636:
140:
272:
31:
297:, one of her colleagues in the Manhattan Project. Two years later, in 1950, a new publication followed in which she attributed the shell closures at the magic numbers to spin-orbit coupling. According to Steven Moszkowski, a student of Goeppert Mayer, the term "magic number" was coined by Wigner: "Wigner too believed in the
263:). It is now believed that the sequence of spherical magic numbers cannot be extended in this way. Further predicted magic numbers are 114, 122, 124, and 164 for protons as well as 184, 196, 236, and 318 for neutrons. However, more modern calculations predict 228 and 308 for neutrons, along with 184 and 196.
1326:
Dvorak, J.; Brüchle, W.; Chelnokov, M.; Dressler, R.; Düllmann, Ch. E.; Eberhardt, K.; Gorshkov, V.; Jäger, E.; Krücken, R.; Kuznetsov, A.; Nagame, Y.; Nebel, F.; Novackova, Z.; Qin, Z.; Schädel, M.; Schausten, B.; Schimpf, E.; Semchenkov, A.; Thörle, P.; Türler, A.; Wegrzecki, M.; Wierczinski, B.;
463:
that are unstable, and represent endpoints beyond which stability drops off rapidly. Nickel-48, discovered in 1999, is the most proton-rich doubly magic nuclide known. At the other extreme, nickel-78 is also doubly magic, with 28 protons and 50 neutrons, a ratio observed only in much heavier
375:
process. Double beta decay in general is so rare that several nuclides exist which are predicted to decay by this mechanism but in which no such decay has yet been observed. Even in nuclides whose double beta decay has been confirmed through observations, half lives usually exceed the
414:-48 are doubly magic because calcium-48 has 20 protons and 28 neutrons while nickel-48 has 28 protons and 20 neutrons. Calcium-48 is very neutron-rich for such a relatively light element, but like calcium-40, it is stabilized by being doubly magic. As an exception, although
289:
became interested in the properties of nuclear fission products, such as decay energies and half-lives. In 1948, she published a body of experimental evidence for the occurrence of closed nuclear shells for nuclei with 50 or 82 protons or 50, 82, and 126 neutrons.
436:(the emission of a He nucleus – also known as an alpha particle – by a heavy element undergoing radioactive decay) is common in part due to the extraordinary stability of helium-4, which makes this type of decay energetically favored in most heavy nuclei over
608:. Hence, the "atomic magic numbers" are 2, 10, 18, 36, 54, 86 and 118. As with the nuclear magic numbers, these are expected to be changed in the superheavy region due to spin/orbit-coupling effects affecting subshell energy levels. Hence
623:
extension of the standard rotation group, the ground state properties (including the magic numbers) for metallic clusters and nuclei were simultaneously determined analytically. A specific potential term is not necessary in this model.
257:
538:
can be solved for the motion of nucleons and energy levels determined. Nuclear shells are said to occur when the separation between energy levels is significantly greater than the local mean separation.
1127:
Kondo, Y.; Achouri, N. L.; Falou, H. Al; Atar, L.; Aumann, T.; Baba, H.; Boretzky, K.; Caesar, C.; Calvet, D.; Chae, H.; Chiga, N.; Corsi, A.; Delaunay, F.; Delbart, A.; Deshayes, Q. (2023-08-31).
371:-208. While only helium-4, oxygen-16, calcium-40, and lead-208 are completely stable, calcium-48 is extremely long-lived and therefore found naturally, disintegrating only by a very inefficient
301:, but he recognized, from the work of Maria Mayer, the very strong evidence for the closed shells. It seemed a little like magic to him, and that is how the words 'Magic Numbers' were coined."
155:
Before this was realized, higher magic numbers, such as 184, 258, 350, and 462, were predicted based on simple calculations that assumed spherical shapes: these are generated by the formula
616:(114) are expected to be more inert than oganesson (118), and the next noble gas after these is expected to occur at element 172 rather than 168 (which would continue the pattern).
