501:
478:
5463:
921:
naturally occurring soluble TNF receptors, the difference being that etanercept, because it is a fusion protein rather than a simple TNF receptor, has a greatly extended half-life in the bloodstream, and therefore a more profound and long-lasting biologic effect than a naturally occurring soluble TNF receptor.
989:
Before the extension it seemed unlikely that a generic would have been available. As a biologic, etanercept is subject to different laws from those applicable to chemical formulations. Many countries do not permit the manufacture of generic biologics. However, the
European Union and the United States
942:
on
September 5, 1989. The fusion protein was developed by Bruce A. Beutler, an academic researcher then at the University of Texas Southwestern Medical Center at Dallas, and colleagues, who patented it and licensed the rights in 1995 to Immunex. Another patent on such fusion protein technology from
920:
TNF receptors that are used to deactivate TNF and blunt the immune response. In addition, TNF receptors are found on the surface of virtually all nucleated cells (red blood cells, which are not nucleated, do not contain TNF receptors on their surface). Etanercept mimics the inhibitory effects of
929:
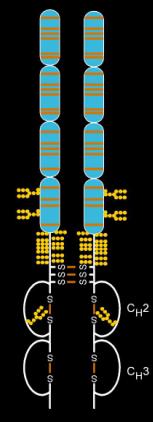
Etanercept is made from the combination of two naturally occurring soluble human 75-kilodalton TNF receptors linked to an Fc portion of an IgG1. The effect is an artificially engineered dimeric fusion protein. Etanercept is a complex molecule containing 6 N-glycans, up to 14 O-glycans and 29
966:
The US retail price of etanercept has risen over time. In 2008, the cost of etanercept was $ 1,500 per month or $ 18,000 per year. By 2011, the cost had exceeded $ 20,000 per year. In 2013, a survey by the
International Federation of Health Plans (IFHP) found that the average US cost for
2295:
Houel S, Hilliard M, Yu YQ, McLoughlin N, Martin SM, Rudd PM, et al. (January 2014). "N- and O-glycosylation analysis of etanercept using liquid chromatography and quadrupole time-of-flight mass spectrometry equipped with electron-transfer dissociation functionality".
2021:
970:
Etanercept is more expensive in the US than in other countries. As of 2013, average monthly costs in surveyed nations ranged from $ 1,017 in
Switzerland to $ 1,646 in Canada, compared to an average monthly cost of $ 2,225 per month in the US.
967:
etanercept was $ 2,225 per month, or $ 26,700 per year. The IFHP report also found wide variation in prices charged to various US health plans, between $ 1,946 per month at the 25th percentile and $ 4,006 per month at the 95th percentile.
621:(TNFα) is the "master regulator" of the inflammatory (immune) response in many organ systems. Autoimmune diseases are caused by an overactive immune response. Etanercept has the potential to treat these diseases by inhibiting TNF-alpha.
2496:
2723:
2599:
644:(IgG1). Third, they linked the DNA for TNF receptor 2 to the DNA for IgG1 Fc. Finally, they expressed the linked DNA to produce a protein that links the protein for TNF receptor 2 to the protein for IgG1 Fc.
2532:
The story behind the production of Enbrel, Amgen's popular rheumatoid arthritis drug, provides insights as to why bioengineered drugs are so expensive." Carol M. Ostrom, Seattle Times, August 18, 2008
901:
and through additional molecular mechanisms that initiate and amplify inflammation. Inhibition of its action by etanercept reduces the inflammatory response, which is especially useful for treating
2230:
Madhusudan S, Muthuramalingam SR, Braybrooke JP, Wilner S, Kaur K, Han C, et al. (September 2005). "Study of etanercept, a tumor necrosis factor-alpha inhibitor, in recurrent ovarian cancer".
1829:
1799:
219:
1318:
1210:
1141:
4608:
656:
978:, Inc. sells the drug outside of the US and Canada. Sales within the US and Canada were $ 3.5 billion in 2010. Sales of etanercept outside the US and Canada were $ 3.3 billion in 2010.
2508:
991:
5518:
4392:
174:
1054:
824:
said that it was "unethical for physicians to practice outside of their area of competence and expertise". Tobinick sued
Novella in response, and lost. Of this treatment, the
1546:
Feldmann M, Maini RN (October 2003). "Lasker
Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases".
2812:
986:
The patent on etanercept was set to expire on
October 23, 2012, but, in the United States, a second patent, granting exclusivity for another 16 years, has been granted.
4624:
2606:
2077:
5086:
2730:
2268:
Smola MG, Soyer HP, Scharnagl E (October 1991). "Surgical treatment of dermatofibrosarcoma protuberans. A retrospective study of 20 cases with review of literature".
2102:
5523:
4552:
1082:
4674:
72:
5106:
2950:
632:. It fuses the TNF receptor to the constant end of the IgG1 antibody. First, the developers isolated the DNA sequence that codes the human gene for soluble
4734:
4385:
2331:
Mukai Y, Nakamura T, Yoshikawa M, Yoshioka Y, Tsunoda S, Nakagawa S, et al. (November 2010). "Solution of the structure of the TNF-TNFR2 complex".
1005:
made an announcement about launching the first biosimilar of
Etanercept in India under the brand name Etacept for the treatment of rheumatic disorders.
828:
advise "there is insufficient evidence to determine its effectiveness and that the treatment may be associated with adverse outcomes and high cost".
5077:
5066:
5042:
4532:
3866:
1731:"Improvement in patient-reported outcomes for patients with ankylosing spondylitis treated with etanercept 50 mg once-weekly and 25 mg twice-weekly"
1328:
1221:
1152:
4421:
2035:
1511:
Text was copied from this source which is copyright
European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
4568:
4378:
2575:
702:
5513:
4700:
2548:
636:, which is a receptor that binds to tumor necrosis factor-alpha. Second, they isolated the DNA sequence that codes the human gene for the
647:
The prototypic fusion protein was first synthesized and shown to be highly active and unusually stable as a modality for blockade of TNF
2677:
5508:
4432:
1589:
5503:
5386:
3809:
3179:
2652:
2865:
2943:
2908:
2482:
1704:
1215:
1146:
1443:
4218:
2191:"Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection"
204:
104:
40:
677:, that binds to TNFα and decreases its role in disorders involving excess inflammation in humans and other animals, including
5097:
4799:
2467:
944:
637:
2081:
1965:
5493:
2142:"A Systematic Review and Meta-Analysis of Injection Site Reactions in Randomized-Controlled Trials of Biologic Injections"
2115:
1994:
825:
729:
686:
602:
5453:
2879:
1652:
Peppel K, Poltorak A, Melhado I, Jirik F, Beutler B (November 1993). "Expression of a TNF inhibitor in transgenic mice".
1022:
In
February 2017, Lifmior was approved for use in the European Union. It was withdrawn from the market in February 2020.
5401:
3015:
2502:
2111:
1972:
1893:
1863:
1836:
1806:
837:
808:
An American physician, Edward Tobinick, has attempted to use etanercept to treat chronic neurological dysfunction after
594:
397:
2699:
1886:
5498:
3124:
2936:
618:
457:
123:
5391:
5033:
2994:
1859:
1605:"A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity"
4992:
4462:
3672:
2841:
2788:
2759:
1770:
1496:
4052:
578:
2631:
67:
etanercept-szzs, etanercept-ykro, Benepali, Brenzys, Erelzi, Etacept, Etera, Eticovo, Lifmior, Nepexto, Rymti
5483:
4745:
4441:
4047:
2019:, Tobinick EL, "Methods for treatment of brain injury utilizing biologics", published 2014-12-02
909:
859:
766:
160:
4457:
496:
5130:
3852:
3278:
2959:
1521:
1246:
817:
741:
682:
614:
446:
167:
1351:"Benepali 25 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC)"
1290:"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)"
4942:
4937:
4888:
4039:
2572:
714:
586:
4527:
4542:
2016:
844:
on etanercept due to a number of serious infections associated with the drug. Serious infections and
5057:
4893:
4598:
4492:
2928:
2387:
1289:
722:
698:
598:
297:
5300:
5008:
4898:
4790:
4507:
4502:
4497:
4487:
3260:
1915:
Maxwell LJ, Zochling J, Boonen A, Singh JA, Veras MM, Tanjong Ghogomu E, et al. (April 2015).
1403:"Erelzi 50 mg solution for injection in pre filled pen - Summary of Product Characteristics (SmPC)"
849:
735:
694:
678:
606:
473:
350:
315:
4761:
4619:
4588:
1830:"Approval of Etanercept for treatment of polyarticular course juvenile rheumatoid arthritis (JRA)"
1377:"Enbrel 25mg solution for injection in pre-filled pen - Summary of Product Characteristics (SmPC)"
4922:
4917:
4883:
4878:
4824:
4639:
4412:
4404:
4209:
3310:
2356:
1677:
1571:
902:
582:
134:
5411:
4715:
4705:
4695:
4629:
4578:
4249:
4107:
1110:
848:, including fatalities, have been reported with the use of etanercept including reactivation of
5340:
5330:
5320:
5257:
4982:
4685:
4259:
3945:
3386:
2529:
5350:
5335:
5252:
5217:
4962:
4467:
4401:
4214:
4204:
3005:
2963:
2813:"Public statement on Lifmior: Withdrawal of the marketing authorisation in the European Union"
2448:
2413:
2348:
2313:
2277:
2247:
2212:
2171:
2060:
1946:
1752:
1669:
1634:
1563:
841:
641:
244:
231:
52:
5277:
4452:
2754:
2700:"The Future of Generic Biologics: Should the United States "Follow-On" the European Pathway?"
5003:
4932:
4927:
4815:
4472:
4010:
3913:
3576:
3472:
2783:
2483:"Lucrative Licensing Deals With Drug, Biotech Firms Are Raising Ethics Issues For Hospitals"
2440:
2431:
Norman P (January 2017). "Enbrel and etanercept biosimilars: a tale of two patent systems".
