657:(CNS) penetrant oxytocin receptor antagonist would show an effect on ejaculation when given systemically. This has been achieved with the Cligosiban which has good CNS penetration. Epelsiban was also investigated as an agent to enhance embryo or blastocyst implantation in women undergoing embryo or blastocyst transfer associated with in vitro fertilization (IVF). and for use in the treatment of adenomyosis. However the development of epelsiban for adenomyosis was terminated in December 2016 for strategic reasons and not because of any safety concerns.
287:
765:
29:
538:
631:(1A2, 2C9, 2C19. 2D6, 3A4 DEF, 3A4 7BQ). In addition, Epelsiban has low intrinsic clearance in all four species (rat, dog. cyno monkey, human), a good PK profile in the rat with a bioavailability of 55%, oral exposure and bioavailability in the cynomolgus monkey comparable to retosiban, and good aqueous solubility (33 mg/ml as the besylate salt).
644:
the contractile effect of oxytocin in human prostatic tissue through its specific oxytocin receptors in a concentration-dependent manner. suggesting a potential role in the treatment of benign prostatic hyperplasia. The selective antagonist
Epelsiban was designed to work on peripheral human oxytocin receptors and not to readily pass the
619:. High in vivo oxytocin antagonist potency was demonstrated in the anesthetized rat model, where uterine contractions were elicited by intravenous administration of oxytocin and reduction in uterine contractility was measured after subsequent intravenous administrations of increasing doses of Epelsiban, which gave an IC50 of 192nM.
643:
also called prostate enlargement. Oxytocin treatment induces prostate enlargement in mice and produces contractions of the prostate through its specific receptor. Oxytocin concentrations are elevated in prostatic tissue from patients with benign prostatic hyperplasia. Epelsiban was found to inhibit
610:
In-vitro binding inhibition data showed that
Epelsiban is a highly potent and selective non-peptide oxytocin antagonist with sub-namomolar potency at the human oxytocin receptor (hOTR) Ki = 0.13 nM and with>50000-fold, >63000-fold, and >31000-fold selectivity over the human V1a, V1b and V2
589:
which achieved good oral bioavailability in the rat (53%) and dog (51%) whilst retaining good oxytocin antagonist potency (Ki= 0.63nM) and >1000 fold selectivity relative to the human vasopressin V1A, V2, V1B receptors. The introduction of polar heterocycles to improve the solubility and human
1163:
Rouget C, Rekik M, Camparo P, Botto H, Rischmann P, Lluel P, Palea S, McCallum SW, Westfall TD (April 2012). "1568 Oxytocin
Produces Contraction of Human Isolated Prostate, an Effect Blocked by the Novel and Selective Oxytocin Receptor Antagonist Gsk557296 Potential Role in Benign Prostatic
899:
Borthwick AD, Liddle J, Davies DE, Exall AM, Hamlett C, Hickey DM, Mason AM, Smith IE, Nerozzi F, Peace S, Pollard D, Sollis SL, Allen MJ, Woollard PM, Pullen MA, Westfall TD, Stanislaus DJ (January 2012). "Pyridyl-2,5-diketopiperazines as potent, selective, and orally bioavailable oxytocin
777:
In screening the GSK compound collection and various libraries, a key consideration was to choose a template with good levels of selectivity over the three vasopressin receptors which are structurally similar to the oxytocin receptor. In addition all templates were also assessed by
786:
for predicted CNS penetration. This was to decrease the risk that templates would be chosen that would cross the blood brain barrier and thus block the central effects of oxytocin both in the foetus and in the mother. This identified the small, conformationally constrained,
585:(PK) profile of this template was achieved by property-based design using an estimated of human oral absorption (EHOA) which focused the SAR on 2,5-DKPs with small exocyclic aromatic rings combined with small amides. This resulted in the 2’,4’-difluoro dimethylamide
1030:
Shinghal R, Barnes A, Mahar KM, Stier B, Giancaterino L, Condreay LD, Black L, McCallum SW (October 2013). "Safety and efficacy of epelsiban in the treatment of men with premature ejaculation: A randomized, double‐blind, placebo‐controlled, fixed‐dose study".
1226:
Wayman C, Russell R, Tang K, Weibly L, Gaboardi S, Fisher L, Allers K, Jackson M, Hawcock T, Robinson N, Wilson L (December 2018). "Cligosiban, a novel brain-penetrant, selective oxytocin receptor antagonist, inhibits ejaculatory physiology in rodents".
360:
1279:
1116:
Mahar KM, Enslin MB, Gress A, Amrine-Madsen H, Cooper M (January 2018). "Single‐and
Multiple‐Day Dosing Studies to Investigate High‐Dose Pharmacokinetics of Epelsiban and Its Metabolite, GSK2395448, in Healthy Female Volunteers".
