20:
36:
535:, endohedral fullerenes represent an atomic trap that is stable at room temperature and for an arbitrarily long time. Atomic or ion traps are of great interest since particles are present free from (significant) interaction with their environment, allowing unique quantum mechanical phenomena to be explored. For example, the compression of the atomic
130:
regardless of whether M was inside or outside the fullerene. In order to allow for more detailed discussions with minimal loss of information, a more explicit notation was proposed in 1991, where the atoms listed to the left of the @ sign are situated inside the network composed of the atoms listed
1099:
Saunders, Martin; Jimenez-Vazquez, Hugo A.; Cross, R. James; Mroczkowski, Stanley; Gross, Michael L.; Giblin, Daryl E.; Poreda, Robert J. (1994). "Incorporation of helium, neon, argon, krypton, and xenon into fullerenes using high pressure".
325:
which is better described as @. These anionic fullerene cages are very stable molecules and do not have the reactivity associated with ordinary empty fullerenes. They are stable in air up to very high temperatures (600 to 850 °C).
886:
Yamada, M; Nakahodo, T; Wakahara, T; Tsuchiya, T; Maeda, Y; Akasaka, T; Kako, M; Yoza, K; Horn, E; Mizorogi, N; Kobayashi, K; Nagase, S (2005). "Positional control of encapsulated atoms inside a fullerene cage by exohedral addition".
277:
and coworkers, the inclusion of a molecule fragment in a fullerene cage had succeeded for the first time. This compound can be prepared by arc-vaporization at temperatures up to 1100 °C of graphite rods packed with
704:) may lead to dissociation of the encapsulated molecules and reaction of their fragments with interiors of the fullerene cage. Such reactions should result in endohedral fullerene adducts, which are currently unknown.
1657:
Sabirov, Denis (2013). "From
Endohedral Complexes to Endohedral Fullerene Covalent Derivatives: A Density Functional Theory Prognosis of Chemical Transformation of Water Endofullerene H2O@C60 upon Its Compression".
296:
Endohedral metallofullerenes are characterised by the fact that electrons will transfer from the metal atom to the fullerene cage and that the metal atom takes a position off-center in the cage. The size of the
1351:
Langlet, R.; Mayer, A.; Geuquet, N.; Amara, H.; Vandescuren, M.; Henrard, L.; Maksimenko, S.; Lambin, Ph. (2007). "Study of the polarizability of fullerenes with a monopole–dipole interaction model".
652:
fullerene as a
Faraday cage, which isolates the encapsulated atom from the external electric field. The mentioned relations should be typical for the more complicated endohedral structures (e.g., C
696:. The encapsulated molecules display unusual physical properties which have been studied by a variety of physical methods. As shown theoretically, compression of molecular endofullerenes (e.g., H
787:
Chai, Yan; Guo, Ting; Jin, Changming; Haufler, Robert E.; Chibante, L. P. Felipe; Fure, Jan; Wang, Lihong; Alford, J. Michael; Smalley, Richard E. (1991). "Fullerenes wlth Metals Inside".
250:
and as different isomer cages. Aside from the dominant presence of mono-metal cages, numerous di-metal endohedral complexes and the tri-metal carbide fullerenes like Sc
737:
Gimenez-Lopez, Maria del Carmen; Chuvilin, Andrey; Kaiser, Ute; Khlobystov, Andrei N. (2010). "Functionalised endohedral fullerenes in single-walled carbon nanotubes".
627:
601:
546:
Contrary to the metallo endohedral compounds, these complexes cannot be produced in an arc. Atoms are implanted in the fullerene starting material using
158:
The choice of the symbol has been explained by the authors as being concise, readily printed and transmitted electronically (the at sign is included in
573:
do not equal to the sum of polarizabilities of a fullerene cage and the trapped atom, i.e. exaltation of polarizability occurs.,. The sign of the Δ
364:
are equal the two encapsulated Ce atoms exhibit a three-dimensional random motion. This is evidenced by the presence of only two signals in the C-
1266:
333:
is utilised in a method to purify compounds from a complex mixture of empty and partly filled fullerenes of different cage size. In this method
817:
Ge, Z; Duchamp, Jc; Cai, T; Gibson, Hw; Dorn, Hc (2005). "Purification of endohedral trimetallic nitride fullerenes in a single, facile step".