66:. As a result, atomic nuclei with a "magic" number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are
1421:
Herrmann, Richard (2010). "Higher dimensional mixed fractional rotation groups as a basis for dynamic symmetries generating the spectrum of the deformed
Nilsson-oscillator".
380:
by orders of magnitude, and emitted beta or gamma radiation is for virtually all practical purposes irrelevant. On the other hand, helium-10 is extremely unstable, and has a
421:
Magic number shell effects are seen in ordinary abundances of elements: helium-4 is among the most abundant (and stable) nuclei in the universe and lead-208 is the heaviest
1468:
Herrmann, Richard (2010). "Fractional phase transition in medium size metal clusters and some remarks on magic numbers in gravitationally and weakly bound clusters".
418:
has 8 protons and 20 neutrons, it is unbound with respect to four-neutron decay and appears to lack closed neutron shells, so it is not regarded as doubly magic.
136:. Unlike the magic numbers 2–126, which are realized in spherical nuclei, theoretical calculations predict that nuclei in the island of stability are deformed.
327:) numbers both equal to one of the magic numbers are called "doubly magic", and are generally very stable against decay. The known doubly magic isotopes are
101:, although 126 is so far only known to be a magic number for neutrons. Atomic nuclei consisting of such a magic number of nucleons have a higher average
293:
It had already been known that nuclei with 20 protons or neutrons were stable: that was evidenced by calculations by
Hungarian-American physicist
781:
Grumann, Jens; Mosel, Ulrich; Fink, Bernd; Greiner, Walter (1969). "Investigation of the stability of superheavy nuclei aroundZ=114 andZ=164".
724:
Grumann, Jens; Mosel, Ulrich; Fink, Bernd; Greiner, Walter (1969). "Investigation of the stability of superheavy nuclei aroundZ=114 andZ=164".
635:
1551:
1204:
158:
546:
for the nucleus, magic numbers are the numbers of nucleons at which a shell is filled. For instance, the magic number 8 occurs when the 1s
862:
452:
of mass number 5 and 8; indeed, all nuclides of those mass numbers decay within fractions of a second to produce alpha particles.
406:, with 20 neutrons and 20 protons, which is the heaviest stable isotope made of the same number of protons and neutrons. Both
476:
455:
Magic effects can keep unstable nuclides from decaying as rapidly as would otherwise be expected. For example, the nuclides
402:
Doubly magic effects may allow the existence of stable isotopes which otherwise would not have been expected. An example is
148:
110:
1563:
1608:
1102:
886:
619:
In 2010, an alternative explanation of magic numbers was given in terms of symmetry considerations. Based on the
649:
488:
139:
1598:
535:
1603:
475:-270, with 108 protons and 162 neutrons, was discovered by an international team of scientists led by the
429:
313:
124:
could theoretically be created with extremely large nuclei and yet not be subject to the extremely rapid
1212:
1487:
1442:
1395:
1348:
1274:
1044:
999:
960:
913:
790:
733:
286:
275:
260:
121:
1185:
1384:"Single-Particle Levels of Spherical Nuclei in the Superheavy and Extremely Superheavy Mass Region"
767:
710:
620:
543:
484:
377:
305:
133:
59:
1503:
1477:
1432:
1060:
1034:
880:
806:
749:
411:
1526:
1233:
271:
1593:
1547:
1364:
1328:
1166:
1148:
949:"On the Consequences of the Symmetry of the Nuclear Hamiltonian on the Spectroscopy of Nuclei"
929:
868:
858:
574:
515:
372:
309:
298:
282:
125:
1495:
1450:
1403:
1356:
1282:
1156:
1140:
1052:
1007:
968:
921:
798:
741:
659:
511:
449:
437:
510:-256 may be doubly magic and spherical due to the difference in size between low- and high-
468:
with one proton and two neutrons (Ni: 28/50 = 0.56; U: 92/146 = 0.63).
