484:
461:
38:
29:
1073:
Fung M, Thornton A, Mybeck K, Wu JH, Hornbuckle K, Muniz E (1 January 2001). "Evaluation of the
Characteristics of Safety Withdrawal of Prescription Drugs from Worldwide Pharmaceutical Markets-1960 to 1999".
1030:
Qureshi ZP, Seoane-Vazquez E, Rodriguez-Monguio R, Stevenson KB, Szeinbach SL (July 2011). "Market withdrawal of new molecular entities approved in the United States from 1980 to 2009".
742:
in the United States, the United
Kingdom, Ireland or Québec, due to the risk of birth defects. In Japan, people may not donate blood for two years after ceasing to use the medication.
1388:
104:
605:
547:
703:
ester). While acitretin is less lipophilic and has a half-life of only 50 hours, it is partly metabolized to etretinate in the body, so that it is still a long-acting
848:
1791:
843:[Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese).
149:
60:
561:
1381:
1126:
760:
677:, so its effects can continue long after dosage stops. It is detectable in the plasma for up to three years following therapy. Etretinate has a low
1238:
981:
945:
1374:
1332:
908:
963:
589:
InChI=1S/C23H30O3/c1-8-26-23(24)14-17(3)11-9-10-16(2)12-13-21-18(4)15-22(25-7)20(6)19(21)5/h9-15H,8H2,1-7H3/b11-9+,13-12+,16-10+,17-14+
836:
883:
581:
1008:
222:
134:
724:
long after use. Therefore, birth control is advised during therapy, and for at least three years after therapy has stopped.
844:
840:
1808:
1119:
360:
1638:
1424:
1349:
440:
1756:
1835:
309:
841:"RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial"
1337:
1112:
817:
92:
479:
1845:
1408:
177:
634:
1840:
682:
300:
1676:
1557:
429:
1671:
201:
1622:
456:
255:
1830:
1400:
1091:
1055:
752:
1577:
1736:
1696:
1691:
1686:
1438:
1850:
1746:
1716:
1602:
1592:
1443:
1397:
1047:
904:
879:
678:
50:
1666:
1587:
1582:
1492:
1487:
1482:
1477:
1472:
409:
349:
1786:
1781:
1776:
1771:
1766:
1761:
1751:
1741:
1731:
1726:
1597:
1497:
1467:
1083:
1039:
496:
169:
1567:
1507:
1502:
653:
market in 1998 due to the high risk of birth defects. It remains on the market in Japan as
369:
264:
878:(in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 728f.
483:
460:
37:
1343:
739:
674:
1824:
1701:
1532:
1135:
1016:
903:(in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. p. 5669.
793:
650:
472:
84:
1095:
1059:
289:
1681:
1643:
1547:
1527:
1522:
1429:
1233:
1222:
1139:
812:
721:
117:
112:
1104:
1562:
1517:
1286:
1281:
1206:
732:
700:
78:
1366:
1087:
1661:
1552:
1254:
1248:
1243:
1201:
1175:
666:
630:
523:
340:
187:
1792:
6-(N-ethyl-N-(5-isobutoxy-4-isopropyl-2-(E)-styrylphenyl)amino)nicotinic acid
1710:
1652:
1612:
1541:
1458:
1453:
1448:
1314:
1291:
1228:
1183:
931:
785:
766:
717:
704:
696:
638:
70:
64:
1051:
964:"Medications taken on a regular basis that exclude you from donating blood"
20:
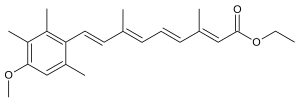
246:)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
1301:
1193:
1165:
728:
670:
642:
320:
329:
1706:
1537:
275:
28:
1043:
789:
646:
420:
389:
546:
537:
400:
738:
If a patient has ever taken etretinate, they are not eligible to
380:
1370:
1108:
156:
1161:
445:
707:
and pregnancy is prohibited for two years after therapy.