2403:
2395:
2340:
2305:
2239:
2202:
2189:
Zalevsky J, Secher T, Ezhevsky SA, Janot L, Steed PM, O'Brien C, et al. (August 2007).
2161:
2153:
1936:
1928:
1778:
1742:
1661:
1624:
1616:
1555:
1179:
894:
748:
652:
513:
324:
279:
4911:
2836:
2374:
Lamanna WC, Mayer RE, Rupprechter A, Fuchs M, Higel F, Fritsch C, et al. (June 2017).
1491:
406:
5467:
5381:
5358:
4903:
4820:
4583:
4309:
4194:
701:, and, potentially, in a variety of other disorders mediated by excess TNFα. It is on the
629:
287:
897:. It mediates the immune response by attracting additional white blood cells to sites of
2656:
2391:
2166:
2141:
1941:
1916:
500:
477:
5488:
5242:
5232:
5159:
4776:
4766:
4690:
4522:
4199:
4067:
3715:
3549:
3528:
3524:
3520:
3148:
2985:
2468:"A three-decade monopoly: How Amgen built a patent thicket around its top-selling drug"
2408:
2375:
1629:
1604:
875:
821:
775:
625:
1708:
1402:
1376:
5477:
4810:
4593:
4512:
4349:
4279:
4189:
4096:
3882:
3582:
3504:
3456:
3436:
3294:
3210:
3061:
3027:
2579:
2078:"Wyeth and Amgen heighten warning of life-threatening infections on skin drug Enbrel"
1350:
1323:
1087:
1059:
1028:
In March 2019, YLB113 (Etanercept biosimilar by YL Biologics) was approved in Japan.
871:
771:
590:
489:
257:
96:
2903:
2360:
1681:
1575:
5416:
5396:
5315:
5305:
5247:
5194:
5124:
5023:
4967:
4654:
4644:
4634:
4537:
4517:
4482:
4477:
4359:
4319:
4304:
4299:
4015:
3965:
3960:
3940:
3820:
3815:
3678:
3663:
3611:
3587:
3534:
3411:
3401:
3220:
3137:
3132:
3090:
3043:
3000:
2967:
2573:"Co-pay hike a painful reality; Miracle drug monthly cost jumps from $ 42 to $ 600"
1932:
912:: those found embedded in white blood cells that respond to TNF by releasing other
898:
813:
187:
182:
1747:
1730:
1263:
82:
1665:
5426:
5421:
5325:
5310:
5282:
5272:
5267:
5222:
5189:
5184:
5179:
5174:
5169:
5154:
5149:
4972:
4957:
4952:
4771:
4756:
4573:
4354:
4294:
4284:
4274:
4174:
4164:
4154:
4149:
4114:
4025:
4020:
3995:
3985:
3975:
3950:
3935:
3930:
3888:
3858:
3844:
3839:
3825:
3768:
3726:
3721:
3707:
3702:
3688:
3668:
3621:
3597:
3555:
3539:
3510:
3494:
3462:
3446:
3441:
3416:
3366:
3356:
3351:
3346:
3335:
3325:
3230:
3142:
3100:
3085:
3079:
3075:
3022:
2207:
2190:
1014:
995:
862:
such as redness and pain are common, occurring in approximately 11.4% of cases.
853:
662:
These investigators also patented the protein, selling all rights to its use to
90:
4370:
2399:
1031:
In April 2019, Eticovo (marketed as Benepali in Europe) received FDA approval.
5262:
5164:
5018:
4563:
4344:
4339:
4324:
4264:
4254:
4239:
4234:
4229:
4179:
4169:
4159:
4124:
4088:
4000:
3990:
3980:
3970:
3955:
3898:
3893:
3872:
3787:
3782:
3740:
3734:
3635:
3592:
3488:
3478:
3431:
3421:
3396:
3391:
3381:
3376:
3371:
3361:
3320:
3305:
3300:
3284:
3225:
3205:
3154:
3114:
3095:
3037:
2344:
2157:
890:
886:
552:
381:
62:
2036:"Another Free Speech Win In Libel Lawsuit Disguised As A Trademark Complaint"
1966:"Approval of Etanercept for treatment of moderate to severe plaque psoriasis"
5436:
5431:
5212:
4659:
4334:
4329:
4314:
4289:
4244:
4224:
4184:
4119:
4083:
4005:
3801:
3754:
3683:
3649:
3616:
3426:
3406:
3315:
3215:
3055:
2243:
1037:
Rymti and Etera were approved for medical use in Australia in October 2020.
690:
610:
76:
2452:
2417:
2352:
2317:
2251:
2216:
2175:
1950:
1775:
World Health Organization model list of essential medicines: 21st list 2019
1756:
1620:
1567:
31:
2444:
2281:
1673:
1638:
4664:
4269:
4144:
3833:
3341:
3251:
3187:
3109:
2530:"What's behind the whopping price tags on the newest generation of drugs:
913:
882:
361:
118:
1783:
953:
Etanercept was approved for use in the European Union in February 2000.
370:
3762:
950:
Etanercept was approved for use in the United States in November 1998.
939:
870:
Etanercept reduces the effect of naturally present TNF, and hence is a
663:
335:
2376:"The structure-function relationship of disulfide bonds in etanercept"
2309:
1019:
In January 2016, Benepali was approved for use in the European Union.
17:
5237:
5227:
4872:
4868:
4864:
4860:
4856:
4852:
4077:
3908:
975:
845:
809:
437:
2497:"Etanercept Product Approval Information - Licensing Action 12/2/98"
998:) which "requires mandatory clinical testing and periodic review".
426:
4907:
4848:
4844:
4840:
4836:
4832:
4828:
4725:
3776:
3629:
1559:
1002:
667:
633:
39:
1034:
In May 2020, Nepexto was approved for use in the European Union.
1025:
In June 2017, Erelzi was approved for use in the European Union.
5292:
5204:
5141:
3748:
3696:
3657:
3643:
3197:
3071:
1887:"Approval of Etanercept for treatment of ankylosing spondylitis"
1705:"Arthritis Drug Effective for Depression in Psoriasis Sufferers"
417:
266:
238:
4374:
2932:
251:
113:
3605:
2104:
Safety Update on TNF- α Antagonists: Infliximab and Etanercept
1800:"Approval of Etanercept for treatment of rheumatoid arthritis"
1297:
674:
1995:"New hope for survivors of stroke and traumatic brain injury"
1860:"Approval of Etanercept for treatment of psoriatic arthritis"
2678:"New Amgen Enbrel patent could block biosimilars until 2028"
1729:
Braun J, McHugh N, Singh A, Wajdula JS, Sato R (June 2007).
462:
213:
145:
2605:. International Federation of Health Plans. Archived from
994:) do have in place a system to approve generic biologics (
799:
chronic severe plaque psoriasis pediatric plaque psoriasis
2549:"Amgen's New Enbrel Patent May Undercut Health Care Plan"
2061:"Practice Advisory: Etanercept for Poststroke Disability"
755:
In the European Union, etanercept is indicated to treat:
657:
University of Texas Southwestern Medical Center at Dallas
1787:. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
1055:"Health Canada New Drug Authorizations: 2016 Highlights"
226:
4533:
Methoxy polyethylene glycol-epoetin beta (CERA/Mircera)
974:
Amgen sells etanercept within the US and Canada, while
774:(rheumatoid-factor-positive or -negative) and extended
703:
World Health Organization's List of Essential Medicines
673:
It is a large molecule, with a molecular weight of 150
666:, a Seattle biotechnology company that was acquired by
589:(TNF), a soluble inflammatory cytokine, by acting as a
992:
Biologics Price Competition and Innovation Act of 2009
5451:
2866:"Japanese approval for Lupin's etanercept biosimilar"
1444:"Enbrel- etanercept solution Enbrel- etanercept kit"
1247:"Australian Public Assessment Report for Etanercept"
1241:
1239:
5371:
5349:
5291:
5203:
5140:
5123:
5116:
5096:
5076:
5056:
5032:
4991:
4798:
4789:
4744:
4724:
4673:
4607:
4551:
4440:
4431:
4411:
4137:
4065:
4038:
3923:
3566:
3268:
3259:
3250:
3243:
3196:
3178:
3169:
3123:
3054:
2984:
2975:
1438:
1436:
1434:
1432:
1430:
1428:
1426:
1424:
762:
severe, active and progressive rheumatoid arthritis
551:
512:
507:
488:
456:
436:
416:
396:
380:
360:
334:
314:
309:
296:
286:
278:
203:
198:
173:
159:
133:
103:
89:
71:
61:
51:
46:
1917:"TNF-alpha inhibitors for ankylosing spondylitis"
1603:Peppel K, Crawford D, Beutler B (December 1991).
938:The first etanercept-related patent was filed by
816:and issued U.S. and foreign patents. Writing for
4625:Granulocyte macrophage colony-stimulating factor
2582:, Margery Eagan, Boston Herald, November 3, 2011
349:
2542:
2540:
2538:
1486:
1484:
793:severe non-radiographic axial spondyloarthritis
323:
2263:
2261:
1482:
1480:
1478:
1476:
1474:
1472:
1470:
1468:
1466:
1464:
1174:
1172:
1170:
759:moderate to severe active rheumatoid arthritis
5519:World Health Organization essential medicines
4386:
2944:
2066:. American Academy of Neurology. 6 June 2016.
8:
2880:"FDA approves etanercept biosimilar Eticovo"
1205:
1203:
1201:
1136:
1134:
1132:
122:
30:
2594:
2592:
2590:
2588:
1921:The Cochrane Database of Systematic Reviews
5392:Leukemia/leukocyte inhibitory factor (LIF)
5137:
5120:
4795:
4437:
4393:
4379:
4371:
3265:
3256:
3247:
3175:
2981:
2951:
2937:
2929:
2140:Kim PJ, Lansang RP, Vender R (July 2023).