1078:
460:
441:
39:
901:
833:. 4th International Conference on the Chemistry and Physics of the Transactinide Elements
691:. 4th International Conference on the Chemistry and Physics of the Transactinide Elements
1491:
1446:
1399:
1352:
1286:
1278:
1161:
1128:
1048:
1003:
964:
917:
794:
737:
1540:
1259:
641:
422:
144:
102:
63:
1587:
1383:
810:
753:
531:
445:
324:
294:
129:
1507:
1064:
1208:
987:
825:
683:
387:
1360:
948:
1499:
1454:
1025:
Audi, Georges (2006). "The history of nuclidic masses and of their evaluation".
609:
433:
30:
1144:
1056:
631:
506: = 164 are not magic numbers, the undiscovered neutron-rich nucleus
492:
407:
403:
344:
340:
98:
1152:
933:
872:
854:
Out of the shadows : contributions of twentieth-century women to physics
1576:
1423:
1011:
827:
Decay modes and a limit of existence of nuclei in the superheavy mass region
654:
613:
605:
578:
527:
480:
415:
381:
336:
17:
1407:
1368:
1170:
972:
925:
852:
768:"Nuclear scientists eye future landfall on a second 'island of stability'"
711:"Nuclear scientists eye future landfall on a second 'island of stability'"
1237:
570:
328:
1039:
132:. Large isotopes with magic numbers of nucleons are said to exist in an
802:
745:
685:
The Impact of
Superheavy Elements on the Chemical and Physical Sciences
593:
558:
energy levels are filled, as there is a large energy gap between the 1p
507:
472:
465:
425:
117:
106:
82:
55:
47:
1301:
581:
356:
352:
348:
332:
252:{\displaystyle 2({\tbinom {n}{1}}+{\tbinom {n}{2}}+{\tbinom {n}{3}})}
86:
78:
74:
51:
27:
Number of protons or neutrons that make a nucleus particularly stable
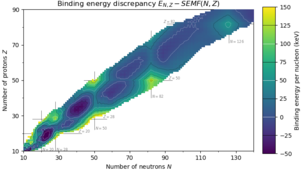
151:. Distinct sharp peaks in the contours appear only at magic numbers.
1258:
Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017).
1482:
1437:
601:
597:
589:
270:
29:
585:
569:
The atomic analog to nuclear magic numbers are those numbers of
368:
94:
456:
364:
360:
90:
308:, which Mayer developed in the following years together with
34:
A graph of isotope stability, with some of the magic numbers.
1577:"A Nearly Complete Explanation of the Nuclear Magic Numbers"
483:
of 9 seconds. Hassium-270 evidently forms part of an
448:. The stability of He also leads to the absence of stable
147:
of isotopes and the binding energy as predicted from the
109:
than one would expect based upon predictions such as the
58:, separately) such that they are arranged into complete
1205:"Twice-magic metal makes its debut - isotope of nickel"
1103:"What is Stable Nuclei - Unstable Nuclei - Definition"
223:
196:
169:
1234:"Tests confirm nickel-78 is a 'doubly magic' isotope"
161:
857:. Byers, Nina. Cambridge: Cambridge Univ. Pr. 2006.
487:, and may even be doubly magic due to the deformed (
1542:The Periodic Table, Its Story and Its Significance
1539:
251:
113:and are hence more stable against nuclear decay.
1260:"The NUBASE2016 evaluation of nuclear properties"
459:-100 and tin-132 are examples of doubly magic
323:Nuclei which have neutron numbers and proton (
73:For protons, this corresponds to the elements
239:
226:
212:
199:
185:
172:
8:
1564:"New magic number "inside atoms" discovered"
304:These magic numbers were the bedrock of the
1027:International Journal of Mass Spectrometry
1481:
1436:
1160:
1038:
238:
225:
222:
211:
198:
195:
184:
171:
168:
160:
1388:Journal of the Physical Society of Japan
526:Magic numbers are typically obtained by
514:orbitals, which alters the shape of the
138:
677:
675:
671:
878:
432:by known experimental observations).
7:
986:Mayer, Maria Goeppert (1949-06-15).
1327:Yakushev, A.; Yeremin, A. (2006).