1608:
1272:
652:
to rodents. As expected, despite this success achieved in mice, oral epelsiban in humans at 50 or 150 mg has not shown satisfactory results in a double blind, placebo-controlled trial. This suggested that a
791:
2,5-DKP scaffold as the preferred template and lead to the success in designing and developing the highly potent and selective, orally active, peripheral oxytocin antagonist
Epelsiban as a clinical candidate.
402:
InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-16-21-8-6-7-9-22(21)17-23)29(36)34(26)27(24-11-10-19(3)31-20(24)4)30(37)33-12-14-38-15-13-33/h6-11,18,23,25-27H,5,12-17H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1
1265:
1069:
Mahar KM, Stier B, Fries M, McCallum SW (November 2015). "A single- and multiple-dose study to investigate the pharmacokinetics of epelsiban and its metabolite, GSK2395448, in healthy female volunteers".
581:) isomers of the monosubstituted aryl 2,5-DKPs with wide range of different functionality had similarly high levels of potency, they all had low bioavailability in the rat. Optimization of the
1601:
1594:
41:
615:(a marketed intravenous peptide oxytocin antagonist) and is 5-fold more potent against the hOTR, and more selective against the human vasopressin receptors, especially V2, than
1574:
627:
Epelsiban has a good Cyp450 profile with no significant inhibition IC50 > 100μM together with no time-dependent inhibition observed against the five Cyp450
374:
1191:
Xu H, Fu S, Chen Y, Chen Q, Gu M, Liu C, Qiao Z, Zhou J, Wang Z (April 2017). "Oxytocin: its role in benign prostatic hyperplasia via the ERK pathway".
2349:
942:
Borthwick AD, Liddle J (January 2013). "Retosiban and
Epelsiban: Potent and Selective Orally available Oxytocin Antagonists". In Domling A (ed.).
764:
2369:
1494:
840:
959:
841:"International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names (Rec. INN): List 67"
499:
649:
421:
394:
602:
to drive the improvements in the later’s pharmacokinetic profile lead to the 2’,6’-dimethyl-3’-pyridyl morpholine amide
Epelsiban.
2364:
2019:
1875:
537:
2123:
1828:
86:
62:
1460:
2379:
2374:
640:
206:
1535:
266:
980:"Inhibition of ejaculation by the non‐peptide oxytocin receptor antagonist GSK 557296: a multi‐level site of action"
2354:
1257:
425:
666:
1531:
645:
534:) and an indanyl group was preferred at C-3, while at C-6, a 4-carbon branched alkyl was shown to be optimal.
1696:
705:. Hydrogenation to remove the Cbz and benzyl protecting groups, enabled cyclization of the linear peptide
654:
467:
255:
1485:
1297:
665:
The cyclic dipeptide
Epelsiban is formed by cyclizing the corresponding linear dipeptide. In the highly
648:. However Epelsiban was found to inhibited brain oxytocin receptors mediating ejaculation, when given
451:
440:
1866:
1621:
1306:
1289:
714:
566:
483:
282:
1746:
847:
436:
115:
2192:
2103:
2098:
1954:
1949:
749:) at the exocyclic amide, the hydrochloric acid hydrolysis of the activated phenolic amide caused
28:
1691:
1686:
1624:
1142:
1095:
2256:
2251:
2207:
1808:
1803:
1758:
1708:
1676:
1656:
1646:
2276:
2093:
1964:
1944:
1793:
1788:
1768:
1586:
2295:
2271:
2266:
2261:
2227:
2113:
2009:
1632:
1617:
1512:
1244:
1208:
1134:
1087:
1048:
1009:
955:
917:
718:
491:
433:
2078:
1452:
944:
Methods and
Principles in Medicinal Chemistry: Protein-Protein Interactions in Drug Discovery
195:
2359:
2222:
2004:
1999:
1984:
1846:
1728:
1400:
1324:
1236:
1200:
1173:
1126:
1079:
1040:
999:
991:
947:
909:
742:
303:
124:
1773:
873:
215:
1666:
1553:
1548:
729:
by activating the acid with the peptide coupling reagent CDI, followed by the addition of
591:
582:
447:
286:
498:, showed potency of Ki = 300nM as a mixture of isomers in the amide side-chain. Initial
2327:
2286:
1851:
1334:
1004:
979:
978:
Clément P, Bernabé J, Compagnie S, Alexandre L, McCallum S, Giuliano F (August 2013).