950:
UK Blood
Transfusion and Tissue Transplantation Services
143:
612:
796:
market in 1998 due to the high risk of birth defects.
759:
bone or joint pain, stiffness; in long-term treatment
784:
The drug was approved by FDA in 1986 to treat severe
637:
that was approved by the FDA in 1986 to treat severe
1621:
1407:
1300:
1274:
1267:
1215:
1192:
1174:
1154:
1147:
535:
522:
495:
490:
471:
439:
419:
399:
379:
359:
339:
319:
308:
299:
274:
254:
213:
200:
186:
176:
168:
133:
128:
103:
91:
77:
59:
49:
44:
569:O=C(OCC)\C=C(\C=C\C=C(\C=C\c1c(cc(OC)c(c1C)C)C)C)C
288:
1076:Therapeutic Innovation & Regulatory Science
1003:
1001:
263:
1409:
1382:
1120:
799:In Japan, the drug remains on market branded
8:
692:) of 120 days, which make dosing difficult.
19:
1623:
869:
867:
865:
1389:
1375:
1367:
1271:
1151:
1127:
1113:
1105:
482:
459:
348:
673:retinoid. It is stored and released from
368:
946:"Donor Selection Guidelines: Etretinate"
761:diffuse idiopathic skeletal hyperostosis
874:Mutschler E, Schäfer-Korting M (2001).
828:
788:. It was subsequently removed from the
645:. It was subsequently removed from the
586:
566:
455:
328:
227:
83:
473:
18:
930:
926:
924:
922:
920:
428:
408:
69:
7:
1610:Retinoic acid metabolism inhibitors:
1032:Pharmacoepidemiology and Drug Safety
116:
388:
279:
751:Side effects are those typical of
14:
1009:"Tigason Drug information sheet"
727:Etretinate should be avoided in
695:Etretinate has been replaced by
507:
36:
27:
851:from the original on 2023-08-27
594:Key:HQMNCQVAMBCHCO-DJRRULDNSA-N
1414:Tooltip Retinoic acid receptor
513:
501:
1:
1809:Receptor/signaling modulators
1648:-Retinoic acid (alitretinoin)
1434:-Retinoic acid (alitretinoin)
772:dry, burning, itching eyelids
699:, the free acid (without the
152:(Other prohibited substances)
641:. It is a second-generation
1628:Tooltip Retinoid X receptor
731:, as it may interfere with
1867:
1757:LE135 (RAR beta selective)
1657:-Retinoic acid (tretinoin)
1463:-Retinoic acid (tretinoin)
1239:Calcipotriol/betamethasone
1088:10.1177/009286150103500134
491:Chemical and physical data
1800:
1327:
602:
577:
557:
218:
55:Tigason, formerly Tegison
35:
26:
1136:Drugs used for psoriasis
847:(published 2023-07-25).
845:Diário Oficial da União
818:List of withdrawn drugs
792:market in 1996 and the
665:Etretinate is a highly
649:market in 1996 and the
196:-form, chain shortening
899:Jasek W, ed. (2007).
876:Arzneimittelwirkungen
683:elimination half-life
161: Withdrawn
1672:Docosahexaenoic acid
1013:RAD-AR Council Japan
1019:on 27 January 2013.