573:leading to discontinuities in the
230:
203:
176:
25:
1079:"The Nobel Prize in Physics 1963"
682:Kratz, J. V. (5 September 2011).
988:"On Closed Shells in Nuclei. II"
634:
120:having magic numbers means that
1186:"The Most Tightly Bound Nuclei"
312:and culminated in their shared
900:Mayer, Maria G. (1948-08-01).
495:-like) shape of this nucleus.
477:Technical University of Munich
246:
165:
128:normally associated with high
1:
1382:Koura, H.; Chiba, S. (2013).
1361:10.1103/PhysRevLett.97.242501
1287:10.1088/1674-1137/41/3/030001
143:The difference between known
68:2, 8, 20, 28, 50, 82, and 126
902:"On Closed Shells in Nuclei"
530:studies; if the form of the
314:1963 Nobel Prize in Physics.
1546:. Oxford University Press.
1500:10.1016/j.physa.2010.03.033
1455:10.1016/j.physa.2009.11.016
1203:W., P. (October 23, 1999).
149:semi-empirical mass formula
111:semi-empirical mass formula
1625:
1558:see chapter 10 especially.
1300:Mason Inman (2006-12-14).
1145:10.1038/s41586-023-06352-6
1129:"First observation of 28O"
1057:10.1016/j.ijms.2006.01.048
947:Wigner, E. (1937-01-15).
116:The unusual stability of
1527:"Shell Model of Nucleus"
650:Magic number (chemistry)
1341:Physical Review Letters
1302:"A Nuclear Magic Trick"
1012:10.1103/PhysRev.75.1969
577:. These occur for the
562:and the next highest 1d
373:double beta minus decay
285:, the German physicist
97:, and the hypothetical
1408:10.7566/JPSJ.82.014201
1329:"Doubly Magic Nucleus
973:10.1103/PhysRev.51.106
926:10.1103/physrev.74.235
885:: CS1 maint: others (
783:Zeitschrift für Physik
726:Zeitschrift für Physik
278:
253:
152:
35:
1538:Scerri, Eric (2007).
1306:Physical Review Focus
464:elements, apart from
444:or any other type of
274:
267:History and etymology
254:
142:
122:transuranium elements
33:
536:Schrödinger equation
502: = 92 and
287:Maria Goeppert Mayer
281:Upon working on the
276:Maria Goeppert Mayer
261:Binomial coefficient
159:
1568:Scientific American
1492:2010PhyA..389.3307H
1447:2010PhyA..389..693H
1400:2013JPSJ...82a4201K
1353:2006PhRvL..97x2501D
1279:2017ChPhC..41c0001A
1240:. September 5, 2014
1049:2006IJMSp.251...85A
1004:1949PhRv...75.1969M
965:1937PhRv...51..106W
918:1948PhRv...74..235M
795:1969ZPhy..228..371G
738:1969ZPhy..228..371G
534:is known, then the
485:island of stability
378:age of the universe
306:nuclear shell model
134:island of stability
1562:Moskowitz, Clara.
824:Koura, H. (2011).
803:10.1007/BF01406719
746:10.1007/BF01406719
471:In December 2006,
279:
249:
244:
217:
190:
153:
36:
1609:Integer sequences
1575:Watkins, Thayer.
1553:978-0-19-530573-9
1476:(16): 3307–3315.
1273:(3): 030001–134.
1267:Chinese Physics C
1139:(7976): 965–970.
998:(12): 1969–1970.
