:
39:
37:
30:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1829:
1818:
1815:
1813:
1810:
1808:
1805:
1804:
1802:
1783:
1780:
1778:
1775:
1773:
1770:
1769:
1767:
1763:
1757:
1754:
1752:
1749:
1747:
1744:
1743:
1741:
1737:
1734:
1732:
1728:
1718:
1715:
1713:
1712:Covalent bond
1710:
1709:
1707:
1703:
1697:
1694:
1692:
1689:
1687:
1684:
1683:
1681:
1677:
1674:
1672:
1668:
1658:
1655:
1653:
1650:
1648:
1645:
1644:
1642:
1640:
1635:
1629:
1626:
1624:
1623:5 (quintuple)
1621:
1619:
1618:4 (quadruple)
1616:
1614:
1611:
1609:
1606:
1604:
1601:
1600:
1598:
1596:
1591:
1585:
1582:
1580:
1577:
1575:
1572:
1570:
1567:
1566:
1564:
1560:
1557:
1553:
1545:
1542:
1541:
1540:
1537:
1535:
1532:
1531:
1528:
1523:
1516:
1511:
1509:
1504:
1502:
1497:
1496:
1493:
1481:
1478:
1476:
1473:
1471:
1468:
1467:
1465:
1461:
1455:
1452:
1450:
1447:
1445:
1442:
1441:
1439:
1437:
1433:
1423:
1420:
1418:
1415:
1413:
1410:
1409:
1407:
1405:
1401:
1395:
1392:
1390:
1387:
1386:
1384:
1380:
1377:
1375:
1371:
1362:
1357:
1355:
1350:
1348:
1343:
1342:
1339:
1333:
1330:
1328:
1325:
1324:
1320:
1313:
1312:0-13-841891-8
1309:
1305:
1299:
1296:
1292:
1288:
1284:
1280:
1276:
1272:
1271:
1263:
1260:
1256:
1252:
1248:
1244:
1240:
1236:
1235:
1227:
1224:
1220:
1216:
1212:
1208:
1205:(4): 762–85,
1204:
1200:
1196:
1189:
1186:
1182:
1180:0-13-035471-6
1176:
1171:
1170:
1161:
1158:
1154:
1153:0-618-37206-7
1150:
1146:
1140:
1137:
1134:
1129:
1126:
1120:
1116:
1113:
1111:
1108:
1106:
1103:
1101:
1098:
1097:
1093:
1091:
1089:
1085:
1065:
1058:, NO, and ClO
1051:
1049:
1045:
1041:
1037:
1033:
1029:
1020:
1018:
1016:
1011:
1008:
1004:
1000:
993:
988:
981:
953:
940:
927:
914:
895:
886:
877:
868:
859:
850:
841:
818:
813:
805:
801:
797:
794:
790:
787:
786:
785:
771:
763:
761:
758:
756:
752:
747:
743:
740:
723:
721:
716:
712:
706:
700:
692:
690:
669:
665:
657:
640:
636:
628:
611:
607:
599:
582:
578:
570:
569:
568:
549:
544:
540:
534:
529:
525:
521:
516:
512:
508:
503:
499:
491:
490:
489:
487:
483:
479:
475:
471:
467:
466:formal charge
461:
460:Formal charge
454:Formal charge
453:
446:
443:
440:
439:
423:
420:
417:
416:
412:
409:
406:
405:
401:
398:
396:The Exception
395:
394:
388:
385:
372:
371:has 4 bonds.
359:
350:
347:
344:
343:
339:
336:
333:
332:
328:
325:
322:
321:
317:
314:
311:
310:
306:
303:
300:
299:
295:
292:
289:
288:
284:
281:
274:
273:
264:
261:
258:
254:
250:
243:
239:
236:
233:
229:
225:
221:
217:
214:
213:
212:
209:
207:
201:
193:
191:
189:
185:
184:second period
181:
176:
172:
170:
166:
162:
158:
154:
150:
146:
142:
138:
134:
130:
126:
122:
118:
114:
110:
106:
100:
95:
82:
72:
68:
64:
60:
56:
52:
46:
45:
40:This section
38:
34:
29:
28:
19:
1695:
1628:6 (sextuple)
1595:multiplicity
1454:CPK coloring
1411:
1303:
1298:
1274:
1268:
1262:
1238:
1232:
1226:
1202:
1198:
1188:
1168:
1160:
1144:
1139:
1128:
1083:
1052:
1024:
1012:
996:
767:
759:
748:
744:
738:
724:
717:
704:
702:
687:
566:
463:
386:
373:
360:
356:
262:
259:
255:
251:
247:
231:
227:
223:
219:
210:
203:
177:
173:
169:shared pairs
164:
160:
124:
120:
116:
112:
108:
104:
103:
76:
63:unverifiable
51:hallucinated
41:
1562:By symmetry
1277:(12): 819,
1064:aromaticity
470:topological
391:Exceptions
1801:Categories
1772:Bonding MO
1756:MO diagram
1613:3 (triple)
1608:2 (double)
1603:1 (single)
1463:Other ways
1241:(3): 317,
1121:References
803:structure.
149:covalently
141:lone pairs
79:March 2024
55:references
1717:Lone pair
1691:Resonance
1579:Delta (δ)
1569:Sigma (σ)
1040:inorganic
887:−
869:−
851:−
705:resonance
693:Resonance
535:−
522:−
478:non-polar
474:resonance
432:, and AlX
178:Although
145:electrons
57:. Please
1739:Concepts
1679:Concepts
1219:95865413
1094:See also
1007:vertices
753:such as
230:−2
137:molecule
131:between
1652:Singlet
1647:Triplet
1584:Phi (φ)
1279:Bibcode
1243:Bibcode
772:ion is
770:nitrite
764:Example
567:where:
182:of the
129:bonding
1574:Pi (π)
1524:theory
1475:SMILES
1310:
1217:
1177:
1151:
992:butane
980:butane
713:below)
345:total:
69:. See
59:remove
1480:InChl
1215:S2CID
799:atom.
739:which
709:(see
188:octet
135:of a
133:atoms
119:, or
1751:LCAO
1639:spin
1308:ISBN
1175:ISBN
1149:ISBN
1042:and
472:and
428:, BX
125:LEDs
1637:By
1593:By
1287:doi
1251:doi
1207:doi
1086:.
792:18.
424:BeX
351:32
143:of
65:or
1803::
1285:,
1275:49
1273:,
1249:,
1239:75
1237:,
1213:,
1203:38
1201:,
1197:,
1017:.
950:CH
937:CH
924:CH
911:CH
892:CH
874:CH
856:CH
838:CH
784:.
774:NO
727:NO
348:40
340:7
334:Cl
329:7
323:Cl
318:7
312:Cl
307:7
301:Cl
296:4
115:,
111:,
1514:e
1507:t
1500:v
1360:e
1353:t
1346:v
1289::
1281::
1253::
1245::
1209::
1155:)
1080:4
1078:H
1076:4
1072:6
1070:H
1068:6
1060:2
1056:2
1054:O
954:3
941:2
928:2
915:3
896:3
878:2
860:2
842:3
780:2
777:−
733:3
730:−
670:n
666:B
641:e
637:U
612:v
608:N
583:f
579:C
550:2
545:n
541:B
530:e
526:U
517:v
513:N
509:=
504:f
500:C
434:3
430:3
426:2
382:4
378:4
369:4
365:4
337:8
326:8
315:8
304:8
293:8
290:C
279:)
277:4
269:4
232:n
228:t
224:n
220:t
123:(
81:)
77:(
73:.
47:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.