23:
1354:Never to phase III
753:hypervitaminosis A
1818:
1817:
1398:Retinoid receptor
1364:
1363:
1323:
1322:
1263:
1262:
932:Drugs.com archive
910:978-3-85200-181-4
679:therapeutic index
635:Hoffmann–La Roche
620:
619:
548:Interactive image
441:CompTox Dashboard
160:
147:
71:Drugs.com archive
16:Chemical compound
1858:
1677:Fluorobexarotene
1629:
1625:
1558:Tazarotenic acid
1415:
1411:
1391:
1384:
1377:
1368:
1272:
1152:
1129:
1122:
1115:
1106:
1100:
1099:
1070:
1064:
1063:
1044:10.1002/pds.2155
1027:
1021:
1020:
1015:. Archived from
1005:
996:
995:
993:
992:
986:www.giveblood.ie
978:
972:
971:
960:
954:
953:
942:
936:
934:
928:
915:
914:
896:
890:
889:
871:
860:
859:
857:
856:
833:
775:unusual bruising
755:, most commonly
720:, and may cause
716:Etretinate is a
616:
615:
608:
550:
530:
515:
509:
503:
486:
475:
464:
463:
449:
447:
432:
412:
392:
372:
352:
332:
312:
292:
282:
281:
267:
205:
158:
155:
145:
142:
120:
87:
73:
40:
31:
24:
22:
1866:
1865:
1861:
1860:
1859:
1857:
1856:
1855:
1836:Withdrawn drugs
1821:
1820:
1819:
1814:
1796:
1627:
1617:
1413:
1403:
1395:
1365:
1360:
1359:
1344:Clinical trials
1319:
1296:
1259:
1211:
1188:
1170:
1143:
1133:
1103:
1072:
1071:
1067:
1029:
1028:
1024:
1007:
1006:
999:
990:
988:
980:
979:
975:
962:
961:
957:
944:
943:
939:
929:
918:
911:
898:
897:
893:
886:
873:
872:
863:
854:
852:
835:
834:
830:
826:
809:
782:
749:
713:
691:
663:
611:
609:
606:(what is this?)
603:
598:
595:
590:
585:
584:
573:
570:
565:
564:
553:
528:
518:
512:
506:
467:
443:
435:
415:
395:
375:
355:
335:
315:
295:
278:
270:
250:
247:
226:
225:
203:
178:Protein binding
170:Pharmacokinetic
164:
124:
94:
17:
12:
11:
5:
1864:
1862:
1854:
1853:
1848:
1843:
1838:
1833:
1823:
1822:
1816:
1815:
1813:
1812:
1805:
1801:
1798:
1797:
1795:
1794:
1789:
1784:
1779:
1774:
1769:
1764:
1759:
1754:
1749:
1744:
1739:
1734:
1729:
1720:
1719:
1714:
1704:
1699:
1694:
1689:
1684:
1679:
1674:
1669:
1664:
1659:
1650:
1641:
1632:
1630:
1619:
1618:
1616:
1615:
1606:
1605:
1600:
1595:
1590:
1585:
1580:
1571:
1570:
1565:
1560:
1555:
1550:
1545:
1535:
1530:
1525:
1520:
1515:
1510:
1505:
1500:
1495:
1490:
1485:
1480:
1475:
1470:
1465:
1456:
1451:
1446:
1441:
1436:
1427:
1418:
1416:
1405:
1404:
1396:
1394:
1393:
1386:
1379:
1371:
1362:
1361:
1358:
1357:
1356:
1355:
1352:
1341:
1335:
1329:
1328:
1325:
1324:
1321:
1320:
1318:
1317:
1312:
1306:
1304:
1298:
1297:
1295:
1294:
1289:
1284:
1278:
1276:
1269:
1265:
1264:
1261:
1260:
1258:
1257:
1252:
1246:
1241:
1236:
1225:
1219:
1217:
1213:
1212:
1210:
1209:
1204:
1198:
1196:
1190:
1189:
1187:
1186:
1180:
1178:
1172:
1171:
1169:
1168:
1158:
1156:
1149:
1145:
1144:
1134:
1132:
1131:
1124:
1117:
1109:
1102:
1101:
1082:(1): 293–317.
1065:
1022:
997:
973:
955:
937:
935:for etretinate
916:
909:
891:
884:
861:
839:(2023-07-24).