575:ionization energy
532:nuclear potential
516:nuclear potential
489:American football
299:liquid drop model
283:Manhattan Project
237:
210:
183:
126:radioactive decay
16:(Redirected from
1616:
1580:
1571:
1557:
1545:
1534:
1512:
1511:
1485:
1465:
1459:
1458:
1440:
1418:
1412:
1411:
1379:
1373:
1372:
1323:
1317:
1316:
1314:
1313:
1297:
1291:
1290:
1264:
1255:
1249:
1248:
1246:
1245:
1230:
1224:
1223:
1221:
1220:
1211:. Archived from
1200:
1194:
1193:
1181:
1175:
1174:
1164:
1124:
1118:
1117:
1115:
1114:
1099:
1093:
1092:
1090:
1089:
1075:
1069:
1068:
1042:
1022:
1016:
1015:
983:
977:
976:
944:
938:
937:
897:
891:
890:
884:
876:
849:
843:
842:
840:
838:
832:
821:
815:
814:
778:
772:
771:
764:
758:
757:
721:
715:
714:
707:
701:
700:
698:
696:
690:
679:
660:Superdeformation
644:
639:
638:
512:angular momentum
438:neutron emission
398:
396:
390:
258:
256:
255:
250:
245:
243:
242:
229:
218:
216:
215:
202:
191:
189:
188:
175:
145:binding energies
21:
1624:
1623:
1619:
1618:
1617:
1615:
1614:
1613:
1584:
1583:
1574:
1561:
1554:
1537:
1524:
1521:
1516:
1515:
1467:
1466:
1462:
1420:
1419:
1415:
1381:
1380:
1376:
1336:
1332:
1325:
1324:
1320:
1311:
1309:
1299:
1298:
1294:
1262:
1257:
1256:
1252:
1243:
1241:
1232:
1231:
1227:
1218:
1216:
1215:on May 24, 2012
1202:
1201:
1197:
1183:
1182:
1178:
1126:
1125:
1121:
1112:
1110:
1101:
1100:
1096:
1087:
1085:
1077:
1076:
1072:
1040:physics/0602050
1024:
1023:
1019:
992:Physical Review
985:
984:
980:
953:Physical Review
946:
945:
941:
906:Physical Review
899:
898:
894:
877:
865:
851:
850:
846:
836:
834:
830:
823:
822:
818:
780:
779:
775:
766:
765:
761:
723:
722:
718:
709:
708:
704:
694:
692:
688:
681:
680:
673:
668:
640:
633:
630:
566:energy levels.
565:
561:
557:
553:
549:
524:
461:isotopes of tin
442:proton emission
394:
392:
385:
321:
269:
224:
197:
170:
157:
156:
46:is a number of
40:nuclear physics
28:
23:
22:
15:
12:
11:
5:
1622:
1620:
1612:
1611:
1606:
1601:
1599:Periodic table
1596:
1586:
1585:
1582:
1581:
1572:
1559:
1552:
1535:
1520:
1519:External links
1517:
1514:
1513:
1460:
1431:(4): 693–704.
1413:
1374:
1347:(24): 242501.
1334:
1330:
1318:
1308:. Vol. 18
1292:
1250:
1225:
1195:
1176:
1119:
1107:Periodic Table
1094:
1083:NobelPrize.org
1070:
1033:(2–3): 85–94.
1017:
978:
959:(2): 106–119.
939:
912:(3): 235–239.
892:
863:
844:
816:
789:(5): 371–386.
773:
759:
732:(5): 371–386.
716:
702:
670:
669:
667:
664:
663:
662:
657:
652:
646:
645:
642:Physics portal
629:
626:
563:
559:
555:
551:
547:
523:
520:
320:
317:
268:
265:
248:
241:
236:
233:
228:
221:
214:
209:
206:
201:
194:
187:
182:
179:
174:
167:
164:
130:atomic numbers
103:binding energy
64:atomic nucleus
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1621:
1610:
1607:
1605:
1604:Radioactivity
1602:
1600:
1597:
1595:
1592:
1591:
1589:
1578:
1573:
1569:
1565:
1560:
1555:
1549:
1544:
1543:
1536:
1532:
1528:
1523:
1522:
1518:
1509:
1505:
1501:
1497:
1493:
1489:
1484:
1479:
1475:
1471:
1464:
1461:
1456:
1452:
1448:
1444:
1439:
1434:
1430:
1426:
1425:
1417:
1414:
1409:
1405:
1401:
1397:
1394:(1): 014201.