781:active and progressive psoriatic arthritis
499:
476:
5387:FMS-like tyrosine kinase 3 ligand (FLT3L)
4816:Interferon alpha (interferon alfa, IFN-α)
2653:"Patent Terms Extended Under 35 USC §156"
2407:
2206:
2165:
2146:Journal of Cutaneous Medicine and Surgery
1940:
1782:
1746:
1628:
405:
27:Biopharmaceutical for autoimmune diseases
5409:Additional cytokine receptor modulators:
1111:"Regulatory Decision Summary for Erelzi"
881:Tumor necrosis factor-alpha (TNFα) is a
5458:
2632:"Portions of the 2010 Financial Report"
1046:
1001:In April 2013, the Indian pharma major
472:
369:
95:
5233:Cucurbitacin I (elatericin B, JSI-124)
490:
29:
5524:Disease-modifying antirheumatic drugs
4569:Granulocyte colony-stimulating factor
2270:European Journal of Surgical Oncology
2114:(FDA). pp. 13–14. Archived from
1777:. Geneva: World Health Organization.
655:, an academic researcher then at the
445:
81:
7:
4928:Interferon kappa (IFN-ε/κ/τ/ζ, IFNK)
4701:Macrophage colony-stimulating factor
1609:The Journal of Experimental Medicine
1526:Union Register of medicinal products
790:severe active ankylosing spondylitis
713:In the United States, etanercept is
340:
186:
5402:Thymic stromal lymphopoietin (TSLP)
1997:. Springer Select. October 31, 2012
796:moderate to severe plaque psoriasis
425:
947:was licensed to Immmunex in 1997.
25:
1327:. 23 October 2014. Archived from
1319:"Rymti Summary Basis of Decision"
1264:"Etanercept Use During Pregnancy"
728:Moderate to Severe Polyarticular
5461:
2909:Therapeutic Goods Administration
1216:Therapeutic Goods Administration
1147:Therapeutic Goods Administration
530:
524:
38:
4933:Interferon omega (IFN-ω, IFNW1)
2600:"2013 Comparative Price Report"
1971:. Letter to Douglas Hunt. U.S.
1892:. Letter to Douglas Hunt. U.S.
1590:"Drugs@FDA: FDA-Approved Drugs"
1115:Drug and Health Products Portal
2547:Pollock A (23 November 2011).
2507:. 1 April 2016. Archived from
2481:Kowalczyk L (March 24, 2002).
2466:Gardner J (November 1, 2021).
1933:10.1002/14651858.CD005468.pub2
1862:. Letter to Sally Gould. U.S.
1835:. Letter to Sally Gould. U.S.
1805:. Letter to Sally Gould. U.S.
1798:Siegel YP (November 2, 1998).
945:Massachusetts General Hospital
542:
536:
518:
1:
2433:Pharmaceutical Patent Analyst
1858:Weiss KD (January 15, 2002).
1694:U.S. Patent number: 5,447,851
1522:"Nepexto Product information"
930:disulfide bridge structures.
826:American Academy of Neurology
730:Juvenile Rheumatoid Arthritis
687:juvenile rheumatoid arthritis
603:juvenile idiopathic arthritis
3016:dihydroorotate dehydrogenase
2503:Food and Drug Administration
2232:Journal of Clinical Oncology
2112:Food and Drug Administration
1973:Food and Drug Administration
1894:Food and Drug Administration
1864:Food and Drug Administration
1837:Food and Drug Administration
1807:Food and Drug Administration
1666:10.4049/jimmunol.151.10.5699
838:Food and Drug Administration
784:enthesitis-related arthritis
595:Food and Drug Administration
573:, sold under the brand name
5514:Drugs developed by Novartis
4777:Thrombopoietin (THPO, MGDF)
4543:Pegol sihematide (EPO-018B)
4463:Carbamylated erythropoietin
2995:purine synthesis inhibitors
2729:. Cipla.com. Archived from
2208:10.4049/jimmunol.179.3.1872
1964:Walton M (April 30, 2004).
1748:10.1093/rheumatology/kem069
908:There are two types of TNF
778:in children and adolescents
619:Tumor necrosis factor alpha
5540:
2400:10.1038/s41598-017-04320-5
1885:Keegan P (July 24, 2003).
1012:
508:Chemical and physical data
5509:Drugs developed by Pfizer
5326:Tofacitinib (tasocitinib)
5283:Tofacitinib (tasocitinib)
5190:Tofacitinib (tasocitinib)
3180:IL-1 receptor antagonists
2842:European Medicines Agency
2789:European Medicines Agency
2760:European Medicines Agency
2345:10.1126/scisignal.2000954
2158:10.1177/12034754231188444
1828:Weiss KD (May 27, 1999).
1771:World Health Organization
1497:European Medicines Agency
1184:European Medicines Agency
37:
5504:Drugs developed by Wyeth
5004:Interferon gamma (IFN-γ)
4053:Anti-lymphocyte globulin
860:Injection site reactions
597:(FDA) approval to treat
579:biologic medical product
4904:Interferon beta (IFN-β)
4048:Anti-thymocyte globulin
2960:Immunosuppressive drugs
2244:10.1200/JCO.2005.04.127
836:On May 2, 2008, the US
787:axial spondyloarthritis
234: / Schedule D
5382:Cardiotrophin 1 (CT-1)
3853:Interleukin-6 receptor
3279:Complement component 5
1621:10.1084/jem.174.6.1483
818:Science-Based Medicine
742:Ankylosing Spondylitis
683:ankylosing spondylitis
659:, and his colleagues.
651:in the early 1990s by
615:ankylosing spondylitis
581:that is used to treat
5379:Additional cytokines:
5306:Decernotinib (VX-509)
4943:Peginterferon alfa-2b
4938:Peginterferon alfa-2a
4458:Asialo erythropoietin
2445:10.4155/ppa-2016-0043
2195:Journal of Immunology
1654:Journal of Immunology
587:tumor necrosis factor
5494:Recombinant proteins
4894:Interferon alfacon-1
4523:Erythropoietin (EPO)
2904:"AusPAR: Etanercept"
2298:Analytical Chemistry
2121:on 24 September 2015
767:idiopathic arthritis
723:Rheumatoid Arthritis
699:rheumatoid arthritis
599:rheumatoid arthritis
585:by interfering with
5041:See IL-28R (IFNLR)
5009:Interferon gamma 1b
4899:Interferon alpha-n3
2846:. 17 September 2018
2793:. 17 September 2018
2764:. 17 September 2018
2392:2017NatSR...7.3951L
1501:. 17 September 2018
957:Society and culture
903:autoimmune diseases
878:that binds to TNF.
874:, functioning as a
866:Mechanism of action
850:latent tuberculosis
747:Moderate to Severe
736:Psoriatic Arthritis
721:Moderate to Severe
695:psoriatic arthritis
679:autoimmune diseases
607:psoriatic arthritis
583:autoimmune diseases
577:among others, is a
247:(Prescription only)
222:(Prescription only)
34:
5499:Immunosuppressants
5397:Oncostatin M (OSM)
4923:Interferon beta 1b
4918:Interferon beta 1a
4889:Interferon alfa n1
4884:Interferon alfa 2b
4879:Interferon alfa 2a
4713:Kinase inhibitors:
4210:Diroximel fumarate
3883:IL-2 receptor/CD25
3311:Certolizumab pegol
2964:Immunosuppressants
2913:. 25 February 2021
2884:www.gabionline.net
2612:on 22 October 2017
2553:The New York Times
2511:on 18 January 2017
2380:Scientific Reports
5449:
5448:
5445:
5444:
5367:
5366:
5134:
5052:
5051:
4785:
4784:
4528:Erythropoietin-Fc
4402:Cytokine receptor
4368:
4367:
4215:Efgartigimod alfa
4205:Dimethyl fumarate
4133:
4132:
4061:
4060:
4034:
4033:
3239:
3238:
3165:
3164:
3006:Mycophenolic acid
2698:Kaldre I (2008).
2333:Science Signaling
2310:10.1021/ac402726h
2238:(25): 5950–5959.
1660:(10): 5699–5703.
1554:(10): 1245–1250.
1357:. 25 January 2021
1270:. 24 January 2020
895:white blood cells
842:black box warning
804:Unrecognized uses
642:immunoglobulin G1
568:
567:
458:CompTox Dashboard
270:
255:
242:
229:
217:
149:
116:
16:(Redirected from
5531:
5466:
5465:
5464:
5457:
5138:
5128:
5121:
4980:Decoy receptors:
4796:
4473:Darbepoetin alfa
4438:
4395:
4388:
4381:
4372:
4011:Telimomab aritox
3914:Zolimomab aritox
3735:CD62L/L-selectin
3473:Immunoglobulin E
3266:
3257:
3248:
3176:
2982:
2953:
2946:
2939:
2930:
2923:
2922:
2920:
2918:
2900:
2894:
2893:
2891:
2890:
2876:
2870:
2869:
2862:
2856:
2855:
2853:
2851:
2833:
2827:
2826:
2824:
2822:
2817:
2809:
2803:
2802:
2800:
2798:
2780:
2774:
2773:
2771:
2769:
2751:
2745:
2744:
2742:
2741:
2735:
2728:
2720:
2714:
2713:
2711:
2710:
2704:www.law.duke.edu
2695:
2689:
2688:
2686:
2685:
2674:
2668:
2667:
2665:
2664:
2655:. Archived from
2649:
2643:
2642:
2640:
2639:
2628:
2622:
2621:
2619:
2617:
2611:
2604:
2596:
2583:
2570:
2564:
2563:
2561:
2559:
2544:
2533:
2527:
2521:
2520:
2518:
2516:
2493:
2487:
2486:
2478:
2472:
2471:
2463:
2457:
2456:
2428:
2422:
2421:
2411:
2371:
2365:
2364:
2328:
2322:
2321:
2292:
2286:
2285:
2265:
2256:
2255:
2227:
2221:
2220:
2210:
2201:(3): 1872–1883.