2343:
2323:
1426:
1372:
1315:
750:
682:
155:
1146:
1099:
2167:
2137:
2053:
2033:
1909:
1456:
1431:
1418:
1385:
1293:
678:
514:
side-chain configuration at C-7. The optimal activity was shown to lie in the (3
2319:
2172:
2157:
2142:
2083:
2058:
2048:
2038:
1974:
1914:
1904:
1899:
1889:
1661:
1543:
1408:
1177:
471:
1240:
951:
462:
implantation in women undergoing embryo or blastocyst transfer associated with
2246:
2212:
2202:
2197:
2187:
2088:
1994:
1939:
1934:
1833:
1723:
1651:
1502:
1470:
1380:
1362:
1347:
788:
730:
599:
459:
336:
186:
2217:
2177:
2152:
2063:
2043:
1969:
1919:
1894:
1856:
1841:
1818:
1798:
1783:
1778:
1753:
1701:
1671:
1464:
1390:
1352:
821:
816:
811:
806:
779:
616:
1248:
1212:
1138:
1091:
1052:
1013:
921:
20:
2162:
1989:
1929:
1823:
1741:
1718:
1681:
1357:
1342:
801:
783:
628:
612:
166:
130:
611:
vasopressin receptors. It is also 100-fold more potent at the hOTR than
175:
2291:
2147:
2073:
1959:
1713:
1436:
1204:
482:
Screening the GSK compound collection and various libraries identified
141:
1044:
995:
913:
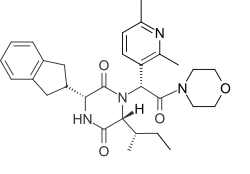
432:) is an orally bioavailable drug which acts as a selective and potent
1979:
1130:
1083:
455:
382:
CC(C)1C(=O)N(C(=O)N1(C2=C(N=C(C=C2)C)C)C(=O)N3CCOCC3)C4CC5=CC=CC=C5C4
246:
235:
359:
350:
900:
antagonists: synthesis, pharmacokinetics, and in vivo potency".
874:"Statement on a Nonproprietary Name Adopted by the USAN Council"
733:. In this short lab-scale synthesis although the linear peptide
226:
1590:
1261:
502:(SAR) studies led to the semi-rigid and chirally pure 2,5-DKP
763:
536:
271:
470:)., and was also investigated for use in the treatment of
846:. World Health Organization. p. 62. Archived from
565:
showed very good levels of selectivity relative to the
713:. Hydrolysis of the phenolic amide, by reaction with
2309:
2236:
2122:
2018:
1874:
1865:
1631:
1530:
1511:
1493:
1483:
1445:
1417:
1399:
1371:
1333:
1314:
1304:
639:Epelsiban was investigated for a potential role in
348:
335:
302:
297:
265:
245:
225:
205:
185:
165:
140:
114:
77:
61:
56:
40:
35:
782:profiling and suitable templates were evaluated
768:Synthetic Route to Oxytocin Antagonist Epelsiban
490:as novel and selective antagonists at the human
154:
753:at the exocyclic position and yielded the acid
709:to occur to give the phenolic cyclic dipeptide
123:
1111:
1109:
1064:
1062:
1025:
1023:
973:
971:
937:
935:
933:
931:
894:
892:
693:, D-alloisoleucine methyl ester hydrochloride
1602:
1273:
1158:
1156:
510:disposed substituents at C-3 and C-6 and the
8:
19:
725:which was converted to the amide Epelsiban
717:(CDI), followed by the addition of aqueous
1871:
1609:
1595:
1587:
1490:
1311:
1280:
1266:
1258:
446:= 0.13 nM). It was initially developed by
285:
194:
1119:Clinical Pharmacology in Drug Development
1072:Clinical Pharmacology in Drug Development
1003:
946:. Weinheim: Wiley-VCH. pp. 225–256.
214:
761:)-stereochemistry as the major product.