1393:
1389:
1385:
1378:
1375:
1370:
1366:
1362:
1358:
1354:
1350:
1346:
1342:
1338:
1322:
1319:
1307:
1303:
1296:
1293:
1288:
1284:
1280:
1276:
1272:
1268:
1261:
1254:
1251:
1239:
1235:
1229:
1226:
1214:
1210:
1206:
1199:
1196:
1191:
1187:
1180:
1177:
1172:
1168:
1163:
1158:
1154:
1150:
1146:
1142:
1138:
1134:
1130:
1123:
1120:
1108:
1104:
1098:
1095:
1084:
1080:
1074:
1071:
1066:
1062:
1058:
1054:
1050:
1046:
1041:
1036:
1032:
1028:
1021:
1018:
1013:
1009:
1005:
1001:
997:
993:
989:
982:
979:
974:
970:
966:
962:
958:
954:
950:
943:
940:
935:
931:
927:
923:
919:
915:
911:
907:
903:
896:
893:
888:
882:
874:
870:
866:
864:0-521-82197-5
860:
856:
855:
848:
845:
829:
828:
820:
817:
812:
808:
804:
800:
796:
792:
788:
784:
777:
774:
769:
763:
760:
755:
751:
747:
743:
739:
735:
731:
727:
720:
717:
712:
706:
703:
687:
686:
678:
676:
672:
665:
661:
658:
656:
653:
651:
648:
647:
643:
637:
632:
627:
625:
622:
617:
615:
611:
607:
603:
599:
595:
591:
587:
583:
580:
576:
572:
567:
545:
540:
537:
533:
529:
521:
519:
517:
513:
509:
505:
501:
496:
494:
490:
486:
482:
478:
474:
469:
467:
462:
458:
453:
451:
447:
446:cluster decay
443:
439:
435:
431:
427:
424:
419:
417:
413:
409:
405:
400:
389:
386:260(40)
383:
379:
374:
370:
366:
362:
358:
354:
350:
346:
342:
338:
334:
330:
326:
318:
316:
315:
311:
307:
302:
300:
296:
295:Eugene Wigner
291:
288:
284:
277:
273:
266:
264:
262:
234:
231:
219:
207:
204:
192:
180:
177:
162:
150:
146:
141:
137:
135:
131:
127:
123:
119:
114:
112:
108:
104:
100:
96:
92:
88:
84:
80:
76:
71:
69:
65:
61:
57:
53:
49:
45:
41:
32:
19:
1567:
1541:
1531:HyperPhysics
1530:
1525:Nave, C. R.
1473:
1469:
1463:
1428:
1422:
1416:
1391:
1387:
1377:
1344:
1340:
1321:
1310:. Retrieved
1305:
1295:
1270:
1266:
1253:
1242:. Retrieved
1228:
1217:. Retrieved
1213:the original
1209:Science News
1198:
1190:HyperPhysics
1189:
1184:Nave, C. R.
1179:
1136:
1132:
1122:
1111:. Retrieved
1109:. 2019-05-22
1106:
1097:
1086:. Retrieved
1082:
1073:
1030:
1026:
1020:
995:
991:
981:
956:
952:
942:
909:
905:
895:
853:
847:
835:. Retrieved
826:
819:
786:
782:
776:
762:
729:
725:
719:
705:
693:. Retrieved
684:
618:
568:
541:
525:
503:
499:
497:
470:
454:
420:
401:
388:yoctoseconds
322:
319:Doubly magic
303:
292:
280:
154:
115:
72:
67:
44:magic number
43:
37:
18:Doubly magic
837:18 November
610:copernicium
579:noble gases
544:shell model
479:, having a
434:Alpha decay
310:Hans Jensen
62:within the
1588:Categories
1312:2006-12-25
1244:2014-09-09
1219:2006-09-29
1113:2019-12-22
1088:2020-06-27
666:References
621:fractional
612:(112) and
522:Derivation
493:rugby ball
408:calcium-48
404:calcium-40
367:-132, and
345:calcium-48
341:calcium-40
99:unbihexium
1483:0907.1953