827:
825:
822:
821:
820:
815:
808:
805:
781:
778:
777:
776:
773:
770:
763:
748:
745:
744:
743:
736:
725:
712:
709:
689:
675:adipose tissue
662:
659:
618:
617:
600:
599:
597:
596:
593:
591:
588:
580:
579:
578:
575:
574:
572:
571:
568:
560:
559:
558:
555:
554:
552:
551:
543:
541:
533:
532:
526:
520:
519:
516:
510:
504:
499:
493:
492:
488:
487:
477:
469:
468:
466:
465:
452:
450:
437:
436:
434:
433:
425:
423:
417:
416:
414:
413:
405:
403:
397:
396:
394:
393:
385:
383:
377:
376:
374:
373:
365:
363:
357:
356:
354:
353:
345:
343:
337:
336:
334:
333:
325:
323:
317:
316:
314:
313:
305:
303:
297:
296:
294:
293:
285:
283:
272:
271:
269:
268:
260:
258:
252:
251:
249:
248:
229:
221:
220:
219:
216:
215:
211:
210:
207:
198:
197:
190:
184:
183:
180:
174:
173:
166:
165:
163:
162:
153:
139:
137:
131:
130:
126:
125:
123:
122:
109:
107:
101:
100:
97:
95:administration
89:
88:
81:
75:
74:
67:
57:
56:
53:
47:
46:
42:
41:
33:
32:
15:
13:
10:
9:
6:
4:
3:
2:
1863:
1852:
1849:
1847:
1846:Phenol ethers
1844:
1842:
1839:
1837:
1834:
1832:
1829:
1828:
1826:
1811:
1810:
1806:
1803:
1802:
1799:
1793:
1790:
1788:
1785:
1783:
1780:
1778:
1775:
1773:
1770:
1768:
1765:
1763:
1760:
1758:
1755:
1753:
1750:
1748:
1745:
1743:
1740:
1738:
1735:
1733:
1730:
1728:
1725:
1722:
1721:
1718:
1715:
1712:
1708:
1705:
1703:
1702:Retinoic acid
1700:
1698:
1695:
1693:
1690:
1688:
1685:
1683:
1680:
1678:
1675:
1673:
1670:
1668:
1665:
1663:
1660:
1658:
1656:
1651:
1649:
1647:
1642:
1640:
1637:
1634:
1633:
1631:
1626:
1620:
1614:
1611:
1608:
1607:
1604:
1601:
1599:
1596:
1594:
1591:
1589:
1586:
1584:
1581:
1579:
1576:
1573:
1572:
1569:
1566:
1564:
1561:
1559:
1556:
1554:
1551:
1549:
1546:
1543:
1539:
1536:
1534:
1533:Retinoic acid
1531:
1529:
1526:
1524:
1521:
1519:
1516:
1514:
1511:
1509:
1506:
1504:
1501:
1499:
1496:
1494:
1491:
1489:
1486:
1484:
1481:
1479:
1476:
1474:
1471:
1469:
1466:
1464:
1462:
1457:
1455:
1452:
1450:
1447:
1445:
1442:
1440:
1437:
1435:
1433:
1428:
1426:
1423:
1420:
1419:
1417:
1412:
1406:
1402:
1399:
1392:
1387:
1385:
1380:
1378:
1373:
1372:
1369:
1353:
1351:
1348:
1347:
1345:
1342:
1339:
1336:
1334:
1331:
1330:
1326:
1316:
1313:
1311:
1308:
1307:
1305:
1303:
1299:
1293:
1290:
1288:
1285:
1283:
1280:
1279:
1277:
1273:
1270:
1266:
1256:
1253:
1250:
1247:
1245:
1242:
1240:
1237:
1235:
1231:
1230:
1226:
1224:
1221:
1220:
1218:
1214:
1208:
1205:
1203:
1200:
1199:
1197:
1195:
1191:
1185:
1182:
1181:
1179:
1177:
1173:
1167:
1163:
1160:
1159:
1157:
1153:
1150:
1146:
1141:
1137:
1130:
1125:
1123:
1118:
1116:
1111:
1110:
1107:
1097:
1093:
1089:
1085:
1081:
1077:
1069:
1066:
1061:
1057:
1053:
1049:
1045:
1041:
1037:
1033:
1026:
1023:
1018:
1014:
1010:
1004:
1002:
998:
987:
983:
982:"Health FAQs"
977:
974:
969:
965:
959:
956:
951:
947:
941:
938:
933:
927:
925:
923:
921:
917:
912:
906:
902:
901:Austria-Codex
895:
892:
887:
885:3-8047-1763-2
881:
877:
870:
868:
866:
862:
850:
846:
842:
838:
832:
829:
823:
819:
816:
814:
811:
810:
806:
804:
802:
797:
795:
794:United States
791:
787:
779:
774:
771:
768:
764:
762:
758:
757:
756:
754:
746:
741:
737:
734:
730:
726:
723:
722:birth defects
719:
715:
714:
710:
708:
706:
702:
698:
693:
688:
684:
680:
676:
672:
668:
660:
658:
656:
652:
651:United States
648:
644:
640:
636:
633:developed by
632:
628:
624:
614:
607:
601:
592:
587:
583:
576:
567:
563:
556:
549:
545:
544:
542:
539:
534:
527:
525:
521:
500:
498:
494:
489:
485:
481:
478:
476:
474:ECHA InfoCard
470:
462:
458:
457:DTXSID0023036
454:
453:
451:
442:
438:
431:
427:
426:
424:
422:
418:
411:
407:
406:
404:
402:
398:
391:
387:
386:
384:
382:
378:
371:
367:
366:
364:
362:
358:
351:
347:
346:
344:
342:
338:
331:
327:
326:
324:
322:
318:
311:
307:
306:
304:
302:
298:
291:
287:
286:
284:
277:
273:
266:
262:
261:
259:
257:
253:
245:
241:
237:
233:
228:
224:
217:
212:
208:
206:
199:
195:
191:
189:
185:
181:
179:
175:
171:
167:
154:
151:
141:
140:
138:
136:
132:
127:
119:
114:
111:
110:
108:
106:
102:
98:
96:
90:
86:
82:
80:
76:
72:
68:
66:
62:
58:
54:
52:
48:
45:Clinical data
43:
39:
34:
30:
25:
1841:Ethyl esters
1807:
1724:Antagonists:
1723:
1682:Isotretinoin
1654:
1645:
1635:
1609:
1575:Antagonists:
1574:
1548:Tamibarotene
1528:Palovarotene
1523:Isotretinoin
1512:
1460:
1431:
1421:
1309:
1234:Calcipotriol
1227:
1223:Fumaric acid
1079:
1075:
1068:
1038:(7): 772–7.
