112:
370:
214:
22:
233:. For steel production, this method uses an inert anode (Carbon, Platinum, Iridium or Chromium-based alloy) and places iron ore in the cathode. The electrochemical reaction in this Molten Oxide cell can reach up to 1600 °C, a temperature that melts iron ore and electrolyte oxide. Then the molten iron ore decompose following this reaction.
392:
In electrorefining, decarburization process happened in electrochemical cell that composed of inert electrode, slag and steel. During the process, current passing through the cell and made slag and steel melted. Oxygen ion from slag decompose and oxidize carbon on steel and to form CO. That
1058:
D.R., George C. Marshall Space Flight Center
Marshall Space Flight Center, AL 35812, National Aeronautics and Space Administration Washington, DC 20546-0001 Curreri, P.A. Ethridge, E.C. Hudson, S.B. Miller, T.Y. Grugel, R.N. Sen, S. Sadoway.
138:. Electrolysis can be used as a final refining stage in pyrometallurgical metal production (electrorefining) and it is also used for reduction of a metal from an aqueous metal salt solution produced by hydrometallurgy (electrowinning).
951:
is come from the slag, based on the reaction above, beside producing CO gas, this method also producing pure silicon (depending on the slag). The benefit of this direct decarburization process is it does not produce
943:
358:. Moreover, if the electricity to run such cells comes from renewable sources, this process may have zero emissions. This technology also can be implemented for producing Nickel, Chromium, and Ferrochromium.
647:
521:
346:
791:
678:
1084:
1088:
350:
The electrolysis reaction will produce molten pure iron as a main product and oxygen as its by-product. Because this process does not add coke in the process, no CO
134:
operations. The electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from aluminium oxide via the
377:
The purpose of this method is to reduce carbon content from steel. This process is suitable for secondary steelmaking industry which recycling steel
1062:
Process
Demonstration For Lunar In Situ Resource Utilization--Molten Oxide Electrolysis (MSFC Independent Research and Development Project No. 5-81)
1175:
Allanore, Antoine; Ortiz, Luis A; Sadoway, Donald R (2011-04-19), Neelameggham, Neale R.; Belt, Cynthia K.; Jolly, Mark; Reddy, Ramana G. (eds.),
32:
1196:
993:
126:
that uses electrical energy to produce metals by electrolysis. It is usually the last stage in metal production and is therefore preceded by
801:
527:
1034:
399:
1330:
172:
by electrodeposition is included in this category, or sometimes electrowinning, or a separate category depending on application.
90:
238:
62:
684:
381:
that has variety of carbon content in their feedstock. This method aim to replace current conventional method that utilizing
47:
69:
361:
Currently
Massachusetts-based Boston Metal company is in a process to scale up this technology to an industrial level.
135:
1320:
1177:"Molten Oxide Electrolysis for Iron Production: Identification of Key Process Parameters for Largescale Development"
147:
76:
382:
355:
58:
1325:
1264:
1115:
973:
385:(BOF) to reduce carbon content of iron by blowing oxygen to make it react with carbon and forming CO
111:
1296:
1176:
1157:
1078:
651:
393:
decarburizing reaction is occurred in three steps as follow. (ads) means adsorbed intermediate
83:
1288:
1280:
1192:
1149:
1141:
1066:
1040:
1030:
989:
169:
1029:. Djokić, Stojan S., Grgur, Branimir N., 1965-. New York: Kluwer Academic/Plenum Publishers.
1272:
1184:
1131:
1123:
981:
197:
187:
213:
165:
131:
1268:
1119:
369:
181:
175:
159:
127:
1314:
1300:
1252:
230:
1161:
222:
154:
226:
21:
1276:
1188:
985:
1103:
123:
1284:
1145:
1070:
1044:
39:
1292:
1153:
1024:
1136:
1127:
1104:"A new anode material for oxygen evolution in molten oxide electrolysis"
225:
in steelmaking is utilizing electrons as the reducing agent instead of
193:
184:, the manufacture of, usually thin, metal parts through electroplating
1060:
146:
Electrometallurgy is the field concerned with the processes of metal
1183:, Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 121–129,
938:{\displaystyle {\ce {C{}+1/2SiO2<=>CO_{(g)}{}+1/2Si_{(}l)}}}
378:
368:
110:
1251:
Judge, William D.; Paeng, Jaesuk; Azimi, Gisele (October 2022).