1230:
Yan, Hong; Yu, Shengping; Wang, Xin; He, Yang; Huang, Wen; Yang, Mingli (2008). "Dipole polarizabilities of noble gas endohedral fullerenes".
162:, which most modern character encoding schemes are based on), and the visual aspects suggesting the structure of an endohedral fullerene.
668:
Closed fullerenes encapsulating small molecules have been synthesized. Representative are the synthesis of the dihydrogen endofullerene H
924:"Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C82(OH)22 and its implication for de novo design of nanomedicine"
1707:
543:. The nitrogen atom can be used as a probe, in order to detect the smallest changes of the electronic structure of its environment.
540:
629:), it is negative (depression of polarizability). The following formula, describing the dependence of Δα on n, has been proposed: Δ
569:
Noble gas endofullerenes are predicted to exhibit unusual polarizability. Thus, calculated values of mean polarizability of Ng@C
47:
peapods. Metal atoms (M = Ho or Sc) are seen as dark spots inside the fullerene molecules; they are doubly encapsulated in the C
983:"Non-destructive inhibition of metallofullerenol Gd@C(82)(OH)(22) on WW domain: implication on signal transduction pathway"
555:
98:) in the name reflects the notion of a small molecule trapped inside a shell. Two types of endohedral complexes exist:
516:, which are observed very easily with the endohedral metallofullerenes, could only be observed in the case of the N@C
1038:
Saunders, M.; Jiménez-Vázquez, H. A.; Cross, R. J.; Poreda, R. J. (1993). "Stable compounds of helium and neon. He@C
365:
922:
Kang SG, Zhou G, Yang P, Liu Y, Sun B, Huynh T, Meng H, Zhao L, Xing G, Chen C, Zhao Y, Zhou R (September 2012).
855:
K.Muthukumar; J.A.Larsson (2008). "Explanation of the different preferential binding sites for Ce and La in M2@C
338:
463:
compound was also demonstrated with pressures of 3 kbars and incorporation of up to 0.1% of the noble gases.
509:
298:
577:
polarizability exaltation depends on the number of atoms in a fullerene molecule: for small fullerenes (
532:
369:
342:
562:
methods. A recent example of endohedral fullerenes includes single molecules of water encapsulated in C
175:
1621:
1512:
1454:
1399:
1360:
1325:
1282:
1239:
1196:
1141:
1057:
994:
935:
330:
203:
171:
119:
1702:
286:
279:
238:. The synthesis in the arc reactor is however unspecific. Besides unfilled fullerenes, endohedral
1614:
Philosophical
Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences
1497:
1478:
1423:
1212:
1165:
1081:
718:
523:
The central atom in these endohedral complexes is located in the center of the cage. While other
404:
91:
1386:
Komatsu, K.; Murata, M.; Murata, Y. (2005). "Encapsulation of
Molecular Hydrogen in Fullerene C
1128:
Kurotobi, Kei; Murata, Yasujiro (2012). "A Single
Molecule of Water Encapsulated in Fullerene C
1639:
1587:
1528:
1470:
1415:
1157:
1102:
1073:
1048:
1020:
963:
904:
834:
764:
559:
498:
is sufficiently stable that exohedral derivatization from the mono- to the hexa adduct of the
239:
75:
606:
580:
368:
spectrum. It is possible to force the metal atoms to a standstill at the equator as shown by
1667:
1629:
1577:
1567:
1520:
1462:
1407:
1368:
1333:
1290:
1247:
1204:
1149:
1110:
1065:
1010:
1002:
953:
943:
896:
868:
826:
796:
754:
746:
713:
551:
513:
334:
179:
115:
512:
of the nitrogen atom in the center to the carbon atoms of the cage takes place. Therefore,
470:
are chemically very inert and commonly exist as individual atoms, this is not the case for
356:
the two metal atoms exhibit a non-bonded interaction. Since all the six-membered rings in C
341:
resin and used as a solid phase against a mobile phase containing the complex mixture in a
302:
1625:
1516:
1458:
1441:
Kurotobi, K.; Murata, Y. (2011). "A Single
Molecule of Water Encapsulated in Fullerene C
1403:
1364:
1337:
1329:
1286:
1243:
1200:
1145:
1061:
998:
939:
1605:
1582:
1547:
1015:
982:
958:
923:
1696:
1216:
547:
536:
528:
274:
1482:
1427:
1308:
Zope, Rajendra R (2008). "Electronic structure and static dipole polarizability of C
1169:
1085:
345:
operation. Only very stable fullerenes such as @ pass through the column unreacted.