1470:Physica A
1438:0806.2300
1424:Physica A
1153:0028-0836
934:0031-899X
881:cite book
873:255313795
811:120251297
754:120251297
695:27 August
655:Superatom
614:flerovium
606:oganesson
571:electrons
528:empirical
498:Although
481:half-life
416:oxygen-28
397:10 s
382:half-life
337:oxygen-16
1594:Isotopes
1508:50477979
1369:17280272
1238:Phys.org
1171:37648757
1162:10630140
1065:13236732
628:See also
430:at least
384:of just
329:helium-4
118:isotopes
56:neutrons
50:(either
48:nucleons
1488:Bibcode
1443:Bibcode
1396:Bibcode
1349:Bibcode
1275:Bibcode
1045:Bibcode
1000:Bibcode
961:Bibcode
914:Bibcode
791:Bibcode
734:Bibcode
594:krypton
542:In the
508:uranium
473:hassium
466:tritium
450:isobars
426:nuclide
107:nucleon
83:calcium
52:protons
1550:
1506:
1367:
1169:
1159:
1151:
1133:Nature
1063:
932:
871:
861:
809:
752:
582:helium
423:stable
412:nickel
393:2.6(4)
363:-100,
357:nickel
353:nickel
349:nickel
333:helium
325:atomic
87:nickel
79:oxygen
75:helium
60:shells
1504:S2CID
1478:arXiv
1433:arXiv
1263:(PDF)
1061:S2CID
1035:arXiv
831:(PDF)
807:S2CID
750:S2CID
689:(PDF)
602:radon
598:xenon
590:argon
491:- or
359:-78,
355:-56,
351:-48,
335:-10,
259:(see
1548:ISBN
1365:PMID
1167:PMID
1149:ISSN
930:ISSN
887:link
869:OCLC
859:ISBN
839:2018
697:2013
604:and
586:neon
554:, 1p
550:, 1p
410:and
369:lead
105:per
95:lead
42:, a
1496:doi
1474:389
1451:doi
1429:389
1404:doi
1357:doi
1335:162
1331:108
1283:doi
1157:PMC
1141:doi
1137:620
1053:doi
1031:251
1008:doi
969:doi
922:doi
799:doi
787:228
742:doi
730:228
564:5/2
560:1/2
556:1/2
552:3/2
548:1/2
457:tin
399:).
365:tin
361:tin
91:tin
54:or
38:In
1590::
1566:.
1529:.
1502:.
1494:.
1486:.
1472:.
1449:.
1441:.
1427:.
1402:.
1392:82
1390:.
1386:.
1363:.
1355:.
1345:97
1343:.
1339:.
1333:Hs
1304:.
1281:.
1271:41
1269:.
1265:.
1236:.
1207:.
1188:.
1165:.
1155:.
1147:.
1135:.
1131:.
1105:.
1081:.
1059:.
1051:.
1043:.
1029:.
1006:.
996:75
994:.
990:.
967:.
957:51
955:.
951:.
928:.
920:.
910:74
908:.
904:.
883:}}
879:{{
867:.
805:.
797:.
785:.
748:.
740:.
728:.
674:^
600:,
596:,
592:,
588:,
584:,
518:.
440:,
347:,
343:,
339:,
331:,
93:,
89:,
85:,
81:,
77:,
70:.
1579:.
1570:.
1556:.
1533:.
1510:.
1498::
1490::
1480::
1457:.
1453::
1445::
1435::
1410:.
1406::
1398::
1371:.
1359::
1351::
1337:"
1315:.
1289:.
1285::
1277::
1247:.
1222:.
1192:.
1173:.
1143::
1116:.
1091:.
1067:.
1055::
1047::
1037::
1014:.
1010::
1002::
975:.
971::
963::
936:.
924::
916::
889:)
875:.
841:.
813:.
801::
793::
770:.
756:.
744::
736::
713:.
699:.
504:N
500:Z
428:(
395:×
391:(
247:)
240:)
235:3
232:n
227:(
220:+
213:)
208:2
205:n
200:(
193:+
186:)
181:1
178:n
173:(
166:(
163:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.