642:{\displaystyle {\ce {C(O_{(ads)}^{-})<=>C(O_{(ads)}^{})}}}
1220:
516:{\displaystyle {\ce {O2- + C <=> C(O^{-}_{(ads)}) +e-}}}
15:
1102:
Allanore, Antoine; Yin, Lan; Sadoway, Donald R. (May 2013).
1253:"Electrorefining for direct decarburization of molten iron"
796:
The total reaction from this cell is following this scheme
931:
895:
834:
779:
714:
631:
341:{\displaystyle {\ce {Fe2O3(l) + e- -> Fe(l) + O2(g)}}}
323:
266:
253:
786:{\displaystyle {\ce {C(O_{(ads)}^{})<=>CO_{(}g)}}}
43:
980:, John Wiley & Sons, Ltd, pp. 523–557, 2016,
903:
858:
814:
742:
587:
446:
804:
687:
654:
530:
402:
241:
190:, the removal of material from a metallic workpiece
178:, the deposition of a layer of one metal on another
956:but CO which is not considered as greenhouse gas.
937:
785:
672:
641:
515:
340:
150:. There are seven categories of these processes:
866:
865:
848:
847:
750:
749:
732:
731:
595:
594:
577:
576:
454:
453:
436:
435:
978:Physical Chemistry of Metallurgical Processes
8:
1083:: CS1 maint: multiple names: authors list (
1023:Popov, K. I. (Konstantin Ivanovich) (2002).
48:introducing citations to additional sources
1087:) CS1 maint: numeric names: authors list (
1135:
930:
918:
917:
898:
894:
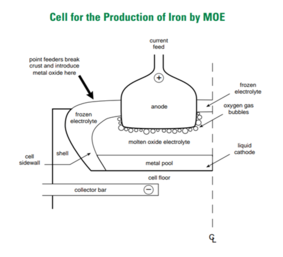
882:
881:
867:
860:
859:
857:
849:
842:
840:
839:
837:
833:
828:
809:
805:
803:
778:
766:
765:
751:
744:
743:
741:
733:
726:
724:
723:
721:
713:
701:
700:
692:
688:
686:
663:
655:
653:
630:
618:
617:
609:
596:
589:
588:
586:
578:
571:
569:
568:
566:
556:
544:
543:
535:
531:
529:
506:
489:
477:
476:
468:
455:
448:
447:
445:
437:
430:
428:
427:
425:
413:
408:
403:
401:
326:
322:
317:
299:
287:
269:
265:
260:
252:
247:
242:
240:
1026:Fundamental aspects of electrometallurgy
212:
38:Relevant discussion may be found on the
965:
841:
725:
570:
429:
1076:
365:Direct Decarburization Electrorefining
196:, industrially known to Knowledge as
7:
1246:
1244:
1242:
1240:
1215:
1213:
1018:
1016:
1014:
1012:
1010:
162:, the extraction of metal from ores
115:Anodized titanium (detail) - a.jpg
14:
1221:"Decarbonizing Steel Production"
31:relies largely or entirely on a
20:
373:Direct Decarburization Reaction
168:, the purification of metals.
926:
919:
889:
883:
868:
843:
774:
767:
752:
727:
717:
708:
702:
693:
634:
625:
619:
610:
597:
572:
562:
551:
545:
536:
495:
484:
478:
469:
456:
431:
354:gas is produced. So no direct
333:
327:
306:
300:
293:
276:
270:
1:
673:{\displaystyle {\ce {+ e-}}}
1347:
1277:10.1038/s41563-021-01106-z
1189:10.1002/9781118061886.ch12
986:10.1002/9781119078326.ch12
209:Molten Oxide Electrolysis
1331:Metallurgical processes
356:greenhouse gas emission
170:Metal powder production
1181:Energy Technology 2011
939:
787:
674:
643:
517:
374:
342:
218:
116:
940:
788:
675:
644:
518:
372:
343:
216:
114:
802:
685:
652:
528:
400:
383:Basic Oxygen Furnace
239:
136:Hall-Hérault process
44:improve this article
1269:2022NatMa..21.1130J
1128:10.1038/nature12134
1120:2013Natur.497..353A
974:"Electrometallurgy"
933:
897:
854:
836:
781:
738:
716:
633:
583:
561:
494:
442:
418:
325:
268:
255:
229:as in conventional
59:"Electrometallurgy"
1321:Chemical processes
935:
913:
912:
877:
873:
824:
823:
783:
761:
757:
696:
670:
639:
613:
602:
539:
513:
472:
461:
404:
375:
338:
313:
256:
243:
219:
132:hydrometallurgical
117:
1263:(10): 1130–1136.