19:
1688:
Movie "Helium atom trapped in fullerene (C60) and dodecahedrane (C20H20)" (Youtube)
499:
282:
215:
1372:
1183:
Sabirov, D.; Bulgakov, R. (2010). "Polarizability exaltation of endofullerenes X@C
1069:
431:
in a noble-gas atmosphere. Under these conditions about one out of every 650,000 C
16:
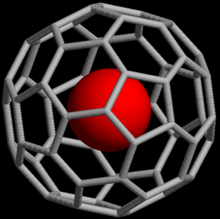
Fullerene molecule with additional atoms, ions, or clusters enclosed within itself
1251:
524:
502:
223:
191:
928:
Proceedings of the
National Academy of Sciences of the United States of America
1687:
1208:
475:
428:
490:
is more surprising. The nitrogen atom is in its electronic initial state (S
1466:
1411:
1153:
948:
467:
396:
231:
219:
211:
195:
152:
79:
63:
24:
1643:
1634:
1591:
1572:
1532:
1474:
1419:
1161:
1077:
1024:
967:
908:
838:
768:
648:
endofullerenes with sufficient accuracy. The calculated data allows using C
471:
372:
when the fullerene is exahedrally functionalized by an electron donation
314:
183:
1114:
800:
35:
1524:
759:
750:
452:
301:
is not always simple to determine. In most cases it is between 2 and 3
235:
227:
187:
95:
1671:
1294:
1006:
900:
830:
265:
In 1999 a discovery drew large attention. With the synthesis of the Sc
872:
440:
436:
373:
207:
199:
148:
1609:
539:
as a consequence of the packing in the cage could be observed with
644:−1). It describes the DFT-calculated mean polarizabilities of Ng@C
505:
456:
448:
159:
155:
trapped inside, and two potassium atoms adhering to the outside."
144:
34:
18:
520:
in a high resolution spectrum as shoulders of the central line.
444:
400:
395:, an endohedral metallofluorenol, can competitively inhibit the
290:
170:
Doping fullerenes with electropositive metals takes place in an
135:
if M were inside the carbon network. A more complex example is K
67:
202:. Also possible are endohedral complexes with elements of the
71:
384:
with 1,1,2,2-tetrakis(2,4,6-trimethylphenyl)-1,2-disilirane.
1318:
Journal of
Physics B: Atomic, Molecular and Optical Physics
1187:(n = 20, 24, 28, 36, 50, and 60; X is a noble gas atom)".
131:
to the right. The example above would then be denoted M@C
558:
can be produced by opening and closing a fullerene by
609:
583:
143:
B), which denotes "a 60-atom fullerene cage with one
478:
and so the formation of the endohedral complexes N@C
403:from activating. It was originally developed as an
621:
595:
439:atom. The formation of endohedral complexes with
550:(nitrogen and phosphorus complexes) or by direct
23:Rendering of a buckminsterfullerene containing a
1610:"Spectroscopy of light-molecule endofullerenes"
126:) with an atom (M) was simply represented as MC
78:enclosed within their inner spheres. The first
8:
1548:"First Synthesis and Characterization of CH
660:and giant fullerene-containing "onions" ).
494:) and is highly reactive. Nevertheless, N@C
242:develop with different cage sizes like La@C
684:, the hydrogen fluoride endofullerene HF@C
1633:
1581:
1571:
1014:
957:
947:
782:
780:
778:
758:
608:
582:
1496:Krachmalnicoff, A.; et al. (2016).
889:Journal of the American Chemical Society
819:Journal of the American Chemical Society
812:
810:
603:), it is positive; for the larger ones (
1560:Angewandte Chemie International Edition
729:
459:as well as numerous adducts of the He@C
850:
848:
7:
1546:Bloodworth, S.; et al. (2019).
508:is possible. In these compounds no
1498:"The dipolar endofullerene HF@C60"
1265:Delaney, P.; Greer, J. C. (2004).