697:, 2,6-dimethylpyridine-3-carboxaldehyde
832:
454:in men and then as an agent to enhance
399:
379:
281:
174:
91:
106:-inden-2-yl)-1--6-piperazine-2,5-dione
18:
254:
7:
1575:Sexual dysfunction pharmacotherapies
234:
145:
14:
541:Discovery and Design of Epelsiban
701:and 2-benzyloxyphenylisonitrile
677:is formed by the four-component
320:
314:
27:
984:British Journal of Pharmacology
500:structure–activity relationship
407:Key:UWHCWRQFNKUYCG-QUZACWSFSA-N
2350:Drugs not assigned an ATC code
1229:The Journal of Sexual Medicine
1033:The Journal of Sexual Medicine
902:Journal of Medicinal Chemistry
569:. However, although all the (3
326:
308:
70:In general: non-regulated
1:
2370:Oxytocin receptor antagonists
598:using intrinsic clearance in
641:benign prostatic hyperplasia
1178:10.1016/j.juro.2012.02.1340
594:(Cyp450) enzyme profile of
450:(GSK) for the treatment of
2396:
1241:10.1016/j.jsxm.2018.10.008
952:10.1002/9783527648207.ch10
486:(2,5-DKPs) exemplified by
298:Chemical and physical data
2178:Vasotocin (argiprestocin)
2173:Vasopressin (argipressin)
2064:Vasotocin (argiprestocin)
2059:Vasopressin (argipressin)
1920:Vasotocin (argiprestocin)
1915:Vasopressin (argipressin)
1702:Vasotocin (argiprestocin)
1566:
737:and the cyclic dipeptide
667:stereoselective synthesis
390:
370:
82:
26:
2365:4-Morpholinyl compounds
2094:ORG-52186 (SCH-740935)
1816:Catabolism inhibitors:
1166:The Journal of Urology
769:
655:central nervous system
542:
1486:premature ejaculation
1419:Melanocortin agonists
1298:premature ejaculation
872:USAN Council (2011).
767:
673:, the linear peptide
567:vasopressin receptors
540:
484:2,5-diketopiperazines
452:premature ejaculation
1622:vasopressin receptor
1307:erectile dysfunction
1290:erectile dysfunction
757:with the required (7
715:carbonyl diimidazole
478:Discovery and Design
2380:Sec-Butyl compounds
2375:3-Pyridyl compounds
1824:Bestatin (ubenimex)
646:blood–brain barrier
606:Mechanism of action
23:
1205:10.1042/CS20170030
853:on October 5, 2016
770:
650:intraventricularly
543:
102:)-3-(2,3-dihydro-1
2355:Diketopiperazines
2337:
2336:
2317:Carrier proteins:
2305:
2304:
2296:lithium carbonate
2284:Other inhibitors:
1836:
1692:PF-06655075 (PF1)
1584:
1583:
1562:
1561:
1479:
1478:
1401:Dopamine agonists
1045:10.1111/jsm.12272
1039:(10): 2506–2517.
996:10.1111/bph.12198
961:978-3-527-33107-9
914:10.1021/jm201287w
741:are a mixture of
719:hydrochloric acid
506:(Ki = 4nM), with
494:(OTR). The lead,
492:oxytocin receptor
434:oxytocin receptor
415:
414:
361:Interactive image
267:CompTox Dashboard
16:Chemical compound
2387:
1872:
1847:o-Phenanthroline
1834:
1611:
1604:
1597:
1588:
1491:
1312:
1282:
1275:
1268:
1259:
1253:
1252:
1223:
1217:
1216:
1193:Clinical Science
1188:
1182:
1181:
1160:
1151:
1150:
1131:10.1002/cpdd.363
1113:
1104:
1103:
1084:10.1002/cpdd.210
1066:
1057:
1056:
1027:
1018:
1017:
1007:
990:(7): 1477–1485.
975:
966:
965:
939:
926:
925:
896:
887:
886:
884:
883:
878:
869:
863:
862:
860:
858:
852:
845:
837:
743:diastereoisomers
689:-indanylglycine
685:(Cbz) protected
623:Pharmacokinetics
526:) series (e.g.,
363:
343:
328:
322:
316:
310:
290:
289:
275:
273:
258:
238:
218:
198:
178:
158:
148:
147:
127:
31:
24:
22:
2395:
2394:
2390:
2389:
2388:
2386:
2385:
2384:
2340:
2339:
2338:
2333:
2301:
2232:
2127:
2118:
2023:
2014:
1879:
1861:
1667:Lipo-oxytocin-1
1627:
1615:
1585:
1580:
1558:
1554:Methylphenidate
1549:Methamphetamine
1526:
1507:
1475:
1441:
1413:
1395:
1367:
1335:PDE5 inhibitors
1329:
1300:
1286:
1256:
1225:
1224:
1220:
1190:
1189:
1185:
1162:
1161:
1154:
1115:
1114:
1107:
1068:
1067:
1060:
1029:
1028:
1021:
977:
976:
969:
962:
941:
940:
929:
898:
897:
890:
881:
879:
876:
871:
870:
866:
856:
854:
850:
843:
839:
838:
834:
830:
798:
775:
663:
637:
625:
608:
592:cytochrome P450
583:pharmacokinetic
480:
466:fertilization (
448:GlaxoSmithKline
444:
411:
408:
403:
398:
397:
386:
383:
378:
377:
366:
341:
331:
325:
319:
313:
293:
283:DTXSID701029864
269:
261:
241:
221:
201:
181:
161:
144:
136:
128:
110:
107:
90:
89:
73:
52:
17:
12:
11:
5:
2393:
2391:
2383:
2382:
2377:
2372:
2367:
2362:
2357:
2352:
2342:
2341:
2335:
2334:
2332:
2331:
2313:
2311:
2307:
2306:
2303:
2302:
2300:
2299:
2289:
2287:Demeclocycline
2280:
2279:
2274:
2269:
2264:
2259:
2254:
2249:
2240:
2238:
2234:
2233:
2231:
2230:
2225:
2220:
2215:
2210:
2205:
2200:
2195:
2190:
2181:
2180:
2175:
2170:
2165:
2160:
2155:
2150:
2145:
2140:
2131:
2129:
2125:
2120:
2119:
2117:
2116:
2107:
2106:
2101:
2096:
2091:
2086:
2081:
2076:
2067:
2066:
2061:
2056:
2051:
2046:
2041:
2036:
2027:
2025:
2021:
2016:
2015:
2013:
2012:
2007:
2002:
1997:
1992:
1987:
1982:
1977:
1972:
1967:
1962:
1957:
1952:
1947:
1942:
1937:
1932:
1923:
1922:
1917:
1912:
1907:
1902:
1897:
1892:
1883:
1881:
1877:
1869:
1863:
1862:
1860:
1859:
1854:
1852:Phosphoramidon
1849:
1844:
1839:
1831:
1826:
1821:
1812:
1811:
1806:
1801:
1796:
1791:
1786:
1781:
1776:
1771:
1766:
1761:
1756:
1744:
1732:
1731:
1726:
1721:
1716:
1711:
1699:
1694:
1689:
1684:
1679:
1674:
1669:
1664:
1659:
1654:
1649:
1637:
1635:
1629:
1628:
1616:
1614:
1613:
1606:
1599:
1591:
1582:
1581:
1579:
1578:
1567:
1564:
1563:
1560:
1559:
1557:
1556:
1551:
1546:
1540:
1538:
1528:
1527:
1525:
1524:
1518:
1516:
1509:
1508:
1506:
1505:
1499:
1497:
1488:
1481:
1480:
1477:
1476:
1474:
1473:
1468:
1449:
1447:
1443:
1442:
1440:
1439:
1434:
1429:
1423:
1421:
1415:
1414:
1412:
1411:
1405:
1403:
1397:
1396:
1394:
1393:
1388:
1383:
1377:
1375:
1373:Alpha blockers
1369:
1368:
1366:
1365:
1360:
1355:
1350:
1345:
1339:
1337:
1331:
1330:
1328:
1327:
1321:
1319:
1316:Prostaglandins
1309:
1302:
1301:
1287:
1285:
1284:
1277:
1270:
1262:
1255:
1254:
1235:(12): 1698–7.
1218:
1199:(7): 595–607.
1183:
1164:Hyperplasia".
1152:
1105:
1078:(6): 418–426.
1058:
1019:
967:
960:
927:
888:
864:
831:
829:
826:
825:
824:
819:
814:
809:
804:
797:
794:
774:
771:
721:gave the acid
662:
659:
636:
633:
624:
621:
607:
604:
479:
476:
442:
413:
412:
410:
409:
406:
404:
401:
393:
392:
391:
388:
387:
385:
384:
381:
373:
372:
371:
368:
367:
365:
364:
356:
354:
346:
345:
339:
333:
332:
329:
323:
317:
311:
306:
300:
299:
295:
294:
292:
291:
278:
276:
263:
262:
260:
259:
251:
249:
243:
242:
240:
239:
231:
229:
223:
222:
220:
219:
211:
209:
203:
202:
200:
199:
191:
189:
183:
182:
180:
179:
171:
169:
163:
162:
160:
159:
151:
149:
138:
137:
135:
134:
129:1159097-48-9 (
120:
118:
112:
111:
109:
108:
93:
85:
84:
83:
80:
79:
75:
74:
72:
71:
67:
65:
59:
58:
54:
53:
51:
50:
46:
44:
38:
37:
33:
32:
15:
13:
10:
9:
6:
4:
3:
2:
2392:
2381:
2378:
2376:
2373:
2371:
2368:
2366:
2363:
2361:
2358:
2356:
2353:
2351:
2348:
2347:
2345:
2329:
2325:
2321:
2318:
2315:
2314:
2312:
2308:
2297:
2293:
2290:
2288:
2285:
2282:
2281:
2278:
2275:
2273:
2270:
2268:
2265:
2263:
2260:
2258:
2255:
2253:
2250:
2248:
2245:
2242:
2241:
2239:
2235:
2229:
2226:
2224:
2221:
2219:
2216:
2214:
2211:
2209:
2206:
2204:
2201:
2199:
2196:
2194:
2191:
2189:
2186:
2183:
2182:
2179:
2176:
2174:
2171:
2169:
2166:
2164:
2161:
2159:
2156:
2154:
2151:
2149:
2146:
2144:
2141:
2139:
2136:
2133:
2132:
2130:
2128:
2121:
2115:
2112:
2109:
2108:
2105:
2102:
2100:
2097:
2095:
2092:
2090:
2087:
2085:
2082:
2080:
2077:
2075:
2072:
2069:
2068:
2065:
2062:
2060:
2057:
2055:
2052:
2050:
2047:
2045:
2042:
2040:
2037:
2035:
2032:
2029:
2028:
2026:
2024:
2017:
2011:
2008:
2006:
2003:
2001:
1998:
1996:
1993:
1991:
1988:
1986:
1983:
1981:
1978:
1976:
1973:
1971:
1968:
1966:
1963:
1961:
1958:
1956:
1953:
1951:
1948:
1946:
1943:
1941:
1938:
1936:
1933:
1931:
1928:
1925:
1924:
1921:
1918:
1916:
1913:
1911:
1908:
1906:
1903:
1901:
1898:
1896:
1893:
1891:
1888:
1885:
1884:
1882:
1880:
1873:
1870:
1868:
1864:
1858:
1855:
1853:
1850:
1848:
1845:
1843:
1840:
1838:
1832:
1830:
1827:
1825:
1822:
1820:
1817:
1814:
1813:
1810:
1807:
1805:
1802:
1800:
1797:
1795:
1792:
1790:
1787:
1785:
1782:
1780:
1777:
1775:
1772:
1770:
1767:
1765:
1762:
1760:
1757:
1755:
1752:
1748:
1747:Tocinoic acid
1745:
1743:
1740:
1737:
1734:
1733:
1730:
1727:
1725:
1722:
1720:
1717:
1715:
1712:
1710:
1707:
1703:
1700:
1698:
1695:
1693:
1690:
1688:
1685:
1683:
1680:
1678:
1675:
1673:
1670:
1668:
1665:
1663:
1660:
1658:
1655:
1653:
1650:
1648:
1645:
1642:
1639:
1638:
1636:
1634:
1630:
1626:
1623:
1619:
1612:
1607:
1605:
1600:
1598:
1593:
1592:
1589:
1577:
1576:
1573:
1569:
1568:
1565:
1555:
1552:
1550:
1547:
1545:
1542:
1541:
1539:
1537:
1533:
1529:
1523:
1520:
1519:
1517:
1514:
1510:
1504:
1501:
1500:
1498:
1496:
1492:
1489:
1487:
1482:
1472:
1469:
1466:
1462:
1458:
1454:
1451:
1450:
1448:
1444:
1438:
1435:
1433:
1430:
1428:
1427:Bremelanotide
1425:
1424:
1422:
1420:
1416:
1410:
1407:
1406:
1404:
1402:
1398:
1392:
1389:
1387:
1384:
1382:
1379:
1378:
1376:
1374:
1370:
1364:
1361:
1359:
1356:
1354:
1351:
1349:
1346:
1344:
1341:
1340:
1338:
1336:
1332:
1326:
1323:
1322:
1320:
1317:
1313:
1310:
1308:
1303:
1299:
1295:
1291:
1283:
1278:
1276:
1271:
1269:
1264:
1263:
1260:
1250:
1246:
1242:
1238:
1234:
1230:
1222:
1219:
1214:
1210:
1206:
1202:
1198:
1194:
1187:
1184:
1179:
1175:
1171:
1167:
1159:
1157:
1153:
1148:
1144:
1140:
1136:
1132:
1128:
1124:
1120:
1112:
1110:
1106:
1101:
1097:
1093:
1089:
1085:
1081:
1077:
1073:
1065:
1063:
1059:
1054:
1050:
1046:
1042:
1038:
1034:
1026:
1024:
1020:
1015:
1011:
1006:
1001:
997:
993:
989:
985:
981:
974:
972:
968:
963:
957:
953:
949:
945:
938:
936:
934:
932:
928:
923:
919:
915:
911:
908:(2): 783–96.