1035:
1031:
1025:
1017:the original
1012:
989:. Retrieved
985:
976:
967:
958:
949:
940:
900:
894:
875:
853:. Retrieved
831:
813:Isotretinoin
800:
798:
783:
765:muscular or
750:
747:Side effects
740:donate blood
694:
686:
664:
661:Pharmacology
654:
626:
625:(trade name
622:
621:
610:
604:
243:
239:
235:
231:
202:Elimination
193:
135:Legal status
129:Legal status
1563:Trifarotene
1518:Fenretinide
1340:from market
1287:Methoxsalen
1282:Trioxysalen
1207:Methoxsalen
1202:Trioxysalen
968:Héma-Québec
733:bone growth
711:Precautions
681:and a long
531: g·mol
480:100.053.727
214:Identifiers
192:Free acid,
188:Metabolites
79:MedlinePlus
51:Trade names
1825:Categories
1662:Bexarotene
1578:BMS-195614
1553:Tazarotene
1513:Etretinate
1401:modulators
1310:Etretinate
1255:Tazarotene
1249:Tacalcitol
1244:Calcitriol
991:2023-10-21
855:2023-08-27
824:References
667:lipophilic
631:medication
623:Etretinate
536:3D model (
524:Molar mass
410:CHEBI:4913
370:65M2UDR9AG
341:ChemSpider
301:IUPHAR/BPS
265:54350-48-0
256:CAS Number
223:IUPAC name
21:Etretinate
1831:Retinoids
1737:LG-100754
1711:vitamin A
1697:LG-100754
1692:LG-101506
1687:LG-100268
1636:Agonists:
1613:Liarozole
1542:vitamin A
1454:Adapalene
1449:Acitretin
1439:AC-261066
1422:Agonists:
1350:Phase III
1338:Withdrawn
1315:Acitretin
1302:Retinoids
1292:Bergapten
1275:Psoralens
1229:vitamin D
1194:Psoralens
1184:Dithranol
1176:Antracens
786:psoriasis
767:abdominal
718:teratogen
705:teratogen
697:acitretin
639:psoriasis
430:ChEMBL464
204:half-life
93:Routes of
65:Drugs.com
1851:Polyenes
1804:See also
1747:UVI-3003
1717:SR-11237
1603:MM-11253
1593:ER-50891
1444:AC-55649
1268:Systemic
1166:Coal tar
1096:73036562
1060:23821961
1052:21574210
849:Archived
807:See also
790:Canadian
729:children
671:aromatic
647:Canadian
643:retinoid
613:(verify)
321:DrugBank
230:Ethyl (2
209:120 days
150:Class F4
105:ATC code
1707:Retinol
1667:CD 3254
1588:CD-2665
1583:BMS-493
1538:Retinol
1493:CD-2314
1488:CD-1530
1483:BMS-961
1478:BMS-753
1473:BMS-493
1148:Topical
801:Tigason
780:History
655:Tigason
629:) is a
627:Tegison
529:354.490
497:Formula
350:4445538
330:DB00926
290:5282375
276:PubChem
182:>99%
121:)
115: (
113:D05BB01
85:a601010
1787:HX-711
1777:PA-452
1772:PA-451
1767:CD3254
1762:LE-540
1752:HX-603
1742:PA-452
1732:HX-630
1727:HX-531
1639:9CDHRA
1598:LE-135
1498:CD-437
1468:AM-580
1425:9CDHRA
1333:WHO-EM
1094:
1058:
1050:
907:
882:
837:Anvisa
769:cramps
562:SMILES
421:ChEMBL
390:D00316
148:
1782:Rhein
1655:trans
1568:TTNPB
1508:EC 23
1503:Ch-55
1461:trans
1216:Other
1092:S2CID
1056:S2CID
701:ethyl
582:InChI
538:JSmol
401:ChEBI
1653:all-
1459:all-
1155:Tars
1048:PMID
905:ISBN
880:ISBN
381:KEGG
361:UNII
310:7599
172:data
99:Oral
61:AHFS
1646:cis
1624:RXR
1432:cis
1410:RAR
1162:Tar
1140:D05
1084:doi
1040:doi
690:1/2
446:EPA
280:CID
118:WHO
1827::
1644:9-
1430:9-
1346::
1164:/
1090:.
1080:35
1078:.
1054:.
1046:.
1036:20
1034:.
1011:.
1000:^
984:.
966:.
948:.
919:^
864:^
803:.
669:,
657:.
511:30
505:23
242:,8
238:,6
234:,4
157:US
144:BR
1713:)
1709:(
1544:)
1540:(
1390:e
1383:t
1376:v
1251:)
1232:(
1142:)
1138:(
1128:e
1121:t
1114:v
1098:.
1086::
1062:.
1042::
994:.
970:.
952:.
913:.
888:.
858:.
735:.
687:t
685:(
540:)
517:3
514:O
508:H
502:C
448:)
444:(
244:E
240:E
236:E
232:E
194:Z
159::
146::
63:/
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.