688:, and the methane endofullerene CH
151:in the geodesic network, a single
14:
981:Kang SG, Huynh T, Zhou R (2012).
527:require complex equipment, e.g.
289:in a nitrogen atmosphere at 300
313:however it can be even about 6
51:molecules and in the nanotubes.
39:Electron microscopy images of M
861:Journal of Materials Chemistry
556:endohedral hydrogen fullerenes
423:are prepared by pressurizing C
1:
1373:10.1016/j.diamond.2007.10.019
1338:10.1088/0953-4075/41/8/085101
1070:10.1126/science.259.5100.1428
789:Journal of Physical Chemistry
1252:10.1016/j.cplett.2008.03.046
305:units, in the case of the La
166:Endohedral metallofullerenes
100:endohedral metallofullerenes
676:, the water endofullerene H
1724:
411:Non-metal doped fullerenes
329:The lack of reactivity in
104:non-metal doped fullerenes
1209:10.1134/S0021364010220054
415:Endohedral complexes He@C
376:group in a reaction of Ce
285:and graphite powder in a
1708:Supramolecular chemistry
1232:Chemical Physics Letters
664:Molecular endofullerenes
1467:10.1126/science.1206376
1412:10.1126/science.1106185
1390:by Organic Synthesis".
1275:Applied Physics Letters
1154:10.1126/science.1206376
949:10.1073/pnas.1204600109
622:{\displaystyle n>30}
596:{\displaystyle n<30}
435:cages was doped with a
147:atom substituted for a
1635:10.1098/rsta.2012.0429
1573:10.1002/anie.201900983
623:
597:
52:
32:
624:
598:
370:x-ray crystallography
343:column chromatography
331:Diels-Alder reactions
204:alkaline earth metals
66:that have additional
56:Endohedral fullerenes
38:
22:
1353:Diamond Relat. Mater
607:
581:
262:were also isolated.
178:. The metals can be
120:buckminsterfullerene
1626:2013RSPTA.37120429L
1517:2016NatCh...8..953K
1459:2011Sci...333..613K
1404:2005Sci...307..238K
1365:2007DRM....16.2145L
1330:2008JPhB...41h5101Z
1287:2004ApPhL..84..431D
1244:2008CPL...456..223Y
1201:2010JETPL..92..662S
1146:2011Sci...333..613K
1115:10.1021/ja00084a089
1062:1993Sci...259.1428S
1056:(5100): 1428–1430.
999:2012NatSR...2E.957K
940:2012PNAS..10915431K
801:10.1021/j100173a002
719:Inclusion compounds
280:scandium(III) oxide
86:complex called La@C
1620:(1998): 20120429.
1525:10.1038/nchem.2563
1271:as a Faraday cage"
987:Scientific Reports
751:10.1039/C0CC02929G
619:
593:
541:ENDOR spectroscopy
405:MRI contrast agent
53:
33:
1672:10.1021/jp310673j
1566:(15): 5038–5043.
1453:(6042): 613–616.
1398:(5707): 238–240.
1359:(12): 2145–2149.
1295:10.1063/1.1640783
1103:J. Am. Chem. Soc.
1007:10.1038/srep00957
901:10.1021/ja054346r
831:10.1021/ja055089t
795:(20): 7564–7568.
714:Fullerene ligands
560:organic chemistry
554:. Alternatively,
337:is modified as a
240:metallofullerenes
180:transition metals
176:laser evaporation
114:In a traditional
1715:
1676:
1675:
1666:(2): 1178–1182.
1660:J. Phys. Chem. C
1654:
1648:
1647:
1637:
1602:
1596:
1595:
1585:
1575:
1543:
1537:
1536:
1505:Nature Chemistry
1502:
1493:
1487:
1486:
1438:
1432:
1431:
1383:
1377:
1376:
1348:
1342:
1341:
1305:
1299:
1298:
1262:
1256:
1255:
1238:(4–6): 223–226.
1227:
1221:
1220:
1180:
1174:
1173:
1125:
1119:
1118:
1109:(5): 2193–2194.
1096:
1090:
1089:
1035:
1029:
1028:
1018:
978:
972:
971:
961:
951:
919:
913:
912:
883:
877:
876:
873:10.1039/b804168g
852:
843:
842:
814:
805:
804:
784:
773:
772:
762:
745:(7): 2116–2118.