907:
903:
895:
893:
889:
875:
868:
865:
849:
842:
836:
833:
827:
823:
820:
818:
815:
813:
810:
808:
805:
803:
800:
799:
795:
793:
790:
785:
781:
772:
766:
762:
760:
756:
752:
751:epimerisation
748:
744:
740:
736:
732:
728:
724:
720:
716:
712:
708:
704:
700:
696:
692:
688:
684:
683:carboxybenzyl
680:
676:
672:
669:of Epelsiban
668:
660:
658:
656:
651:
647:
642:
634:
632:
630:
622:
620:
618:
614:
605:
603:
601:
597:
593:
588:
584:
580:
576:
572:
568:
564:
560:
556:
552:
548:
539:
535:
533:
529:
525:
521:
517:
513:
509:
505:
501:
497:
493:
489:
485:
477:
475:
473:
469:
465:
461:
457:
453:
449:
445:
438:
435:
431:
430:GSK-557,296-B
427:
423:
419:
405:
400:
396:
389:
380:
376:
369:
362:
358:
357:
355:
352:
347:
340:
338:
334:
307:
305:
301:
296:
288:
284:
280:
279:
277:
268:
264:
257:
256:ChEMBL2037511
253:
252:
250:
248:
244:
237:
233:
232:
230:
228:
224:
217:
213:
212:
210:
208:
204:
197:
193:
192:
190:
188:
184:
177:
173:
172:
170:
168:
164:
157:
153:
152:
150:
143:
139:
132:
126:
122:
121:
119:
117:
113:
105:
101:
97:
92:
88:
81:
76:
69:
68:
66:
64:
60:
55:
48:
47:
45:
43:
39:
36:Clinical data
34:
30:
25:
2316:
2283:
2244:Antagonists:
2243:
2193:JNJ-17079166
2185:Antagonists:
2184:
2168:Terlipressin
2138:Desmopressin
2134:
2110:
2104:TASP-0390325
2099:TASP-0233278
2071:Antagonists:
2070:
2054:Terlipressin
2034:Desmopressin
2030:
1955:JNJ-17308616
1950:JNJ-17079166
1927:Antagonists:
1926:
1910:Terlipressin
1886:
1815:
1763:
1751:Non-peptide:
1750:
1738:
1736:Antagonists:
1735:
1706:Non-peptide:
1705:
1643:
1640:
1571:
1570:
1521:
1457:acecarbromal
1432:Melanotan II
1386:Phentolamine
1232:
1228:
1221:
1196:
1192:
1186:
1172:(4S): e635.
1169:
1165:
1125:(1): 33–43.
1122:
1118:
1075:
1071:
1036:
1032:
987:
983:
943:
905:
901:
880:. Retrieved
867:
855:. Retrieved
848:the original
835:
776:
758:
754:
746:
738:
734:
726:
722:
710:
706:
702:
698:
694:
690:
686:
679:Ugi reaction
674:
670:
664:
638:
635:Pharmacology
626:
609:
595:
586:
578:
574:
570:
562:
558:
554:
550:
546:
544:
531:
527:
523:
519:
515:
511:
507:
503:
495:
487:
481:
463:
429:
428:, code name
417:
416:
103:
99:
95:
63:Legal status
57:Legal status
2320:Neurophysin
2158:Ornipressin
2143:Felypressin
2084:Brezivaptan
2049:Ornipressin
2039:Felypressin
1975:Relcovaptan
1905:Selepressin
1900:Ornipressin
1890:Felypressin
1867:Vasopressin
1837:-Methionine
1687:PF-06478939
1662:Demoxytocin
1544:Amphetamine
1515:antagonists
1409:Apomorphine
1325:Alprostadil
472:adenomyosis
344: g·mol
125:872599-83-2
78:Identifiers
2344:Categories
2257:RWJ-339489
2252:Ribuvaptan
2247:Balovaptan
2213:Satavaptan
2208:RWJ-351647
2203:Mozavaptan
2198:Lixivaptan
2188:Conivaptan
2089:Nelivaptan
1995:WAY-267464
1940:Conivaptan
1935:Balovaptan
1809:WAY-162720
1804:SSR-126768
1759:Cligosiban
1724:WAY-267464
1677:Nacartocin
1657:Cargutocin
1652:Carbetocin
1647:Aspartocin
1625:modulators
1503:Dapoxetine
1471:Papaverine
1381:Moxisylyte
1363:Vardenafil
1348:Sildenafil
1288:Drugs for
882:2011-10-28
828:References
789:homochiral
731:morpholine
600:microsomes
460:blastocyst
437:antagonist
349:3D model (
337:Molar mass
216:T2EZ19HX73
187:ChemSpider