1198:978-1-118-06188-6
1114:(7449): 353–356.
995:978-1-119-07832-6
925:
916:
911:
888:
880:
875:
827:
822:
808:
773:
764:
759:
707:
699:
691:
662:
624:
616:
608:
604:
550:
542:
534:
505:
483:
475:
467:
463:
424:
407:
332:
316:
305:
298:
286:
275:
259:
246:
148:electrodeposition
128:pyrometallurgical
120:Electrometallurgy
109:
108:
94:
1338:
1305:
1304:
1257:Nature Materials
1248:
1235:
1234:
1232:
1231:
1217:
1208:
1207:
1206:
1205:
1172:
1166:
1165:
1139:
1099:
1093:
1092:
1082:
1074:
1055:
1049:
1048:
1020:
1005:
1004:
1003:
1002:
970:
944:
942:
941:
936:
934:
932:
929:
923:
922:
914:
904:
899:
896:
893:
892:
886:
878:
876:
874:
872:
871:
864:
856:
855:
853:
846:
838:
835:
832:
825:
815:
810:
806:
792:
790:
789:
784:
782:
780:
777:
771:
770:
762:
760:
758:
756:
755:
748:
740:
739:
737:
730:
722:
720:
715:
712:
711:
705:
697:
689:
679:
677:
676:
671:
669:
668:
667:
660:
648:
646:
645:
640:
638:
637:
632:
629:
628:
622:
614:
606:
605:
603:
601:
600:
593:
585:
584:
582:
575:
567:
565:
560:
555:
554:
548:
540:
532:
522:
520:
519:
514:
512:
511:
510:
503:
498:
493:
488:
487:
481:
473:
465:
464:
462:
460:
459:
452:
444:
443:
441:
434:
426:
422:
417:
412:
405:
347:
345:
344:
339:
337:
336:
330:
324:
321:
314:
309:
303:
296:
292:
291:
284:
279:
273:
267:
264:
257:
254:
251:
244:
198:chemical milling
188:Electropolishing
104:
101:
95:
93:
52:
24:
16:
1346:
1345:
1341:
1340:
1339:
1337:
1336:
1335:
1311:
1310:
1309:
1308:
1250:
1249:
1238:
1229:
1227:
1219:
1218:
1211:
1203:
1201:
1199:
1174:
1173:
1169:
1101:
1100:
1096:
1075:
1057:
1056:
1052:
1037:
1022:
1021:
1008:
1000:
998:
996:
972:
971:
967:
962:
955:
950:
800:
799:
683:
682:
659:
650:
649:
526:
525:
502:
398:
397:
388:
367:
353:
283:
237:
236:
211:
206:
204:Research trends
166:Electrorefining
144:
122:is a method in
105:
99:
96:
53:
51:
37:
25:
12:
11:
5:
1344:
1342:
1334:
1333:
1328:
1323:
1313:
1312:
1307:
1306:
1236:
1209:
1197:
1167:
1094:
1050:
1035:
1006:
994:
964:
963:
961:
958:
953:
948:
928:
921:
910:
907:
902:
891:
885:
870:
863:
852:
845:
831:
821:
818:
813:
794:
793:
776:
769:
754:
747:
736:
729:
719:
710:
704:
695:
680:
666:
658:
636:
627:
621:
612:
599:
592:
581:
574:
564:
559:
553:
547:
538:
523:
509:
501:
497:
492:
486:
480:
471:
458:
451:
440:
433:
421:
416:
411:
386:
366:
363:
351:
335:
329:
320:
312:
308:
302:
295:
290:
282:
278:
272:
263:
250:
210:
207:
205:
202:
201:
200:
191:
185:
182:Electroforming
179:
176:Electroplating
173:
163:
160:Electrowinning
157:
143:
140:
107:
106:
42:. Please help
28:
26:
19:
13:
10:
9:
6:
4:
3:
2:
1343:
1332:
1329:
1327:
1324:
1322:
1319:
1318:
1316:
1302:
1298:
1294:
1290:
1286:
1282:
1278:
1274:
1270:
1266:
1262:
1258:
1254:
1247:
1245:
1243:
1241:
1237:
1226:
1222:
1216:
1214:
1210:
1200:
1194:
1190:
1186:
1182:
1178:
1171:
1168:
1163:
1159:
1155:
1151:
1147:
1143:
1138:
1133:
1129:
1125:
1121:
1117:
1113:
1109:
1105:
1098:
1095:
1090:
1086:
1080:
1072:
1068:
1064:
1063:
1054:
1051:
1046:
1042:
1038:
1036:0-306-47564-2
1032:
1028:
1027:
1019:
1017:
1015:
1013:
1011:
1007:
997:
991:
987:
983:
979:
975:
969:
966:
959:
957:
945:
908:
905:
900:
861:
850:
829:
819:
816:
811:
797:
745:
734:
681:
664:
656:
590:
579:
557:
524:
507:
499:
490:
449:
438:
419:
414:
409:
396:
395:
394:
390:
384:
380:
371:
364:
362:
359:
357:
348:
318:
310:
288:
280:
261:
248:
234:
232:
231:blast furnace
228:
224:
221:Molten Oxide
215:
208:
203:
199:
195:
192:
189:
186:
183:
180:
177:
174:
171:
167:
164:
161:
158:
156:
153:
152:
151:
149:
141:
139:
137:
133:
129:
125:
121:
113:
103:
100:February 2019
92:
89:
85:
82:
78:
75:
71:
68:
64:
61: –
60:
56:
55:Find sources:
49:
45:
41:
35:
34:
33:single source
29:This article
27:
23:
18:
17:
1326:Electrolysis
1260:
1256:
1228:. Retrieved
1225:Boston Metal
1224:
1202:, retrieved
1180:
1170:
1137:1721.1/82073
1111:
1107:
1097:
1061:
1053:
1025:
999:, retrieved
977:
968:
946:
798:
795:
391:
376:
360:
349:
235:
223:Electrolysis
220:
155:Electrolysis
145:
119:
118:
97:
87:
80:
73:
66:
54:
30:
217:MOE scheme
1315:Categories
1230:2022-11-22
1204:2022-11-22
1001:2020-09-24
960:References
124:metallurgy
70:newspapers
1301:237947963
1285:1476-4660
1146:1476-4687
1079:cite book
1071:703646739
869:⇀
862:−
851:−
844:↽
753:⇀
746:−
735:−
728:↽
665:−
598:⇀
591:−
580:−
573:↽
558:−
508:−
491:−
457:⇀
450:−
439:−
432:↽
415:−
294:⟶
289:−
142:Processes
40:talk page
1293:34580434
1154:23657254
1045:51893969
1265:Bibcode
1162:4379353
1116:Bibcode
947:The SiO
194:Etching
84:scholar
1299:
1291:
1283:
1195:
1160:
1152:
1144:
1108:Nature
1069:
1043:
1033:
992:
86:
79:
72:
65:
57:
1297:S2CID
1158:S2CID
379:scrap
91:JSTOR
77:books
1289:PMID
1281:ISSN
1193:ISBN
1150:PMID
1142:ISSN
1089:link
1085:link
1067:OCLC
1041:OCLC
1031:ISBN
990:ISBN
227:coke
63:news
1273:doi
1185:doi
1132:hdl
1124:doi
1112:497
982:doi
826:SiO
706:ads
623:ads
549:ads
482:ads
130:or
46:by
1317::
1295:.
1287:.
1279:.
1271:.
1261:21
1259:.
1255:.
1239:^
1223:.
1212:^
1191:,
1179:,
1156:.
1148:.
1140:.
1130:.
1122:.
1110:.
1106:.
1081:}}
1077:{{
1065:.
1039:.
1009:^
988:,
976:,
952:CO
915:Si
879:CO
763:CO
389:.
297:Fe
245:Fe
1303:.
1275::
1267::
1233:.
1187::
1164:.
1134::
1126::
1118::
1091:)
1073:.
1047:.
984::
954:2
949:2
927:)
924:l
920:(
909:2
906:1
901:+
890:)
887:g
884:(
830:2
820:2
817:1
812:+
807:C
775:)
772:g
768:(
718:)
709:)
703:(
698:O
694:(
690:C
661:e
657:+
635:)
626:)
620:(
615:O
611:(
607:C
563:)
552:)
546:(
541:O
537:(
533:C
504:e
500:+
496:)
485:)
479:(
474:O
470:(
466:C
423:C
420:+
410:2
406:O
387:2
352:2
334:)
331:g
328:(
319:2
315:O
311:+
307:)
304:l
301:(
285:e
281:+
277:)
274:l
271:(
262:3
258:O
249:2
102:)
98:(
88:·
81:·
74:·
67:·
50:.
36:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.