2186:
2180:
2179:
2169:
2137:
2131:
2130:
2128:
2126:
2120:
2109:
2099:
2093:
2092:
2090:
2089:
2080:. Archived from
2074:
2068:
2067:
2065:
2057:
2051:
2050:
2048:
2047:
2032:
2026:
2025:
2024:
2020:
2013:
2007:
2006:
2004:
2002:
1991:
1985:
1984:
1982:
1980:
1970:
1961:
1955:
1954:
1944:
1912:
1906:
1905:
1903:
1901:
1891:
1882:
1876:
1875:
1873:
1871:
1855:
1849:
1848:
1846:
1844:
1834:
1825:
1819:
1818:
1816:
1814:
1804:
1795:
1789:
1788:
1786:
1767:
1761:
1760:
1750:
1726:
1720:
1719:
1717:
1716:
1707:. Archived from
1701:
1695:
1692:
1686:
1685:
1649:
1643:
1642:
1632:
1615:(6): 1483–1489.
1600:
1594:
1593:
1586:
1580:
1579:
1543:
1537:
1536:
1534:
1532:
1518:
1512:
1510:
1508:
1506:
1488:
1459:
1458:
1456:
1454:
1440:
1419:
1418:
1416:
1414:
1399:
1393:
1392:
1390:
1388:
1373:
1367:
1366:
1364:
1362:
1347:
1341:
1340:
1338:
1336:
1315:
1309:
1308:
1306:
1304:
1294:nctr-crs.fda.gov
1286:
1280:
1279:
1277:
1275:
1260:
1254:
1253:
1251:
1243:
1234:
1233:
1231:
1229:
1220:. Archived from
1207:
1196:
1195:
1193:
1191:
1176:
1165:
1164:
1162:
1160:
1151:. Archived from
1138:
1127:
1126:
1124:
1122:
1107:
1101:
1100:
1098:
1096:
1079:
1073:
1072:
1070:
1068:
1051:
749:Plaque Psoriasis
744:(AS) (July 2003)
653:Bruce A. Beutler
624:Etanercept is a
611:plaque psoriasis
563:
561:
544:
538:
532:
526:
520:
503:
492:
481:
480:
466:
464:
449:
429:
409:
373:
353:
343:
342:
327:
301:
268:
265:
260:
253:
250:
240:
237:
228:
225:
215:
212:
190:
147:
144:
126:
115:
112:
99:
85:
42:
35:
33:
21:
5539:
5538:
5534:
5533:
5532:
5530:
5529:
5528:
5474:
5473:
5472:
5462:
5460:
5452:
5450:
5441:
5363:
5359:Deucravacitinib
5345:
5287:
5199:
5127:
5112:
5092:
5072:
5048:
5028:
4987:
4821:Interferon alfa
4781:
4740:
4720:
4669:
4603:
4599:Pegnartograstim
4584:Lipegfilgrastim
4547:
4493:Epoetin epsilon
4427:
4407:
4399:
4369:
4364:
4310:Rozanolixizumab
4195:Deucravacitinib
4129:
4057:
4030:
3919:
3568:
3562:
3270:
3235:
3192:
3171:
3161:
3119:
3059:
3050:
2986:Antimetabolites
2977:
2971:
2957:
2927:
2926:
2916:
2914:
2902:
2901:
2897:
2888:
2886:
2878:
2877:
2873:
2864:
2863:
2859:
2849:
2847:
2835:
2834:
2830:
2820:
2818:
2815:
2811:
2810:
2806:
2796:
2794:
2782:
2781:
2777:
2767:
2765:
2755:"Benepali EPAR"
2753:
2752:
2748:
2739:
2737:
2733:
2726:
2722:
2721:
2717:
2708:
2706:
2697:
2696:
2692:
2683:
2681:
2676:
2675:
2671:
2662:
2660:
2651:
2650:
2646:
2637:
2635:
2630:
2629:
2625:
2615:
2613:
2609:
2602:
2598:
2597:
2586:
2571:
2567:
2557:
2555:
2546:
2545:
2536:
2528:
2524:
2514:
2512:
2495:
2494:
2490:
2480:
2479:
2475:
2465:
2464:
2460:
2430:
2429:
2425:
2373:
2372:
2368:
2330:
2329:
2325:
2294:
2293:
2289:
2267:
2266:
2259:
2229:
2228:
2224:
2188:
2187:
2183:
2139:
2138:
2134:
2124:
2122:
2118:
2107:
2101:
2100:
2096:
2087:
2085:
2076:
2075:
2071:
2063:
2059:
2058:
2054:
2045:
2043:
2034:
2033:
2029:
2022:
2015:
2014:
2010:
2000:
1998:
1993:
1992:
1988:
1978:
1976:
1968:
1963:
1962:
1958:
1927:(4): CD005468.
1914:
1913:
1909:
1899:
1897:
1889:
1884:
1883:
1879:
1869:
1867:
1857:
1856:
1852:
1842:
1840:
1832:
1827:
1826:
1822:
1812:
1810:
1802:
1797:
1796:
1792:
1769:
1768:
1764:
1741:(6): 999–1004.
1728:
1727:
1723:
1714:
1712:
1703:
1702:
1698:
1693:
1689:
1651:
1650:
1646:
1602:
1601:
1597:
1588:
1587:
1583:
1548:Nature Medicine
1545:
1544:
1540:
1530:
1528:
1520:
1519:
1515:
1504:
1502:
1490:
1489:
1462:
1452:
1450:
1442:
1441:
1422:
1412:
1410:
1401:
1400:
1396:
1386:
1384:
1375:
1374:
1370:
1360:
1358:
1349:
1348:
1344:
1334:
1332:
1317:
1316:
1312:
1302:
1300:
1288:
1287:
1283:
1273:
1271:
1262:
1261:
1257:
1249:
1245:
1244:
1237:
1227:
1225:
1224:on 13 June 2021
1209:
1208:
1199:
1189:
1187:
1186:. 24 March 2020
1178:
1177:
1168:
1158:
1156:
1155:on 13 June 2021
1140:
1139:
1130:
1120:
1118:
1109:
1108:
1104:
1094:
1092:
1081:
1080:
1076:
1066:
1064:
1063:. 14 March 2017
1053:
1052:
1048:
1043:
1017:
1011:
984:
964:
959:
936:
927:
893:, two types of
868:
840:(FDA) placed a
834:
832:Adverse effects
806:
725:(RA) (Nov 1998)
711:
630:recombinant DNA
559:
557:
547:
541:
535:
529:
523:
484:
460:
452:
432:
412:
392:
376:
356:
339:
330:
299:
288:Bioavailability
280:Pharmacokinetic
274:
258:
194:
162:
155:
136:
129:
28:
23:
22:
15:
12:
11:
5:
5537:
5535:
5527:
5526:
5521:
5516:
5511:
5506:
5501:
5496:
5491:
5486:
5484:TNF inhibitors
5476:
5475:
5471:
5470:
5447:
5446:
5443:
5442:
5440:
5439:
5434:
5429:
5424:
5419:
5414:
5405:
5404:
5399:
5394:
5389:
5384:
5375:
5373:
5369:
5368:
5365:
5364:
5362:
5361:
5355:
5353:
5347:
5346:
5344:
5343:
5338:
5333:
5328:
5323:
5318:
5313:
5308:
5303:
5301:Cercosporamide
5297:
5295:
5289:
5288:
5286:
5285:
5280:
5275:
5270:
5265:
5260:
5255:
5250:
5245:
5243:Deuruxolitinib
5240:
5235:
5230:
5225:
5220:
5215:
5209:
5207:
5201:
5200:
5198:
5197:
5192:
5187:
5182:
5177:
5172:
5167:
5162:
5160:Deuruxolitinib
5157:
5152:
5146:
5144:
5135:
5118:
5114:
5113:
5111:
5110:
5102:
5100:
5094:
5093:
5091:
5090:
5082:
5080:
5074:
5073:
5071:
5070:
5062:
5060:
5054:
5053:
5050:
5049:
5047:
5046:
5038:
5036:
5034:IFNLR (λ, III)
5030:
5029:
5027:
5026:
5021:
5012:
5011:
5006:
4997:
4995:
4989:
4988:
4986:
4985:
4976:
4975:
4970:
4965:
4960:
4955:
4946:
4945:
4940:
4935:
4930:
4925:
4920:
4915:
4901:
4896:
4891:
4886:
4881:
4876:
4818:
4813:
4804:
4802:
4800:IFNAR (α/β, I)
4793:
4787:
4786:
4783:
4782:
4780:
4779:
4774:
4769:
4767:Promegapoietin
4764:
4759:
4750:
4748:
4746:Thrombopoietin
4742:
4741:
4739:
4738:
4730:
4728:
4722:
4721:
4719:
4718:
4709:
4708:
4703:
4698:
4693:
4691:Interleukin-34
4688:
4679:
4677:
4671:
4670:
4668:
4667:
4662:
4657:
4648:
4647:
4642:
4637:
4632:
4627:
4622:
4613:
4611:
4605:
4604:
4602:
4601:
4596:
4591:
4586:
4581:
4576:
4571:
4566:
4557:
4555:
4549:
4548:
4546:
4545:
4540:
4535:
4530:
4525:
4520:
4515:
4510:
4505:
4500:
4495:
4490:
4485:
4480:
4475:
4470:
4465:
4460:
4455:
4446:
4444:
4442:Erythropoietin
4435:
4429:
4428:
4426:
4425:
4417:
4415:
4409:
4408:
4400:
4398:
4397:
4390:
4383:
4375:
4366:
4365:
4363:
4362:
4357:
4352:
4347:
4342:
4337:
4332:
4327:
4322:
4317:
4312:
4307:
4302:
4297:
4292:
4287:
4282:
4277:
4272:
4267:
4262:
4257:
4252:
4247:
4242:
4237:
4232:
4227:
4222:
4219:+hyaluronidase
4212:
4207:
4202:
4200:Deuruxolitinib
4197:
4192:
4187:
4182:
4177:
4172:
4167:
4162:
4157:
4152:
4147:
4141:
4139:
4135:
4134:
4131:
4130:
4128:
4127:
4122:
4117:
4112:
4111:
4110:
4105:
4093:
4092:
4091:
4086:
4073:
4071:
4063:
4062:
4059:
4058:
4056:
4055:
4050:
4044:
4042:
4036:
4035:
4032:
4031:
4029:
4028:
4023:
4018:
4013:
4008:
4003:
3998:
3993:
3988:
3983:
3978:
3973:
3968:
3963:
3958:
3953:
3948:
3943:
3938:
3933:
3927:
3925:
3921:
3920:
3918:
3917:
3904:
3903:
3902:
3901:
3896:
3891:
3878:
3877:
3876:
3875:
3863:
3862:
3861:
3849:
3848:
3847:
3842:
3830:
3829:
3828:
3823:
3818:
3806:
3805:
3804:
3793:
3792:
3791:
3790:
3785:
3773:
3772:
3771:
3759:
3758:
3757:
3745:
3744:
3743:
3731:
3730:
3729:
3724:
3712:
3711:
3710:
3705:
3693:
3692:
3691:
3686:
3681:
3676:
3673:+hyaluronidase
3666:
3654:
3653:
3652:
3640:
3639:
3638:
3626:
3625:
3624:
3619:
3614:
3602:
3601:
3600:
3595:
3590:
3585:
3572:
3570:
3564:
3563:
3561:
3560:
3559:
3558:
3545:
3544:
3543:
3542:
3537:
3516:
3515:
3514:
3513:
3500:
3499:
3498:
3497:
3484:
3483:
3482:
3481:
3468:
3467:
3466:
3465:
3452:
3451:
3450:
3449:
3444:
3439:
3434:
3429:
3424:
3419:
3414:
3409:
3404:
3399:
3394:
3389:
3384:
3379:
3374:
3369:
3364:
3359:
3354:
3349:
3344:
3331:
3330:
3329:
3328:
3323:
3318:
3313:
3308:
3303:
3290:
3289:
3288:
3287:
3274:
3272:
3263:
3254:
3245:
3241:
3240:
3237:
3236:
3234:
3233:
3228:
3223:
3218:
3213:
3208:
3202:
3200:
3194:
3193:
3191:
3190:
3184:
3182:
3173:
3167:
3166:
3163:
3162:
3160:
3159:
3158:
3157:
3149:PDE4 inhibitor
3145:
3140:
3135:
3129:
3127:
3121:
3120:
3118:
3117:
3112:
3106:
3105:
3104:
3103:
3098:
3093:
3088:
3067:
3065:
3052:
3051:
3049:
3048:
3047:
3046:
3033:
3032:
3031:
3030:
3025:
3011:
3010:
3009:
3008:
3003:
2990:
2988:
2979:
2973:
2972:
2958:
2956:
2955:
2948:
2941:
2933:
2925:
2924:
2895:
2871:
2857:
2828:
2804:
2784:"Lifmior EPAR"
2775:
2746:
2724:"Cipla - Home"
2715:
2690:
2669:
2644:
2623:
2584:
2578:2013-01-18 at
2565:
2534:
2522:
2488:
2473:
2458:
2423:
2366:
2323:
2304:(1): 576–584.
2287:
2276:(5): 447–453.
2257:
2222:
2181:
2152:(4): 358–367.
2132:
2094:
2069:
2052:
2027:
2008:
1986:
1956:
1907:
1877:
1850:
1820:
1790:
1762:
1721:
1696:
1687:
1644:
1595:
1581:
1538:
1513:
1460:
1420:
1394:
1368:
1342:
1331:on 27 May 2023
1310:
1281:
1255:
1235:
1197:
1180:"Nepexto EPAR"
1166:
1128:
1117:. 6 April 2017
1102:
1074:
1045:
1044:
1042:
1039:
1010:
1007:
983:
980:
963:
960:
958:
955:
943:Brian Seed at
935:
932:
926:
923:
876:decoy receptor
867:
864:
833:
830:
822:Steven Novella
805:
802:
801:
800:
797:
794:
791:
788:
785:
782:
779:
776:oligoarthritis
769:
763:
760:
753:
752:
745:
739:
733:
726:
710:
707:
634:TNF receptor 2
626:fusion protein
566:
565:
555:
549:
548:
545:
539:
533:
527:
521:
516:
510:
509:
505:
504:
494:
486:
485:
483:
482:
469:
467:
454:
453:
451:
450:
442:
440:
434:
433:
431:
430:
422:
420:
414:
413:
411:
410:
402:
400:
394:
393:
391:
390:
386:
384:
378:
377:
375:
374:
366:
364:
358:
357:
355:
354:
346:
344:
332:
331:
329:
328:
320:
318:
312:
311:
307:
306:
303:
294:
293:
290:
284:
283:
276:
275:
273:
272:
263:
248:
235:
223:
209:
207:
201:
200:
196:
195:
193:
192:
179:
177:
171:
170:
165:
163:administration
157:
156:
154:
153:
151:
141:
139:
131:
130:
128:
127:
109:
107:
101:
100:
93:
87:
86:
79:
69:
68:
65:
59:
58:
55:
49:
48:
44:
43:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
5536:
5525:
5522:
5520:
5517:
5515:
5512:
5510:
5507:
5505:
5502:
5500:
5497:
5495:
5492:
5490:
5487:
5485:
5482:
5481:
5479:
5469:
5459:
5455:
5438:
5435:
5433:
5430:
5428:
5425:
5423:
5420:
5418:
5415:
5413:
5410:
5407:
5406:
5403:
5400:
5398:
5395:
5393:
5390:
5388:
5385:
5383:
5380:
5377:
5376:
5374:
5370:
5360:
5357:
5356:
5354:
5352:
5348:
5342:
5339:
5337:
5334:
5332:
5329:
5327:
5324:
5322:
5319:
5317:
5314:
5312:
5309:
5307:
5304:
5302:
5299:
5298:
5296:
5294:
5290:
5284:
5281:
5279:
5276:
5274:
5271:
5269:
5266:
5264:
5261:
5259:
5256:
5254:
5251:
5249:
5246:
5244:
5241:
5239:
5236:
5234:
5231:
5229:
5226:
5224:
5221:
5219:
5216:
5214:
5211:
5210:
5208:
5206:
5202:
5196:
5193:
5191:
5188:
5186:
5183:
5181:
5178:
5176:
5173:
5171:
5168:
5166:
5163:
5161:
5158:
5156:
5153:
5151:
5148:
5147:
5145:
5143:
5139:
5136:
5132:
5126:
5122:
5119:
5115:
5108:
5104:
5103:
5101:
5099:
5095:
5088:
5084:
5083:
5081:
5079:
5075:
5068:
5064:
5063:
5061:
5059:
5055:
5044:
5040:
5039:
5037:
5035:
5031:
5025:
5022:
5020:
5017:
5014:
5013:
5010:
5007:
5005:
5002:
4999:
4998:
4996:
4994:
4993:IFNGR (γ, II)
4990:
4984:
4981:
4978:
4977:
4974:
4971:
4969:
4966:
4964:
4961:
4959:
4956:
4954:
4951:
4948:
4947:
4944:
4941:
4939:
4936:
4934:
4931:
4929:
4926:
4924:
4921:
4919:
4916:
4913:
4909:
4905:
4902:
4900:
4897:
4895:
4892:
4890:
4887:
4885:
4882:
4880:
4877:
4874:
4870:
4866:
4862:
4858:
4854:
4850:
4846:
4842:
4838:
4834:
4830:
4826:
4822:
4819:
4817:
4814:
4812:
4811:Albinterferon
4809:
4806:
4805:
4803:
4801:
4797:
4794:
4792:
4788:
4778:
4775:
4773:
4770:
4768:
4765:
4763:
4760:
4758:
4755:
4752:
4751:
4749:
4747:
4743:
4736:
4732:
4731:
4729:
4727:
4723:
4717:
4714:
4711:
4710:
4707:
4704:
4702:
4699:
4697:
4694:
4692:
4689:
4687:
4684:
4681:
4680:
4678:
4676:
4672:
4666:
4663:
4661:
4658:
4656:
4653:
4650:
4649:
4646:
4643:
4641:
4638:
4636:
4633:
4631:
4628:
4626:
4623:
4621:
4618:
4615:
4614:
4612:
4610:
4609:GM-CSF (CSF2)
4606:
4600:
4597:
4595:
4594:Pegfilgrastim
4592:
4590:
4587:
4585:
4582:
4580:
4577:
4575:
4572:
4570:
4567:
4565:
4562:
4559:
4558:
4556:
4554:
4550:
4544:
4541:
4539:
4536:
4534:
4531:
4529:
4526:
4524:
4521:
4519:
4516:
4514:
4513:Epoetin theta
4511:
4509:
4508:Epoetin omega
4506:
4504:
4503:Epoetin kappa
4501:
4499:
4498:Epoetin gamma
4496:
4494:
4491:
4489:
4488:Epoetin delta
4486:
4484:
4481:
4479:
4476:
4474:
4471:
4469:
4466:
4464:
4461:
4459:
4456:
4454:
4451:
4448:
4447:
4445:
4443:
4439:
4436:
4434:
4430:
4423:
4419:
4418:
4416:
4414:
4410:
4406:
4403:
4396:
4391:
4389:
4384:
4382:
4377:
4376:
4373:
4361:
4358:
4356:
4353:
4351:
4350:Tildrakizumab
4348:
4346:
4343:
4341:
4338:
4336:
4333:
4331:
4328:
4326:
4323:
4321:
4318:
4316:
4313:
4311:
4308:
4306:
4303:
4301:
4298:
4296:
4293:
4291:
4288:
4286:
4283:
4281:
4280:Pegcetacoplan
4278:
4276:
4273:
4271:
4268:
4266:
4263:
4261:
4258:
4256:
4253:
4251:
4248:
4246:
4243:
4241:
4238:
4236:
4233:
4231:
4228:
4226:
4223:
4220:
4216:
4213:
4211:
4208:
4206:
4203:
4201:
4198:
4196:
4193:
4191:
4190:Darvadstrocel
4188:
4186:
4183:
4181:
4178:
4176:
4173:
4171:
4168:
4166:
4163:
4161:
4158:
4156:
4153:
4151:
4148:
4146:
4143:
4142:
4140:
4136:
4126:
4123:
4121:
4118:
4116:
4113:
4109:
4106:
4104:
4101:
4100:
4099:
4098:
4097:TNF inhibitor
4094:
4090:
4087:
4085:
4082:
4081:
4080:
4079:
4075:
4074:
4072:
4069:
4064:
4054:
4051:
4049:
4046:
4045:
4043:
4041:
4037:
4027:
4024:
4022:
4019:
4017:
4014:
4012:
4009:
4007:
4004:
4002:
3999:
3997:
3994:
3992:
3989:
3987:
3984:
3982:
3979:
3977:
3974:
3972:
3969:
3967:
3964:
3962:
3959:
3957:
3954:
3952:
3949:
3947:
3944:
3942:
3939:
3937:
3934:
3932:
3929:
3928:
3926:
3922:
3915:
3911:
3910:
3906:
3905:
3900:
3897:
3895:
3892:
3890:
3887:
3886:
3885:
3884:
3880:
3879:
3874:
3871:
3870:
3869:
3868:
3864:
3860:
3857:
3856:
3855:
3854:
3850:
3846:
3843:
3841:
3838:
3837:
3836:
3835:
3831:
3827:
3824:
3822:
3819:
3817:
3814:
3813:
3812:
3811:
3807:
3803:
3800:
3799:
3798:
3795:
3794:
3789:
3786:
3784:
3781:
3780:
3779:
3778:
3774:
3770:
3767:
3766:
3765:
3764:
3763:CD147/Basigin
3760:
3756:
3753:
3752:
3751:
3750:
3746:
3742:
3739:
3738:
3737:
3736:
3732:
3728:
3725:
3723:
3720:
3719:
3718:
3717:
3713:
3709:
3706:
3704:
3701:
3700:
3699:
3698:
3694:
3690:
3687:
3685:
3682:
3680:
3677:
3674:
3670:
3667:
3665:
3662:
3661:
3660:
3659:
3655:
3651:
3648:
3647:
3646:
3645:
3641:
3637:
3634:
3633:
3632:
3631:
3627:
3623:
3620:
3618:
3615:
3613:
3610:
3609:
3608:
3607:
3603:
3599:
3596:
3594:
3591:
3589:
3586:
3584:
3583:Muromonab-CD3
3581:
3580:
3579:
3578:
3574:
3573:
3571:
3565:
3557:
3554:
3553:
3552:
3551:
3547:
3546:
3541:
3538:
3536:
3533:
3532:
3531:
3530:
3526:
3522:
3518:
3517:
3512:
3509:
3508:
3507:
3506:
3502:
3501:
3496:
3493:
3492:
3491:
3490:
3486:
3485:
3480:
3477:
3476:
3475:
3474:
3470:
3469:
3464:
3461:
3460:
3459:
3458:
3457:Interleukin 5
3454:
3453:
3448:
3445:
3443:
3440:
3438:
3437:Tildrakizumab
3435:
3433:
3430:
3428:
3425:
3423:
3420:
3418:
3415:
3413:
3410:
3408:
3405:
3403:
3400:
3398:
3395:
3393:
3390:
3388:
3385:
3383:
3380:
3378:
3375:
3373:
3370:
3368:
3365:
3363:
3360:
3358:
3355:
3353:
3350:
3348:
3345:
3343:
3340:
3339:
3338:
3337:
3333:
3332:
3327:
3324:
3322:
3319:
3317:
3314:
3312:
3309:
3307:
3304:
3302:
3299:
3298:
3297:
3296:
3292:
3291:
3286:
3283:
3282:
3281:
3280:
3276:
3275:
3273:
3271:(noncellular)
3267:
3264:
3262:
3258:
3255:
3253:
3249:
3246:
3244:Extracellular
3242:
3232:
3229:
3227:
3224:
3222:
3219:
3217:
3214:
3212:
3211:Ridaforolimus
3209:
3207:
3204:
3203:
3201:
3199:
3195:
3189:
3186:
3185:
3183:
3181:
3177:
3174:
3170:Intracellular
3168:
3156:
3153:
3152:
3151:
3150:
3146:
3144:
3141:
3139:
3136:
3134:
3131:
3130:
3128:
3126:
3122:
3116:
3113:
3111:
3108:
3107:
3102:
3099:
3097:
3094:
3092:
3089:
3087:
3084:
3083:
3082:
3081:
3077:
3073:
3069:
3068:
3066:
3063:
3057:
3053:
3045:
3042:
3041:
3040:
3039:
3035:
3034:
3029:
3028:Teriflunomide
3026:
3024:
3021:
3020:
3018:
3017:
3013:
3012:
3007:
3004:
3002:
2999:
2998:
2997:
2996:
2992:
2991:
2989:
2987:
2983:
2980:
2976:Intracellular
2974:
2969:
2965:
2961:
2954:
2949:
2947:
2942:
2940:
2935:
2934:
2931:
2912:
2910:
2905:
2899:
2896:
2885:
2881:
2875:
2872:
2867:
2861:
2858:
2845:
2843:
2838:
2837:"Erelzi EPAR"
2832:
2829:
2814:
2808:
2805:
2792:
2790:
2785:
2779:
2776:
2763:
2761:
2756:
2750:
2747:
2736:on 2013-05-01
2732:
2725:
2719:
2716:
2705:
2701:
2694:
2691:
2679:
2673:
2670:
2659:on 2010-02-24
2658:
2654:
2648:
2645:
2633:
2627:
2624:
2608:
2601:
2595:
2593:
2591:
2589:
2585:
2581:
2580:archive.today
2577:
2574:
2569:
2566:
2554:
2550:
2543:
2541:
2539:
2535:
2531:
2526:
2523:
2510:
2506:
2504:
2498:
2492:
2489:
2484:
2477:
2474:
2469:
2462:
2459:
2454:
2450:
2446:
2442:
2438:
2434:
2427:
2424:
2419:
2415:
2410:
2405:
2401:
2397:
2393:
2389:
2385:
2381:
2377:
2370:
2367:
2362:
2358:
2354:
2350:
2346:
2342:
2339:(148): ra83.
2338:
2334:
2327:
2324:
2319:
2315:
2311:
2307:
2303:
2299:
2291:
2288:
2283:
2279:
2275:
2271:
2264:
2262:
2258:
2253:
2249:
2245:
2241:
2237:
2233:
2226:
2223:
2218:
2214:
2209:
2204:
2200:
2196:
2192:
2185:
2182:
2177:
2173:
2168:
2163:
2159:
2155:
2151:
2147:
2143:
2136:
2133:
2117:
2113:
2106:
2105:
2098:
2095:
2084:on 2008-05-05
2083:
2079:
2073:
2070:
2062:
2056:
2053:
2041:
2040:Above the Law
2037:
2031:
2028:
2018:
2012:
2009:
1996:
1990:
1987:
1974:
1967:
1960:
1957:
1952:
1948:
1943:
1938:
1934:
1930:
1926:
1922:
1918:
1911:
1908:
1895:
1888:
1881:
1878:
1865:
1861:
1854:
1851:
1838:
1831:
1824:
1821:
1808:
1801:
1794:
1791:
1785:
1780:
1776:
1772:
1766:
1763:
1758:
1754:
1749:
1744:
1740:
1736:
1732:
1725:
1722:
1711:on 2007-10-20
1710:
1706:
1700:
1697:
1691:
1688:
1683:
1679:
1675:
1671:
1667:
1663:
1659:
1655:
1648:
1645:
1640:
1636:
1631:
1626:
1622:
1618:
1614:
1610:
1606:
1599:
1596:
1591:
1585:
1582:
1577:
1573:
1569:
1565:
1561:
1560:10.1038/nm939
1557:
1553:
1549:
1542:
1539:
1527:
1523:
1517:
1514:
1500:
1498:
1493:
1492:"Enbrel EPAR"
1487:
1485:
1483:
1481:
1479:
1477:
1475:
1473:
1471:
1469:
1467:
1465:
1461:
1449:
1445:
1439:
1437:
1435:
1433:
1431:
1429:
1427:
1425:
1421:
1409:. 25 May 2021
1408:
1404:
1398:
1395:
1383:. 8 June 2021
1382:
1378:
1372:
1369:
1356:
1352:
1346:
1343:
1330:
1326:
1325:
1324:Health Canada
1320:
1314:
1311:
1299:
1295:
1291:
1285:
1282:
1269:
1265:
1259:
1256:
1248:
1242:
1240:
1236:
1223:
1219:
1217:
1212:
1206:
1204:
1202:
1198:
1185:
1181:
1175:
1173:
1171:
1167:
1154:
1150:
1148:
1143:
1137:
1135:
1133:
1129:
1116:
1112:
1106:
1103:
1090:
1089:
1088:Health Canada
1084:
1078:
1075:
1062:
1061:
1060:Health Canada
1056:
1050:
1047:
1040:
1038:
1035:
1032:
1029:
1026:
1023:
1020:
1016:
1008:
1006:
1004:
999:
997:
993:
987:
981:
979:
977:
972:
968:
961:
956:
954:
951:
948:
946:
941:
933:
931:
924:
922:
919:
915:
911:
906:
904:
900:
896:
892:
888:
884:
879:
877:
873:
872:TNF inhibitor
865:
863:
861:
857:
855:
851:
847:
843:
839:
831:
829:
827:
823:
819:
815:
811:
803:
798:
795:
792:
789:
786:
783:
780:
777:
773:
772:polyarthritis
770:
768:
764:
761:
758:
757:
756:
750:
746:
743:
740:
737:
734:
731:
727:
724:
720:
719:
718:
716:
708:
706:
704:
700:
696:
692:
688:
684:
680:
676:
671:
669:
665:
660:
658:
654:
650:
645:
643:
639:
635:
631:
627:
622:
620:
616:
612:
608:
604:
600:
596:
592:
591:TNF inhibitor
588:
584:
580:
576:
572:
556:
554:
550:
517:
515:
511:
506:
502:
498:
495:
493:
491:ECHA InfoCard
487:
479:
475:
474:DTXSID8040850
471:
470:
468:
459:
455:
448:
447:ChEMBL1201572
444:
443:
441:
439:
435:
428:
424:
423:
421:
419:
415:
408:
404:
403:
401:
399:
395:
388:
387:
385:
383:
379:
372:
368:
367:
365:
363:
359:
352:
348:
347:
345:
337:
333:
326:
322:
321:
319:
317:
313:
308:
304:
302:
295:
291:
289:
285:
281:
277:
271: Rx-only
264:
261:
249:
246:
236:
233:
224:
221:
211:
210:
208:
206:
202:
197:
189:
184:
181:
180:
178:
176:
172:
169:
166:
164:
158:
152:
143:
142:
140:
138:
132:
125:
120:
111:
110:
108:
106:
102:
98:
94:
92:
88:
84:
80:
78:
74:
70:
66:
64:
60:
56:
54:
50:
47:Clinical data
45:
41:
36:
19:
5417:Lestaurtinib
5408:
5378:
5316:Ritlecitinib
5248:Lestaurtinib
5195:Upadacitinib
5024:Fontolizumab
5015:
5000:
4979:
4968:Rontalizumab
4949:
4807:
4762:Pegacaristim
4753:
4712:
4682:
4675:M-CSF (CSF1)
4655:Mavrilimumab
4651:
4645:Sargramostim
4635:Molgramostim
4620:Ecogramostim
4616:
4589:Nartograstim
4560:
4553:G-CSF (CSF3)
4538:Peginesatide
4518:Epoetin zeta
4483:Epoetin beta
4478:Epoetin alfa
4449:
4360:Upadacitinib
4320:Satralizumab
4305:Ritlecitinib
4300:Risankizumab
4102:
4095:
4076:
4016:Teprotumumab
3966:Inebilizumab
3961:Fontolizumab
3941:Atorolimumab
3909:T-lymphocyte
3907:
3881:
3865:
3851:
3832:
3821:Lerdelimumab
3816:Bertilimumab
3808:
3796:
3775:
3761:
3747:
3733:
3714:
3695:
3679:Pascolizumab
3664:Obinutuzumab
3656:
3642:
3628:
3612:Clenoliximab
3604:
3588:Otelixizumab
3575:
3548:
3535:Lebrikizumab
3519:
3503:
3487:
3471:
3455:
3412:Satralizumab
3402:Risankizumab
3334:
3293:
3277:
3269:Serum target
3221:Temsirolimus
3147:
3138:Pomalidomide
3133:Lenalidomide
3091:Pimecrolimus
3070:
3044:Methotrexate
3036:
3014:
3001:Azathioprine
2993:
2978:(initiation)
2915:. Retrieved
2907:
2898:
2887:. Retrieved
2883:
2874:
2860:
2848:. Retrieved
2840:
2831:
2819:. Retrieved
2807:
2795:. Retrieved
2787:
2778:
2766:. Retrieved
2758:
2749:
2738:. Retrieved
2731:the original
2718:
2707:. Retrieved
2703:
2693:
2682:. Retrieved
2680:. 2011-11-25
2672:
2661:. Retrieved
2657:the original
2647:
2636:. Retrieved
2626:
2614:. Retrieved
2607:the original
2568:
2556:. Retrieved
2552:
2525:
2513:. Retrieved
2509:the original
2500:
2491:
2476:
2461:
2436:
2432:
2426:
2383:
2379:
2369:
2336:
2332:
2326:
2301:
2297:
2290:
2273:
2269:
2235:
2231:
2225:
2198:
2194:
2184:
2149:
2145:
2135:
2123:. Retrieved
2116:the original
2103:
2097:
2086:. Retrieved
2082:the original
2072:
2055:
2044:. Retrieved
2042:. 2017-02-24
2039:
2030:
2011:
2001:November 17,
1999:. Retrieved
1989:
1977:. Retrieved
1959:
1924:
1920:
1910:
1898:. Retrieved
1880:
1868:. Retrieved
1853:
1841:. Retrieved
1823:
1811:. Retrieved
1793:
1784:10665/325771
1774:
1765:
1738:
1735:Rheumatology
1734:
1724:
1713:. Retrieved
1709:the original
1699:
1690:
1657:
1653:
1647:
1612:
1608:
1598:
1584:
1551:
1547:
1541:
1529:. Retrieved
1525:
1516:
1503:. Retrieved
1495:
1451:. Retrieved
1447:
1411:. Retrieved
1406:
1397:
1385:. Retrieved
1380:
1371:
1359:. Retrieved
1354:
1345:
1333:. Retrieved
1329:the original
1322:
1313:
1301:. Retrieved
1293:
1284:
1272:. Retrieved
1267:
1258:
1226:. Retrieved
1222:the original
1214:
1211:"Rymti ARTG"
1188:. Retrieved
1183:
1157:. Retrieved
1153:the original
1145:
1142:"Etera ARTG"
1119:. Retrieved
1114:
1105:
1093:. Retrieved
1091:. 8 May 2018
1086:
1077:
1065:. Retrieved
1058:
1049:
1036:
1033:
1030:
1027:
1024:
1021:
1018:
1000:
988:
985:
973:
969:
965:
952:
949:
937:
928:
917:
907:
899:inflammation
885:produced by
880:
869:
858:
856:infections.
835:
814:brain injury
807:
754:
751:(April 2004)
712:
709:Medical uses
672:
661:
648:
646:
628:produced by
623:
593:. It has US
574:
570:
569:
305:70–132 hours
298:Elimination
205:Legal status
199:Legal status
168:Subcutaneous
105:License data
5427:Quizartinib
5422:Midostaurin
5311:Peficitinib
5273:Ruxolitinib
5268:Peficitinib
5223:Baricitinib
5185:Ruxolitinib
5180:Peficitinib
5175:Oclacitinib
5170:Momelotinib
5155:Baricitinib
5150:Abrocitinib
5058:Interleukin
5016:Antibodies:
4973:Sifalimumab
4958:Faralimomab
4953:Anifrolumab
4950:Antibodies:
4772:Romiplostim
4757:Eltrombopag
4726:SCF (c-Kit)
4652:Antibodies:
4640:Regramostim
4574:Lenograstim
4355:Tofacitinib
4295:Ravulizumab
4285:Pirfenidone
4275:Peficitinib
4175:Canakinumab
4165:Briakinumab
4155:Bimekizumab
4150:Baricitinib
4115:Aflibercept
4026:Vepalimomab
4021:Vapaliximab
3996:Rovelizumab
3986:Pexelizumab
3976:Morolimumab
3951:Cedelizumab
3936:Anifrolumab
3931:Alemtuzumab
3889:Basiliximab
3859:Tocilizumab
3845:Vedolizumab
3840:Natalizumab
3826:Metelimumab
3769:Gavilimomab
3727:Toralizumab
3722:Teneliximab
3708:Lumiliximab
3703:Gomiliximab
3689:Ublituximab
3669:Ocrelizumab
3622:Zanolimumab
3598:Visilizumab
3556:Secukinumab
3540:Ustekinumab
3511:Elsilimomab
3495:Faralimomab
3463:Mepolizumab
3447:Ustekinumab
3442:Tocilizumab
3417:Secukinumab
3367:Canakinumab
3357:Briakinumab
3352:Bimekizumab
3347:Basiliximab
3336:Interleukin
3326:Nerelimomab
3231:Zotarolimus
3172:(reception)
3143:Thalidomide
3101:Voclosporin
3086:Ciclosporin
3080:Calcineurin
3076:Cyclophilin
3023:Leflunomide
3019:inhibitors
2616:24 November
2386:(1): 3951.
2125:20 December
1252:. Feb 2021.
1083:"Arthritis"
1015:Biosimilars
1009:Biosimilars
996:biosimilars
891:macrophages
887:lymphocytes
854:hepatitis B
564: g·mol
497:100.224.383
325:185243-69-0
310:Identifiers
292:58–76% (SC)
91:MedlinePlus
63:Biosimilars
53:Trade names
5478:Categories
5412:Emfilermin
5263:Pacritinib
5165:Filgotinib
5131:inhibitors
5019:Emapalumab
4791:Interferon
4716:Agerafenib
4706:Mirimostim
4696:Lanimostim
4630:Milodistim
4579:Leridistim
4564:Filgrastim
4405:modulators
4345:Sutimlimab
4340:Spesolimab
4325:Siltuximab
4265:Olokizumab
4255:Ixekizumab
4250:Itacitinib
4240:Guselkumab
4235:Fingolimod
4230:Filgotinib
4180:Crovalimab
4170:Brodalumab
4160:Blisibimod
4125:Rilonacept
4108:Opinercept
4103:Etanercept
4089:Belatacept
4040:Polyclonal
4001:Siplizumab
3991:Reslizumab
3981:Ofatumumab
3971:Maslimomab
3956:Emapalumab
3899:Inolimomab
3894:Daclizumab
3873:Odulimomab
3788:Ruplizumab
3783:Frexalimab
3741:Aselizumab
3636:Efalizumab
3593:Teplizumab
3489:Interferon
3479:Omalizumab
3432:Spesolimab
3422:Siltuximab
3397:Rilonacept
3392:Olokizumab
3382:Ixekizumab
3377:Guselkumab
3372:Daclizumab
3362:Brodalumab
3321:Infliximab
3306:Afelimomab
3301:Adalimumab
3285:Eculizumab
3261:Monoclonal
3252:Antibodies
3226:Umirolimus
3206:Everolimus
3155:Apremilast
3115:Gusperimus
3096:Tacrolimus
3064:inhibitors
3056:Macrolides
3038:antifolate
2889:2024-07-16
2740:2019-06-05
2709:2019-06-05
2684:2019-07-14
2663:2009-12-09
2638:2019-06-05
2439:(1): 5–7.