734:
628:
626:
625:
620:
602:
600:
599:
594:
552:ion implantation
399:in the oncogene
339:cyclopentadienyl
335:Merrifield resin
116:chemical formula
94:in 1985. The @ (
1723:
1722:
1718:
1717:
1716:
1714:
1713:
1712:
1693:
1692:
1684:
1679:
1656:
1655:
1651:
1604:
1603:
1599:
1555:
1551:
1545:
1544:
1540:
1511:(10): 953–957.
1500:
1495:
1494:
1490:
1444:
1440:
1439:
1435:
1389:
1385:
1384:
1380:
1350:
1349:
1345:
1315:
1311:
1307:
1306:
1302:
1270:
1264:
1263:
1259:
1229:
1228:
1224:
1195:(10): 662–665.
1186:
1182:
1181:
1177:
1140:(6042): 613–6.
1131:
1127:
1126:
1122:
1098:
1097:
1093:
1045:
1041:
1037:
1036:
1032:
980:
979:
975:
934:(38): 15431–6.
921:
920:
916:
895:(42): 14570–1.
885:
884:
880:
867:(28): 3347–51.
859:(M = Ce, La)".
858:
854:
853:
846:
825:(46): 16292–8.
816:
815:
808:
786:
785:
776:
736:
735:
731:
727:
710:
703:
699:
695:
691:
687:
683:
679:
675:
671:
666:
659:
655:
651:
647:
639:
605:
604:
579:
578:
572:
565:
519:
510:charge transfer
497:
493:
489:
485:
481:
462:
434:
426:
422:
418:
413:
394:
390:
383:
379:
363:
359:
355:
351:
324:
320:
312:
308:
299:charge transfer
272:
268:
261:
257:
253:
249:
245:
168:
142:
138:
134:
129:
125:
112:
89:
85:
50:
46:
42:
30:
17:
12:
11:
5:
1721:
1719:
1711:
1710:
1705:
1695:
1694:
1691:
1690:
1683:
1682:External links
1680:
1678:
1677:
1649:
1597:
1553:
1549:
1538:
1488:
1442:
1433:
1387:
1378:
1343:
1313:
1309:
1300:
1268:
1257:
1222:
1184:
1175:
1129:
1120:
1091:
1043:
1039:
1030:
973:
914:
878:
856:
844:
806:
774:
728:
726:
723:
722:
721:
716:
709:
706:
701:
697:
693:
689:
685:
681:
677:
673:
669:
665:
662:
657:
653:
649:
645:
637:
618:
615:
612:
592:
589:
586:
570:
563:
533:magnetic traps
517:
495:
491:
487:
483:
479:
460:
432:
424:
420:
416:
412:
409:
392:
388:
381:
377:
361:
357:
353:
349:
322:
318:
310:
306:
270:
266:
259:
255:
251:
247:
243:
167:
164:
140:
136:
132:
127:
123:
111:
108:
87:
83:
60:endofullerenes
58:, also called
48:
44:
40:
28:
15:
13:
10:
9:
6:
4:
3:
2:
1720:
1709:
1706:
1704:
1701:
1700:
1698:
1689:
1686:
1685:
1681:
1673:
1669:
1665:
1661:
1653:
1650:
1645:
1641:
1636:
1631:
1627:
1623:
1619:
1615:
1611:
1607:
1606:Levitt, M. H.
1601:
1598:
1593:
1589:
1584:
1579:
1574:
1569:
1565:
1561:
1557:
1542:
1539:
1534:
1530:
1526:
1522:
1518:
1514:
1510:
1506:
1499:
1492:
1489:
1484:
1480:
1476:
1472:
1468:
1464:
1460:
1456:
1452:
1448:
1437:
1434:
1429:
1425:
1421:
1417:
1413:
1409:
1405:
1401:
1397:
1393:
1382:
1379:
1374:
1370:
1366:
1362:
1358:
1354:
1347:
1344:
1339:
1335:
1331:
1327:
1324:(8): 085101.