116:CAS Number
87:IUPAC name
2277:YM-222546
2218:Tolvaptan
2153:Lypressin
2135:Agonists:
2044:Lypressin
2031:Agonists:
1970:PF-184563
1965:LY-307174
1945:FR-218944
1895:Lypressin
1887:Agonists:
1857:Puromycin
1842:Leupeptin
1819:Amastatin
1799:Retosiban
1794:Nolasiban
1789:L-372,662
1784:L-371,257
1779:L-368,899
1769:Erlosiban
1764:Epelsiban
1754:Barusiban
1672:Merotocin
1641:Agonists:
1572:See also:
1522:Epelsiban
1465:vitamin E
1461:quebracho
1391:Yohimbine
1353:Tadalafil
857:4 October
822:Retosiban
817:L-371,257
812:L-368,899
807:Barusiban
780:in silico
661:Synthesis
617:retosiban
557:) series
418:Epelsiban
21:Epelsiban
2272:VMAX-382
2267:VMAX-372
2262:VMAX-367
2237:Unsorted
2228:YM-35471
2163:TC OT 39
2114:TASP-699
2111:Ligands:
2010:YM-35471
1990:TC OT 39
1930:Atosiban
1742:Atosiban
1739:Peptide:
1719:TC OT 39
1682:Oxytocin
1644:Peptide:
1633:Oxytocin
1618:Oxytocin
1513:Oxytocin
1358:Udenafil
1343:Avanafil
1249:30527053
1213:28130436
1147:41083332
1139:28556598
1100:23903528
1092:27137713
1053:23937679
1014:23530818
922:22239250
802:Atosiban
796:See also
784:in vitro
629:isozymes
613:atosiban
464:in vitro
167:DrugBank
156:11634973
131:besylate
42:ATC code
2360:Indanes
2292:Lithium
2148:LIT-001
2079:ABT-558
2074:ABT-436
1960:LIT-001
1714:LIT-001
1453:Afrodor
1437:PL-6983
1005:3724105
773:History
681:of the
342:518.658
304:Formula
196:9809717
176:DB11934
142:PubChem
2310:Others
2223:YM-471
2005:YM-471
2000:YM-218
1985:SRX251
1980:SRX246
1729:WJ0679
1446:Others
1296:) and
1247:
1211:
1145:
1137:
1098:
1090:
1051:
1012:
1002:
958:
920:
545:The (3
456:embryo
375:SMILES
247:ChEMBL
236:D10117
1774:IX-01
1495:SSRIs
1294:G04BE
1143:S2CID
1096:S2CID
877:(PDF)
851:(PDF)
844:(PDF)
395:InChI
351:JSmol
1829:EDTA
1697:TGOT
1620:and
1536:DRIs
1484:For
1305:For
1245:PMID
1209:PMID
1135:PMID
1088:PMID
1049:PMID
1010:PMID
956:ISBN
918:PMID
859:2016
426:USAN
227:KEGG
207:UNII
49:None
1709:CA7
1532:DRA
1318:(E)
1237:doi
1201:doi
1197:131
1174:doi
1170:187
1127:doi
1080:doi
1041:doi
1000:PMC
992:doi
988:169
948:doi
910:doi
577:, 7
573:, 6
553:, 7
549:, 6
522:, 7
518:, 6
508:cis
468:IVF
458:or
422:INN
272:EPA
146:CID
2346::
2328:II
2326:,
2022:1B
1878:1A
1749:;
1704:;
1463:,
1459:,
1243:.
1233:15
1231:.
1207:.
1195:.
1168:.
1155:^
1141:.
1133:.
1121:.
1108:^
1094:.
1086:.
1074:.
1061:^
1047:.
1037:10
1035:.
1022:^
1008:.
998:.
986:.
982:.
970:^
954:.
930:^
916:.
906:55
904:.
891:^
755:10
747:RS
745:(7
727:11
723:10
671:11
561:,
530:,
474:.
424:,
318:38
312:30
98:,6
94:(3
2330:)
2324:I
2322:(
2298:)
2294:(
2126:2
2124:V
2020:V
1876:V
1835:L
1610:e
1603:t
1596:v
1534:/
1467:)
1455:(
1292:(
1281:e
1274:t
1267:v
1251:.
1239::
1215:.
1203::
1180:.
1176::
1149:.
1129::
1123:7
1102:.
1082::
1076:4
1055:.
1043::
1016:.
994::
964:.
950::
924:.
912::
885:.
861:.
759:R
739:9
735:8
711:9
707:8
703:7
699:6
695:5
691:4
687:R
675:8
596:3
587:3
579:R
575:R
571:R
563:3
559:2
555:R
551:R
547:R
532:3
528:2
524:R
520:R
516:R
512:R
504:2
496:1
488:1
443:i
441:K
439:(
420:(
353:)
330:4
327:O
324:4
321:N
315:H
309:C
274:)
270:(
133:)
104:H
100:R
96:R
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.