2088:2008-05-02
2046:2022-06-27
2017:US 8900583
1715:2008-01-10
1041:References
1013:See also:
738:(Jan 2002)
732:(May 1999)
571:Etanercept
553:Molar mass
407:OP401G7OJC
382:ChemSpider
316:CAS Number
124:Etanercept
32:Etanercept
5437:Sunitinib
5432:Sorafenib
5341:ZM-449829
5331:WHI-P 154
5321:TCS-21311
5258:NSC-33994
5213:Atiprimod
5001:Agonists:
4983:Bifarcept
4808:Agonists:
4754:Agonists:
4686:Cilmostim
4683:Agonists:
4660:Namilumab
4617:Agonists:
4561:Agonists:
4450:Agonists:
4413:Chemokine
4335:Sirukumab
4330:Siponimod
4315:Sarilumab
4290:Ponesimod
4260:Netakimab
4245:Iptacopan
4225:Etrasimod
4185:Danicopan
4120:Alefacept
4084:Abatacept
4006:Talizumab
3946:Begelomab
3802:Belimumab
3755:Galiximab
3684:Rituximab
3650:Erlizumab
3617:Keliximab
3427:Sirukumab
3407:Sarilumab
3387:Netakimab
3316:Golimumab
3216:Sirolimus
2634:. Sec.gov
1979:April 14,
1900:April 14,
1870:April 14,
1843:April 14,
1813:April 14,
1274:13 August
1268:Drugs.com
962:Economics
925:Structure
914:cytokines
910:receptors
765:juvenile
715:indicated
691:psoriasis
670:in 2002.
300:half-life
161:Routes of
135:Pregnancy
83:Monograph
77:Drugs.com
5468:Medicine
5336:ZM-39923
5253:NSC-7908
5218:AZD-1480
5109:instead.
5089:instead.
5069:instead.
5045:instead.
4963:MEDI-545
4737:instead.
4665:Otilimab
4468:CNTO-530
4424:instead.
4270:Ozanimod
4145:Avacopan
4138:Unsorted
3924:Unsorted
3834:Integrin
3567:Cellular
3342:Anakinra
3188:Anakinra
3110:Abetimus
2962: /
2576:Archived
2558:10 March
2453:28201948
2418:28638112
2361:24226117
2353:21081755
2318:24308717
2252:16135466
2217:17641054
2176:37533141
2167:10486173
1951:25887212
1942:11200207
1773:(2019).
1757:17389658
1682:10859938
1576:52860838
1568:14520364
1453:17 April
1448:DailyMed
1335:10 March
1121:13 April
1095:13 April
883:cytokine
681:such as
362:DrugBank
175:ATC code
137:category
119:DailyMed
5278:SD-1008
4453:ARA-290
4066:-cept (
2917:12 June
2850:2 April
2821:2 April
2797:2 April
2768:2 April
2409:5479810
2388:Bibcode
2282:1936291
2110:. U.S.
1674:7693816
1639:1660525
1630:2119031
1531:3 March
1505:2 April
1413:12 June
1387:12 June
1361:12 June
1228:12 June
1190:4 March
1159:12 June
1067:7 April
982:Patents
940:Immunex
934:History
918:soluble
664:Immunex
649:in vivo
640:end of
514:Formula
371:DB00005
351:7847807
336:PubChem
262:Rx-only
259:WARNING
230::
191:)
185: (
183:L04AB01
150: D
121::
97:a602013
5454:Portal
5372:Others
5238:CYT387
5228:CHZ868
5117:Others
4873:IFNA21
4869:IFNA17
4865:IFNA16
4861:IFNA14
4857:IFNA13
4853:IFNA10
4078:CTLA-4
4068:Fusion
3569:target
3550:IL-17A
3527:, and
3060:other
2515:4 June
2451:
2416:
2406:
2359:
2351:
2316:
2280:
2250:
2215:
2174:
2164:
2023:
1949:
1939:
1755:
1680:
1672:
1637:
1627:
1574:
1566:
1303:22 Oct
976:Pfizer
916:, and
846:sepsis
810:stroke
575:Enbrel
438:ChEMBL
427:D00742
338:
256:
243:
232:℞-only
218:
117:
57:Enbrel
18:Enbrel
5489:Amgen
4912:IFNB3
4908:IFNB1
4849:IFNA8
4845:IFNA7
4841:IFNA6
4837:IFNA5
4833:IFNA4
4829:IFNA2
4825:IFNA1
3867:LFA-1
3777:CD154
3630:CD11a
3529:IL-23
3525:IL-13
3521:IL-12
3125:IMiDs
2911:(TGA)
2844:(EMA)
2816:(PDF)
2791:(EMA)
2762:(EMA)
2734:(PDF)
2727:(PDF)
2610:(PDF)
2603:(PDF)
2505:(FDA)
2501:U.S.
2357:S2CID
2119:(PDF)
2108:(PDF)
2064:(pdf)
1975:(FDA)
1969:(PDF)
1896:(FDA)
1890:(PDF)
1866:(FDA)
1839:(FDA)
1833:(PDF)
1809:(FDA)
1803:(PDF)
1678:S2CID
1572:S2CID
1499:(EMA)
1407:(emc)
1381:(emc)
1355:(emc)
1250:(PDF)
1218:(TGA)
1149:(TGA)
1003:Cipla
717:for:
668:Amgen
5351:TYK2
5293:JAK3
5205:JAK2
5142:JAK1
5107:here
5105:See
5087:here
5085:See
5078:TGFβ
5067:here
5065:See
5043:here
4735:here
4733:See
4422:here
4420:See
3797:BLyS
3749:CD80
3716:CD40
3697:CD23
3658:CD20
3644:CD18
3505:IL-6
3198:mTOR
3072:FKBP
3062:IL-2
2919:2021
2852:2020
2823:2020
2799:2020
2770:2020
2618:2017
2560:2023
2517:2020
2449:PMID
2414:PMID
2349:PMID
2314:PMID
2278:PMID
2248:PMID
2213:PMID
2172:PMID
2127:2013
2003:2018
1981:2015
1947:PMID
1925:2015
1902:2015
1872:2015
1845:2015
1815:2015
1753:PMID
1670:PMID
1635:PMID
1564:PMID
1533:2023
1507:2020
1455:2021
1415:2021
1389:2021
1363:2021
1337:2023
1305:2023
1276:2020
1230:2021
1192:2023
1161:2021
1123:2024
1097:2024
1069:2024
889:and
852:and
812:and
613:and
605:and
528:3475
522:2224
418:KEGG
398:UNII
389:None
282:data
73:AHFS
5125:JAK
5098:TNF
4433:CSF
3810:CAT
3606:CD4
3577:CD3
3295:TNF
2968:L04
2441:doi
2404:PMC
2396:doi
2341:doi
2306:doi
2240:doi
2203:doi
2199:179
2162:PMC
2154:doi
1937:PMC
1929:doi
1779:hdl
1743:doi
1662:doi
1658:151
1625:PMC
1617:doi
1613:174
1556:doi
1298:FDA
675:kDa
562:.07
560:235
540:698
534:621
463:EPA
341:SID
245:POM
188:WHO
5480::
4910:,
4871:,
4867:,
4863:,
4859:,
4855:,
4851:,
4847:,
4843:,
4839:,
4835:,
4831:,
4827:,
3523:,
2906:.
2882:.
2839:.
2786:.
2757:.
2702:.
2587:^
2551:.
2537:^
2499:.
2447:.
2435:.
2412:.
2402:.
2394:.
2382:.
2378:.
2355:.
2347:.
2335:.
2312:.
2302:86
2300:.
2274:17
2272:.
2260:^
2246:.
2236:23
2234:.
2211:.
2197:.
2193:.
2170:.
2160:.
2150:27
2148:.
2144:.
2038:.
1945:.
1935:.
1923:.
1919:.
1751:.
1739:46
1737:.
1733:.
1676:.
1668:.
1656:.
1633:.
1623:.
1611:.
1607:.
1570:.
1562:.
1550:.
1524:.
1494:.
1463:^
1446:.
1423:^
1405:.
1379:.
1353:.
1321:.
1296:.
1292:.
1266:.
1238:^
1213:.
1200:^
1182:.
1169:^
1144:.
1131:^
1113:.
1085:.
1057:.
905:.
820:,
705:.
697:,
693:,
689:,
685:,
638:Fc
617:.
609:,
601:,
558:51
546:36
267:EU
252:US
239:UK
227:CA
220:S4
214:AU
146:AU
114:US
5456::
5133:)
5129:(
4914:)
4906:(
4875:)
4823:(
4394:e
4387:t
4380:v
4221:)
4217:(
4070:)
3916:)
3912:(
3675:)
3671:(
3078:/
3074:/
3058:/
2970:)
2966:(
2952:e
2945:t
2938:v
2921:.
2892:.
2868:.
2854:.
2825:.
2801:.
2772:.
2743:.
2712:.
2687:.
2666:.
2641:.
2620:.
2562:.
2519:.
2485:.
2470:.
2455:.
2443::
2437:6
2420:.
2398::
2390::
2384:7
2363:.
2343::
2337:3
2320:.
2308::
2284:.
2254:.
2242::
2219:.
2205::
2178:.
2156::
2129:.
2091:.
2049:.
2005:.
1983:.
1953:.
1931::
1904:.
1874:.
1847:.
1817:.
1781::
1759:.
1745::
1718:.
1684:.
1664::
1641:.
1619::
1592:.
1578:.
1558::
1552:9
1535:.
1509:.
1457:.
1417:.
1391:.
1365:.
1339:.
1307:.
1278:.
1232:.
1194:.
1163:.
1125:.
1099:.
1071:.
990:(
543:S
537:O
531:N
525:H
519:C
465:)
461:(
269::
254::
241::
216::
148::
75:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.