1323:
1319:
1304:
1301:
1296:
1292:
1288:
1284:
1280:
1276:
1272:
1261:
1258:
1253:
1249:
1245:
1241:
1237:
1233:
1226:
1223:
1218:
1214:
1210:
1206:
1202:
1198:
1194:
1190:
1179:
1176:
1171:
1167:
1163:
1159:
1155:
1151:
1147:
1143:
1139:
1135:
1124:
1121:
1116:
1112:
1108:
1105:
1104:
1095:
1092:
1087:
1083:
1079:
1075:
1071:
1067:
1063:
1059:
1055:
1051:
1050:
1034:
1031:
1026:
1022:
1017:
1012:
1008:
1004:
1000:
996:
992:
988:
984:
977:
974:
969:
965:
960:
955:
950:
945:
941:
937:
933:
929:
925:
918:
915:
910:
906:
902:
898:
894:
890:
882:
879:
874:
870:
866:
862:
851:
849:
845:
840:
836:
832:
828:
824:
820:
813:
811:
807:
802:
798:
794:
790:
783:
781:
779:
775:
770:
766:
761:
756:
752:
748:
744:
740:
733:
730:
724:
720:
717:
715:
712:
711:
707:
705:
663:
661:
643:
636:
632:
616:
613:
610:
590:
587:
584:
576:
567:
561:
557:
553:
549:
548:gas discharge
544:
542:
538:
537:wave function
534:
530:
529:laser cooling
526:
521:
515:
511:
507:
504:
501:
477:
473:
469:
464:
458:
454:
450:
446:
442:
438:
430:
410:
408:
406:
402:
398:
385:
375:
371:
367:
346:
344:
340:
336:
332:
327:
317:such as in Sc
316:
304:
300:
294:
292:
288:
287:K-H generator
284:
281:
276:
263:
241:
237:
233:
229:
225:
221:
217:
216:alkali metals
213:
209:
205:
201:
197:
193:
189:
185:
181:
177:
173:
165:
163:
161:
156:
154:
150:
146:
121:
117:
109:
107:
105:
101:
97:
93:
81:
77:
73:
69:
65:
61:
57:
37:
26:
21:
1663:
1659:
1652:
1617:
1613:
1600:
1563:
1559:
1541:
1508:
1504:
1491:
1450:
1446:
1436:
1395:
1391:
1381:
1356:
1352:
1346:
1321:
1317:
1303:
1278:
1274:
1260:
1235:
1231:
1225:
1192:
1188:
1178:
1137:
1133:
1123:
1106:
1101:
1094:
1053:
1047:
1033:
990:
986:
976:
931:
927:
917:
892:
888:
881:
864:
860:
822:
818:
792:
788:
742:
739:Chem. Commun
738:
732:
667:
641:
634:
630:
574:
568:
545:
525:atomic traps
522:
500:malonic acid
465:
414:
386:
347:
328:
295:
283:iron nitride
264:
226:metals like
169:
157:
118:notation, a
113:
103:
99:
59:
55:
54:
760:10347/32317
514:C-couplings
468:noble gases
224:tetravalent
192:lanthanides
190:as well as
172:arc reactor
92:synthesized
1703:Fullerenes
1697:Categories
1281:(3): 431.
725:References
476:phosphorus
275:Harry Dorn
64:fullerenes
1217:119757902
1189:JETP Lett
427:to ca. 3
397:WW domain
315:electrons
232:zirconium
220:potassium
212:strontium
196:lanthanum
153:potassium
80:lanthanum
27:atom (M@C
25:noble gas
1644:23918717
1608:(2013).
1592:30773760
1533:27657872
1483:33694477
1475:21798946
1428:24964146
1420:15653499
1170:33694477
1162:21798946
1086:41794612
1078:17801275
1042:and Ne@C
1025:23233876
968:22949663
909:16231899
839:16287323
769:21183975
708:See also
472:nitrogen
419:and Ne@C
184:scandium
110:Notation
76:clusters
1622:Bibcode
1583:6492075
1513:Bibcode
1455:Bibcode
1447:Science
1400:Bibcode
1392:Science
1361:Bibcode
1326:Bibcode
1283:Bibcode
1240:Bibcode
1197:Bibcode
1142:Bibcode
1134:Science
1058:Bibcode
1049:Science
1016:3518810
995:Bibcode
993:: 957.
959:3458392
936:Bibcode
486:and P@C
453:krypton
246:or La@C
236:hafnium
228:uranium
188:yttrium
174:or via
96:at sign
1642:
1590:
1580:
1531:
1481:
1473:
1426:
1418:
1215:
1168:
1160:
1084:
1076:
1023:
1013:
966:
956:
907:
837:
767:
466:While
441:helium
437:helium
303:charge
208:barium
200:cerium
149:carbon
62:, are
1501:(PDF)
1479:S2CID
1424:S2CID
1213:S2CID
1166:S2CID
1082:S2CID
506:ester
503:ethyl
482:, N@C
457:xenon
449:argon
374:silyl
348:In Ce
218:like
206:like
194:like
182:like
160:ASCII
145:boron
74:, or
68:atoms
1640:PMID
1588:PMID
1529:PMID
1471:PMID
1416:PMID
1158:PMID
1074:PMID
1021:PMID
964:PMID
905:PMID
835:PMID
765:PMID
614:>
588:<
474:and
455:and
445:neon
401:YAP1
391:(OH)
387:Gd@C
291:Torr
234:and
222:and
210:and
198:and
139:(K@C
102:and
90:was
72:ions
1668:doi
1664:117
1630:doi
1618:371
1578:PMC
1568:doi
1521:doi
1463:doi
1451:333
1445:".
1408:doi
1396:307
1369:doi
1334:doi
1316:".
1314:240
1291:doi
1248:doi
1236:456
1205:doi
1150:doi
1138:333
1132:".
1111:doi
1107:116
1066:doi
1054:259
1046:".
1011:PMC
1003:doi
954:PMC
944:doi
932:109
897:doi
893:127
869:doi
827:doi
823:127
797:doi
755:hdl
747:doi
680:O@C
658:240
531:or
492:3/2
429:bar
366:NMR
321:N@C
273:by
269:N@C
43:N@C
1699::
1662:.
1638:.
1628:.
1616:.
1612:.
1586:.
1576:.
1564:58
1562:.
1558:.
1554:60
1552:@C
1527:.
1519:.
1507:.
1503:.
1477:.
1469:.
1461:.
1449:.
1443:60
1422:.
1414:.
1406:.
1394:.
1388:60
1367:.
1357:16
1355:.
1332:.
1322:41
1320:.
1312:@C
1310:60
1289:.
1279:84
1277:.
1273:.
1269:60
1267:"C
1246:.
1234:.
1211:.
1203:.
1193:92
1191:.
1164:.
1156:.
1148:.
1136:.
1130:60
1080:.
1072:.
1064:.
1052:.
1044:60
1040:60
1019:.
1009:.
1001:.
989:.
985:.
962:.
952:.
942:.
930:.
926:.
903:.
891:.
865:18
863:.
857:80
847:^
833:.
821:.
809:^
793:95
791:.
777:^
763:.
753:.
743:47
741:.
702:60
700:@C
694:60
692:@C
686:60
682:60
674:60
672:@C
656:@C
654:60
650:60
646:60
640:(2
638:Ng
633:=
617:30
591:30
571:60
566:.
564:60
518:60
496:60
488:60
484:70
480:60
461:60
451:,
447:,
443:,
433:60
425:60
421:60
417:60
407:.
393:22
389:82
382:80
380:@C
360:-I
358:80
354:80
352:@C
323:80
311:80
309:@C
293:.
271:80
260:80
258:@C
248:82
244:60
230:,
214:,
186:,
141:59
133:60
128:60
124:60
122:(C
106:.
88:60
84:60
70:,
49:80
45:80
31:).
29:60
1674:.
1670::
1646:.
1632::
1624::
1594:.
1570::
1556:"
1550:4
1535:.
1523::
1515::
1509:8
1485:.
1465::
1457::
1430:.
1410::
1402::
1375:.
1371::
1363::
1340:.
1336::
1328::
1297:.
1293::
1285::
1254:.
1250::
1242::
1219:.
1207::
1199::
1185:n
1172:.
1152::
1144::
1117:.
1113::
1088:.
1068::
1060::
1027:.
1005::
997::
991:2
970:.
946::
938::
911:.
899::
875:.
871::
841:.
829::
803:.
799::
771:.
757::
749::
698:2
690:4
678:2
670:2
642:e
635:α
631:α
611:n
585:n
575:α
378:2
362:h
350:2
319:3
307:2
267:3
256:2
254:C
252:3
137:2
82:C
41:3
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.